Abstract
Summary: A detachable coil was assessed for magnetic field interactions, heating (body RF coil, SAR of 2.0-W/kg), and artifacts at 3-Tesla. The coil showed no magnetic field interactions and heating was negligible (+0.3°C). Therefore, this coil is safe for a patient undergoing MR imaging at 3T or less. While artifacts may impair the ability to properly visualize anatomy in proximity to this implant, careful selection of imaging parameters can mitigate this problem.
The use of detachable coils is a safe and effective alternative to surgical clipping of intracranial aneurysms (1–3). Accordingly, there has been increased use of these implants (1–3). MR imaging is frequently used to examine patients who have undergone coil embolization by using standard imaging techniques as well as MR angiography (MRA), perfusion-weighted imaging, and diffusion-weighted imaging (4–6).
Since these implants are made from metallic materials, there are potential safety problems (e.g., magnetic field interactions and heating) and related issues (e.g., artifacts) that must be considered (7–9). Importantly, imaging systems with 3T magnets are now being used on a routine basis and have a greater tendency to impact safety and artifact size for metallic implants as compared with lower-field-strength systems (8, 9). In consideration of the increasing use of embolization coils and the likelihood that patients with these implants may require MR imaging examinations, this investigation determined magnetic field interactions, heating, and artifacts at 3T for a detachable coil used for treatment of cerebral aneurysms.
Materials
Detachable Coil
A detachable coil used for treatment of cerebral aneurysms (TruFill DCS Orbit Detachable Coil, Cordis Neurovascular, Inc., Miami Lakes, FL) was assessed in this investigation. This implant is made from 92% platinum and 8% tungsten. The outer diameter of this coil is 0.3 mm, the length is 30 cm, the coiled-shape diameter is 12 cm, and the mass is 225 mg.
Magnetic Field Interactions
Magnetic field interactions were determined for the coil using a shielded, 3T MR system (General Electric Medical Systems, Milwaukee, WI).
Translational Attraction.
Translational attraction was assessed for the coil by using the deflection angle method, as previously described (9). The implant was formed into a 12-mm-diameter coil and attached to a test fixture to measure the deflection angle of the 3T MR system. The test fixture has a protractor with 1° graduated markings mounted to the top of the structure and included a bubble level to ensure proper orientation in the system for the test procedure. The coil was suspended from the protractor by a 20-cm length of string (weight, <1% of the weight of the implant) that was attached at the 0° indicator (9, 10).
Measurements were obtained at the position in the 3T MR system that produced the greatest magnetically induced deflection angle (9, 10). This point was determined by using gauss line plots, magnetic field measurements, and visual inspection to identify the location of the highest spatial gradient (9, 10). For the 3T MR system, the direction of the static magnetic field is horizontal and the highest spatial gradient, 3.25 T/m, occurs at a position that is 96 cm from the isocenter (9). The coil was held on the test fixture so that the string was vertical and then released. The deflection angle from the vertical direction to the nearest 1° was measured three times and an average value was calculated (9, 10)
Torque.
Magnetic field-induced torque was assessed qualitatively for the coil by using a previously described methodology (9). This procedure used a flat plastic material with a millimeter grid on the bottom (9). The coil was formed into a 12-mm diameter and placed on the test platform in an orientation that was 45° relative to the static magnetic field of the 3T system. The test apparatus with the coil was positioned in the center of the imaging, where the effect of torque from the static magnetic field is the greatest (based on the known characteristics of the 3T MR system used for this assessment) (9). The coil was observed for movement with respect to alignment or rotation relative to the static magnetic field. The coil was then moved 45° relative to its previous position and again observed for alignment or rotation (9). This process was repeated to encompass a full 360° rotation of positions for the coil in the 3T MR system. A qualitative scale was applied to the results to characterize torque (9): 0, no torque; +1, mild or low torque (the implant slightly changed orientation but did not align to the magnetic field); +2, moderate torque (the implant aligned gradually to the magnetic field); +3, strong torque (the implant showed rapid and forceful alignment to the magnetic field); +4, very strong torque (the implant showed very rapid and very forceful alignment to the magnetic field).
