Abstract
BACKGROUND AND PURPOSE: Aneurysmal subarachnoid hemorrhage (SAH) affects six to eight people per 100,000 annually, yet the optimum management of this condition remains controversial. Although the International Subarachnoid Aneurysm Trial (ISAT) explored this area, only 28% of patients from our center were randomized in that study. Our purpose was to evaluate the treatment and outcomes of patients not recruited into ISAT.
METHODS: Procedural data, adverse events, additional procedures, and length of hospital stay were recorded for 122 patients who came to our center with aneurysmal SAH. Modified Rankin Scale (MRS) was assessed at 2 months and at 1 year by a postal questionnaire and telephone interview.
RESULTS: Nine patients were treated conservatively, 67 underwent surgical clipping, and 46 underwent endovascular therapy. At 2 months, a good grade (MRS 0–2) was achieved in 67% of patients treated with endovascular therapy and in 45% of patients treated with surgery. At 1 year, a good grade was achieved in 72% in the endovascular group and 49% in the surgical group.
CONCLUSION: Endovascular therapy was a safe and effective treatment in a series of subjects who were not randomized in ISAT and in whom endovascular therapy was chosen over surgical clipping. The outcomes at 2 months and 1 year for those subjects treated with endovascular therapy were superior to the outcomes in those treated with surgical clipping. Our study was small and from a single center, but in this population the outcomes of endovascular treatment were similar to those reported by ISAT.
Aneurysmal subarachnoid hemorrhage (SAH) is a significant cause of mortality and continuing morbidity in the population worldwide. Recent studies show the annual incidence of SAH in Western Europe and North America is six to eight per 100,000 population (1). The natural history of the disease is such that more than 30% of patients will die within the first 24 hours of the bleed and another 25–30% will die in the subsequent 4 weeks without some form of intervention (1). Aneurysmal rebleeding and delayed ischemic neurologic deficits attributable to clinical vasospasm are major causes of morbidity and mortality in those patients who survive the initial effects of aneurysm rupture (2–4).
The conventional method of treating intracranial aneurysms has been surgical clipping. Although surgery is invasive and associated with high morbidity rates, this approach invariably provides a definitive solution (2–4). During the past 10 years, new management strategies in the form of endovascular therapy have been developed in an attempt to balance these risks and to optimize clinical outcomes for patients with aneurysmal SAH. Since the introduction of Guglielmi detachable coils in 1991, endovascular treatment has assumed increasing importance in the management of intracranial aneurysms; yet, this form of treatment is not without risk and is associated with immediate and delayed thromboembolic and ischemic complications (5–8).
In October 2002, results were published from the International Subarachnoid Aneurysm Trial (ISAT) in which surgery was compared with endovascular therapy in the treatment of ruptured intracranial aneurysms (9). ISAT was stopped prematurely after an interim analysis that showed a significantly better outcome for patients treated with endovascular coiling than for those treated with neurosurgical clipping. Randomization to ISAT internationally was at approximately 20% of the target population, which has led to some criticism as the patients represented a select subgroup of which 88% of patients were of good clinical status, 93% of the target aneurysms were 10 mm or smaller, and 97% of aneurysms were in the anterior circulation (10). Although these results are important, the optimum management of the entire group of patients with SAH seen in clinical practice remains far from clear.
The aim of this study was to explore the management strategies and clinical outcomes of the 72% of patients who came to our unit with aneurysmal SAH but were not entered into ISAT.
Methods
The study population comprised all patients who came to our unit with a diagnosis of aneurysmal SAH, confirmed with angiographic imaging, but were excluded from ISAT or other clinical trials investigating the treatment of ruptured intracranial aneurysms. Ethical approval was gained from the local research ethics committee, and the study was registered with the Hospital Trust. Informed consent was obtained from the patients.
Clinical variables and a definitive reason for a specified management strategy were recorded. Endovascular or neurosurgical procedural data were noted. Immediate outcomes, adverse events, and additional procedures during hospital stay were documented, and results of all subsequent angiography were recorded.