MR Imaging-Related Heating
An in vitro experiment was performed at 3T to determine MR imaging-related heating for the coil (11, 12). The coil (formed into a coil shape and of 12-mm diameter) was placed in a plastic phantom that approximated the size and shape of the human head and torso (dimensions: head portion - width, 16.5-cm; length, 29.2-cm; height, 16.5-cm; torso portion - width, 43.2-cm; length, 61.0-cm; height, 16.5-cm (12). The phantom was filled with a gelling agent (hydroxyetheyl-cellulose) in an aqueous solution (91.48% H2O) with 0.12% NaCl (12). A plastic frame placed on the bottom of the phantom had small adjustable posts that were used to position the coil according to its intended in vivo use (12). This experimental set-up lacks blood flow and, thus, simulates an extreme condition with regard to MR imaging-related heating for the coil.
Temperatures were recorded by a fluoroptic thermometry system (Model 3100, Luxtron, Santa Clara, CA). Fluoroptic thermometry probes (0.5-mm diameter) were positioned on the coil to record positions on this implant that would be associated with the greatest heating during MR imaging (i.e., based on results from pilot experiments), as follows: probe 1, placed in direct contact with one “free” end of the coil; probe 2, placed in direct contact with the middle portion of the coil. Probe 3 was also placed in the gelled saline at a position approximately 60 cm from the coil to record a reference temperature. Fluoroptic thermometry probe positions were verified immediately before and after the heating experiment.
MR imaging was performed at 3T on the gelled-saline filled phantom with the coil by using a transmit RF body coil to produce a relatively high level of RF energy, as follows: gradient echo; axial plane; repetition time, 8-ms; echo time, 4-ms; flip angle, 20°; FOV, 35 cm; imaging matrix, 256 × 128; section thickness, 5 mm; number of section locations, 8; phase direction, anterior to posterior; transmitter gain, 180. These imaging parameters produced a whole-body-averaged specific absorption rate (SAR) of 2.0-W/kg and spatial peak SAR of 4.0-W/kg. Of note is that this level of exposure to RF energy exceeds that typically used for most clinical MR imaging procedures involving the brain at 3T. To simulate MR imaging-related heating for the coil, the “landmark” position (i.e., the center position or anatomic region for MR imaging) and section locations imaged were selected to encompass the entire area of the coil. The room temperature and system’s bore temperature were at a constant level throughout the heating experiment and the system’s fan was off. After recording 5-minute baseline temperatures, MR imaging was performed for 20 minutes with temperatures recorded at 20-second intervals.
Artifacts
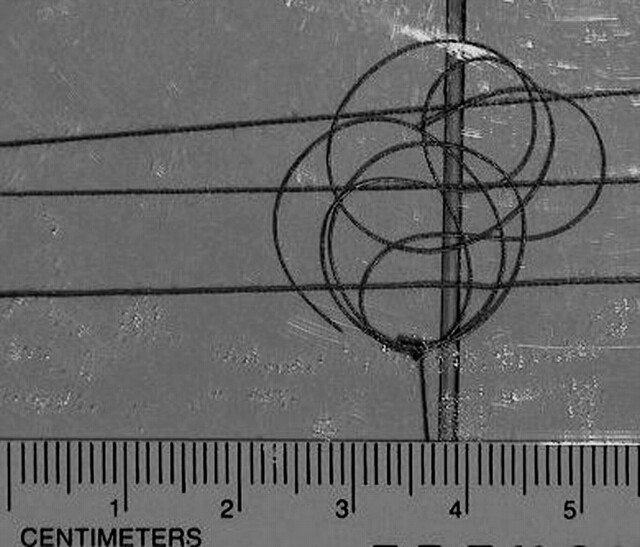