For ease of comparison with ISAT, the Modified Rankin Scale (MRS) (9) was used to assess clinical outcome. Patient outcome (MRS) was determined from postal questionnaires sent to the surviving patients or their next of kin at 2 months and at 1 year after ictus. These were followed by a telephone interview. This dual approach allowed detailed information to be gained and validated from all patients on their current health status.
Our center was a high recruiter to ISAT; the decision not to randomize patients into ISAT was made by the consultant interventional neuroradiologist, the consultant neurosurgeon, and the patient, based on a variety of factors as documented in Table 1.
TABLE 1:
Reasons for nonrandomization into ISAT
| Reason | No. (%) of Patients |
|---|---|
| Surgical clipping (n = 67) | |
| Anatomy of aneurysm unsuitable, wide neck | 22 (33) |
| Radiologist decision not suitable for embolization | 13 (19) |
| Intracranial haematoma to be removed at the same time | 8 (12) |
| Aneurysm size unsuitable | 6 (9) |
| Unavailability of radiologist or angiography room | 6 (9) |
| Anatomy, tortuous vessels | 5 (7) |
| Patient requested surgery | 3 (4) |
| Vessel could not be separated from aneurysm neck | 2 (3) |
| Patient pregnant | 1 (1) |
| Management not discussed with neuroradiologist on call | 1 (1) |
| Endovascular coiling (n = 46) | |
| Posterior circulation aneurysm | 18 (39) |
| Patient requested endovascular therapy | 10 (22) |
| Patient age or grade | 7 (15) |
| Surgeon did not believe aneurysm was suitable for clipping | 5 (11) |
| Anatomy unsuitable for surgery | 3 (6) |
| Preexistent medical condition | 1 (2) |
| Aneurysm partially treated | 1 (2) |
| Outside referral after failed clipping | 1 (2) |
At the time the study was performed, three consultant neuroradiologists and seven consultant neurosurgeons were involved in clinical decision making. The experience of these consultants varied from 15 years or more to newly appointed consultants with limited experience. All procedures were carried out under the supervision of a consultant neuroradiologist or a consultant neurosurgeon, but in some instances were performed by trainees.
The methods of the current study did not involve randomization; consequently, inferential analysis comparing the outcome of the two treatment groups was not possible. One of the main study objectives was to explore the process of clinical reasoning that led to a decision not to randomize a patient into ISAT and to the subsequent treatment, namely, surgical clipping, endovascular coiling, or conservative management. Both descriptive and inferential analysis (chi-square, Mann Whitney and logistic regression) within individual treatment groups have been used to provide the following results. A P value < .05 was significant. Results from this study were also compared with those results published from ISAT.
Results
A total of 122 patients were registered in the study between February 10, 2001 and May 2, 2002. The age of the patients ranged from 26 to 87 years (mean, 55 years). There were 88 women (72%) and 34 (28%) men. Within the sample population, nine patients were treated conservatively. Of these nine patients, seven died before discharge, with time from ictus to death ranging from 7 to 31 days (mean, 18.7 days), and one patient died between the 2-month and 1-year follow-up. The remaining patient survived, but in a vegetative state (MRS grade 5). Sixty-seven patients underwent surgical clipping (including three that had failed embolizations), and 46 patients received endovascular therapy (including one who had a failed surgical clipping).
Presentation
The data showed that choice of treatment strategy was not dependent on age (age ranges of the endovascular and surgical groups were similar; mean ages, 53.4 years and 54.2 years, respectively), nor was the choice dependent on clinical grade (no significant relationship was noted between World Federation of Neurologic Surgeons [WFNS] clinical grading scale and patient treatment). Of those patients with a poor clinical grade (WFNS grades 3–6) at presentation, 81% of those treated surgically had an intracerebral hematoma.
Anatomic Considerations
No significant difference in aneurysm size, extent of bleed as seen on CT scan, or evidence of vasospasm as shown by angiographic imaging was seen between the treatment groups (Table 2). We found a statistically significant difference in the width of the neck of the aneurysm between the chosen management strategies. With the technology available at that time, wide-necked aneurysms were problematic for endovascular coiling, and consequently significantly more were treated surgically (χ2 analysis, P = .043).