Artifacts were characterized for the coil by performing MR imaging of the implant (coil-shaped) with it attached to a flat plastic frame and placed inside of a gadolinium-doped, saline-filled, plastic phantom (Fig 1) (13), based on recommendations from the ASTM International (14). Gadolinium-doped saline was used to provide a high signal intensity background for the evaluation of artifacts in this study and has been used in other artifact assessments for metallic implants (13). MR images were obtained by using a 3T MR system (General Electric Medical Systems), a transmit-receive RF head coil (i.e., similar to what would be used for MR imaging of the brain) and the following parameters: T1-weighted, spin-echo pulse sequence; TR, 500 ms; TE, 20 ms; matrix size, 256 × 256; section thickness, 10 mm; FOV, 20 cm; NEX, 2; and gradient echo pulse sequence; TR, 100 ms; TE, 15 ms; flip angle, 30°; matrix size, 256 × 256; section thickness, 10 mm; FOV, 20 cm; NEX, 2 (13). Imaging planes were oriented to encompass the long axis and short axis of the coil-shaped implant. The frequency encoding direction was parallel to the plane of imaging for each condition. Similar pulse sequences have been used for artifact evaluations of implants (13). Planimetry software was used to measure (accuracy and resolution +10%) the cross-sectional area of the largest artifact size (outer dimensions of the coiled-shape implant, determined during pilot experiments) for the coil, for each pulse sequence and for each orientation of the section location (13). Image display parameters (i.e., window and level settings, magnification, etc.) were used in a consistent manner to facilitate valid measurements of artifact size.
Fig 1.
The detachable coil (TruFill DCS Orbit Detachable Coil) shown attached to a plastic frame for the assessment of artifacts at 3T.
Results
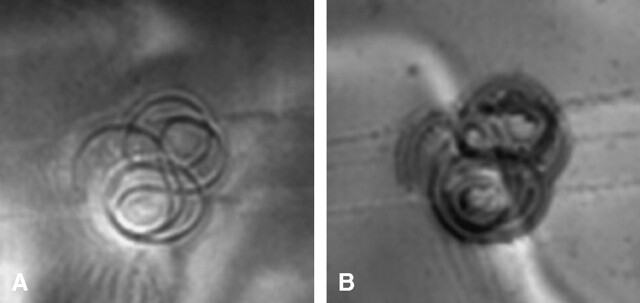
The average deflection angle was 0° and the qualitative torque value was 0 (no torque) for the detachable coil. Findings for the MR imaging-related heating experiment showed that the highest temperature change was +0.3°C, as recorded by probes 1 and 2. The highest temperature change measured by the reference probe was also +0.3°C. Artifact test results are summarized in Table 1. The artifacts were seen as signal intensity voids that were slightly larger than the size and shape of the coil, with the gradient echo pulse sequence showing larger artifacts than those obtained with the T1-weighted, spin-echo pulse sequence (Fig 2).
TABLE 1:
Artifact size for the detachable coil associated with MR imaging at 3T
| Pulse Sequence | Plane Orientation | Signal Void (mm2) |
|---|---|---|
| T1-SE | long axis | 671 |
| short axis | 20 | |
| GRE | long axis | 787 |
| short axis | 85 |
Imaging plane relative to the coil; T1-SE indicates T1-weighted spin echo; GRE, gradient echo.
Fig 2.
Artifacts characterized for the detachable coil.
A, T1-weighted MR image (TR/TE, 500/20; section thickness, 10-mm; FOV, 20-cm) of the detachable coil at 3T.
B, Gradient echo MR image (TR/TE/flip angle, 100/20/30; section thickness, 10-mm; FOV, 20-cm) of the detachable coil at 3T.
Discussion
Magnetic Field Interactions
The coil that underwent in vitro testing showed a deflection angle of 0° and no torque during exposure to a 3T magnetic field. Because this embolization coil lacks magnetic field interactions, a patient with this implant may undergo MR imaging at 3T or less without concerns of movement or migration. Notably, while certain intravascular implants that exhibit “weak” magnetic field interactions have labeling statements that indicate patients must wait 6–8 weeks before undergoing an MR imaging examination (8, 9), a waiting period is unnecessary for this coil.
MR Imaging-Related Heating
MR imaging can produce excessive temperature elevations in implants made from conductive materials that form a closed loop or that have an elongated shape (7, 8, 12). Accordingly, this is a theoretical safety concern for coils, particularly with regard to the RF energy used in association with a 3T MR system (7, 8). The TruFill DCS Orbit Detachable Coil evaluated for MR imaging-related heating at 3T and a whole-body-averaged SAR of 2.0-W/kg showed a temperature change (+0.3°C) that was the same as that of the “background” heating of the gelled-saline-filled phantom. This small amount of heating will not present a risk to a patient undergoing MR imaging under comparable conditions used in this study. The absence of heating is likely related to the small diameter of loops formed by the coil (loops formed by this implant in situ will be even smaller), which are not conducive to the coupling of RF fields.
Artifacts
The extent of the artifact associated with a metallic implant is dependent on the magnetic susceptibility of the material, the configuration of the implant, the field strength of the system, the pulse sequence used for MR imaging, and other factors (7, 8, 14). Using similar imaging parameters, larger artifacts will occur for a metallic implant at 3T as compared with 1.5T. Because of the importance of using MR imaging to monitor embolized aneurysms (4–6), it is desirable to use coils made with materials that have a low magnetic susceptibility.
Artifacts at 3T were relatively minor for the TruFill DCS Orbit Detachable Coil in comparison to the size and shape of this implant; only localized signal intensity losses were evident (Fig 2). The gradient echo pulse sequence produced larger artifacts than those of the T1-weighted pulse sequence, similar to what has been reported for other implants (8, 13). Thus, depending on the pulse sequence used for imaging, the extent of the artifact may impair the ability to visualize cranial anatomy located in the same area or in proximity to this coil.
Hennenmeyer et al (7) studied the platinum Guglielmi Detachable Coil (GDC) at 3T and reported that imaging artifacts were minimal. Therefore, obtaining high-spatial-resolution structural and functional MR images is likely feasible in patients with GDCs (7). Given the small percentage of tungsten (8%) present in the TruFill DCS Orbit Detachable Coil and its impact on imaging artifacts, similar results are expected when imaging a patient with this implant at 3T. Notably, the selection of parameters known to minimize artifact size should mitigate the extent of signal intensity loss associated with this coil at 3T, allowing the use of imaging techniques that are most advantageous for monitoring aneurysms (15, 16).
Other Considerations
The present study was performed to determine if this coil is “MR-safe.” Unfortunately, there is a tendency in the MR community to use the terms “MR-safe” and “MR-compatible” in an interchangeable manner, causing undue confusion (17). Furthermore, these terms are sometimes exploited or misused in marketing efforts by implant manufacturers, adding to the confusion that exists (8). The terms MR-safe and MR-compatible are defined by the ASTM International, as follows (10, 11): MR-safe - The device, when used in the MR environment, has been demonstrated to present no additional risk to the patient or other individuals, but may affect the quality of the diagnostic information. MR-compatible - The device, when used in the MR environment, is MR safe and has been demonstrated to neither significantly affect the quality of the diagnostic information nor have its operations affected by the MR device. The MR conditions used to test the implant should be specified in conjunction with the terms MR-safe and MR-compatible, since an implant that is safe or compatible by using one set of conditions may not be found to be so under other conditions (10, 11). In general, MR safety testing involves an evaluation of magnetic field interactions and heating for an implant while MR compatibility testing requires these tests and characterization of artifacts. Furthermore, for mechanical, magnetic, or electronic devices, a functional assessment may also be needed (17). From a practical consideration, the MR community is predominantly concerned if an implant or device is, in fact, MR-safe.
Footnotes
Supported by an unrestricted research grant from Cordis Neurovascular, Inc., Miami Lakes, FL.
References
- 1.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 2.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acuteintracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 3.Tournade A, Courtheoux P, Sengel C, Ozgulle S, Tajahmady T. Saccular intracranial aneurysms: endovascular treatment with mechanical detachable spiral coils. Radiology 1997;202:481–486 [DOI] [PubMed] [Google Scholar]
- 4.Vallee J-N, Pierot L, Bonafe A, Turjman F, Flandroy P, Berge J, Rodesch G, Bracard S. Endovascular treatment of intracranial wide-necked aneurysms using three-dimensional coils: predictors of immediate anatomic and clinical results. AJNR Am J Neuroradiol 2004;25:298–306 [PMC free article] [PubMed] [Google Scholar]
- 5.Kahara VJ, Seppanen SK, Ryymin PS, Mattila P, Laasonen EM. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:1470–1475 [PMC free article] [PubMed] [Google Scholar]
- 6.Biondi A, Oppenheim C, Vivas E, Casasco A, Lalam T, Sourour N, Jean LL, Dormont D, Marsault C. Cerebral aneurysms treated by Guglielmi detachable coils: evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2000;21:957–963 [PMC free article] [PubMed] [Google Scholar]
- 7.Hennemeyer CT, Wicklow K, Feinberg DA, Derdeyn CP. In vitro evaluation of platinum Guglielmi detachable coils at 3 T with a porcine model: Safety issues and artifacts. Radiology 2001;219:732–737 [DOI] [PubMed] [Google Scholar]
- 8.Shellock FG. Reference manual for magnetic resonance safety, implants, and devices: 2005 edition . Biomedical Research Publishing Group, Los Angeles, CA,2005
- 9.Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-Tesla MR system. J Magn Reson Imaging 2002;16:721–732 [DOI] [PubMed] [Google Scholar]
- 10.American Society for Testing and Materials (ASTM) International: F2052. Standard test method for measurement of magnetically induced displacement force on passive implants in the magnetic resonance environment. In: Annual Book of ASTM Standards, Volume 13.01 Medical Devices; West Conshohocken, PA,2001;pp:1576–1580
- 11.American Society for Testing and Materials (ASTM) International. F2182 Test method for measurement of radio frequency induced heating near passive implants during magnetic resonance imaging. American Society for Testing and Materials (ASTM) International, West Conshohocken, PA2003
- 12.Rezai AR, Finelli D, Nyenhuis JA, Hrdlick G, Tkach J, Ruggieri P, Stypulkowski PH, Sharan A, Shellock FG. Neurostimulator for deep brain stimulation: Ex vivo evaluation of MRI-related heating at 1.5-Tesla. J Magn Reson Imaging 2002;15:241–250 [DOI] [PubMed] [Google Scholar]
- 13.Shellock FG, Kanal E. Aneurysm clips: Evaluation of MR imaging artifacts at 1.5 Tesla. Radiology 1998;209:563–566 [DOI] [PubMed] [Google Scholar]
- 14.American Society for Testing and Materials (ASTM) International: F2119 Test method for evaluation of MR image artifacts from passive implants. American Society for Testing and Materials (ASTM) International, West Conshohocken, PA2003
- 15.Hug J, Nagel E, Bornstedt A, Schackenburg B, Oswald H, Fleck E. Coronary arterial stents: safety and artifacts during MR imaging. Radiology 2000;216:781–787 [DOI] [PubMed] [Google Scholar]
- 16.Suh JS, Jeong EK, Shin KY, et al. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol 1998;171:1207–1213. [DOI] [PubMed] [Google Scholar]
- 17.Shellock FG, Crues JV. Commentary: MR safety and the American College of Radiology White Paper. AJR Am J Roentgenology 2002;178:1349–1352 [DOI] [PubMed] [Google Scholar]