TABLE 2:
Clinical variables at admission
| Variable | Endovascular (n = 46) | Surgery (n = 67) |
|---|---|---|
| WFNS grade at admission | ||
| 1 | 18 (39) | 27 (40) |
| 2 | 14 (30) | 23 (34) |
| 3 | 6 (13) | 6 (9) |
| 4 | 3 (6) | 2 (2) |
| 5 | 3 (6) | 9 (13) |
| 6 | 2 (4) | 2 (2) |
| Aneurysm size (mm) | ||
| <2 | 0 (0) | 2 (3) |
| 2–5 | 24 (52) | 32 (48) |
| 6–10 | 18 (39) | 24 (36) |
| >10 | 4 (9) | 9 (13) |
| Width of aneurysm neck (mm)* | ||
| <1 | 8 (17) | 10 (15) |
| 1–4 | 29 (63) | 29 (43) |
| >4 | 9 (20) | 28 (42) |
| CT Fisher grade | ||
| No blood | 3 (6) | 9 (13) |
| <1 mm | 2 (4) | 5 (7) |
| >/= 1 mm | 9 (20) | 20 (30) |
| Intraventricular or intraparenchymal | 32 (70) | 33 (49) |
| Vasospasm | ||
| No spasm | 24 (52) | 28 (42) |
| Vasospasm | 22 (48) | 39 (58) |
Note.—Data are number (%) of patients.
Significantly more wide-necked aneurysms were treated surgically (χ2 P = .043).
Subsequent analysis demonstrated expected differences between the treatment groups in location of aneurysm, with more posterior circulation aneurysms treated with endovascular therapy and more middle cerebral artery aneurysms treated surgically (selection bias due to recruitment to ISAT) (Table 3).
TABLE 3:
Location of ruptured aneurysm
| Artery | Endovascular | Surgery | Total |
|---|---|---|---|
| Anterior cerebral | 10 (22) | 19 (28) | 29 |
| Middle cerebral | 1 (2) | 35 (52) | 36 |
| Posterior communicating | 12 (26) | 10 (15) | 22 |
| Posterior cerebral | 19 (41) | 2 (3) | 21 |
| Internal carotid | 4 (9) | 1 (1) | 5 |
| Total | 46 (41) | 67 (59) | 113 |
Note.—Data are number (%) of patients.
Clinical Management
On analysis of definitive reasoning for management strategy, it became obvious that no clear guidelines were followed, with the exception of the accepted preference for endovascular therapy for aneurysms located in the posterior cerebral circulation. The treatment path was dependent on the clinical decision made by the team responsible for each patient (reasons for preferred management are shown in Table 1). The variability in patient treatment reflected consultant experience and highlighted the lack of documented evidence, at that time, on which to base clinical guidelines.
The time from ictus to treatment differed between the two treatment groups: mean time from ictus to treatment was 4.5 days for endovascular therapy compared with 2.73 days for surgery (Mann-Whitney P = .017). The center received referrals from outside hospitals for endovascular therapy; this contributed to the longer delay in treatment in this group.
Clinical Outcome
The outcome at 2 months following endovascular therapy in our study demonstrated that 31 (67%) of 46 patients had achieved a good clinical grade (MRS grade 0–2). In comparison, in ISAT internationally 74.6% (715/959) of the endovascular group achieved a good outcome. At 1 year, a further improvement was seen in our patients in the endovascular group, with 72% (33 patients) achieving a good outcome. ISAT reported a similar improvement (76.3% [611/801]).
After endovascular therapy, four patients (9%) died in the acute stage before discharge and another two patients (4%) died between 2 months and 1 year. This gave a mortality of 13% (6 patients) in our study, compared with ISAT, which reported a mortality of 8.1% (65 patients) at 1 year. In our study, all patients who died following endovascular therapy had a poor clinical grade at presentation.
At 2 months, the outcome following surgical clipping in our study showed that 30 (45%) of 67 patients had achieved a good clinical grade (MRS grade 0–2). In comparison, in ISAT 63.6% (602/947) of patients achieved a good outcome. At 1 year, further improvement was seen in our surgical patients, with 49% (33 patients) achieving a good outcome. ISAT also reported an improvement to 69.4% (550/793) (Fig 1).
Fig 1.
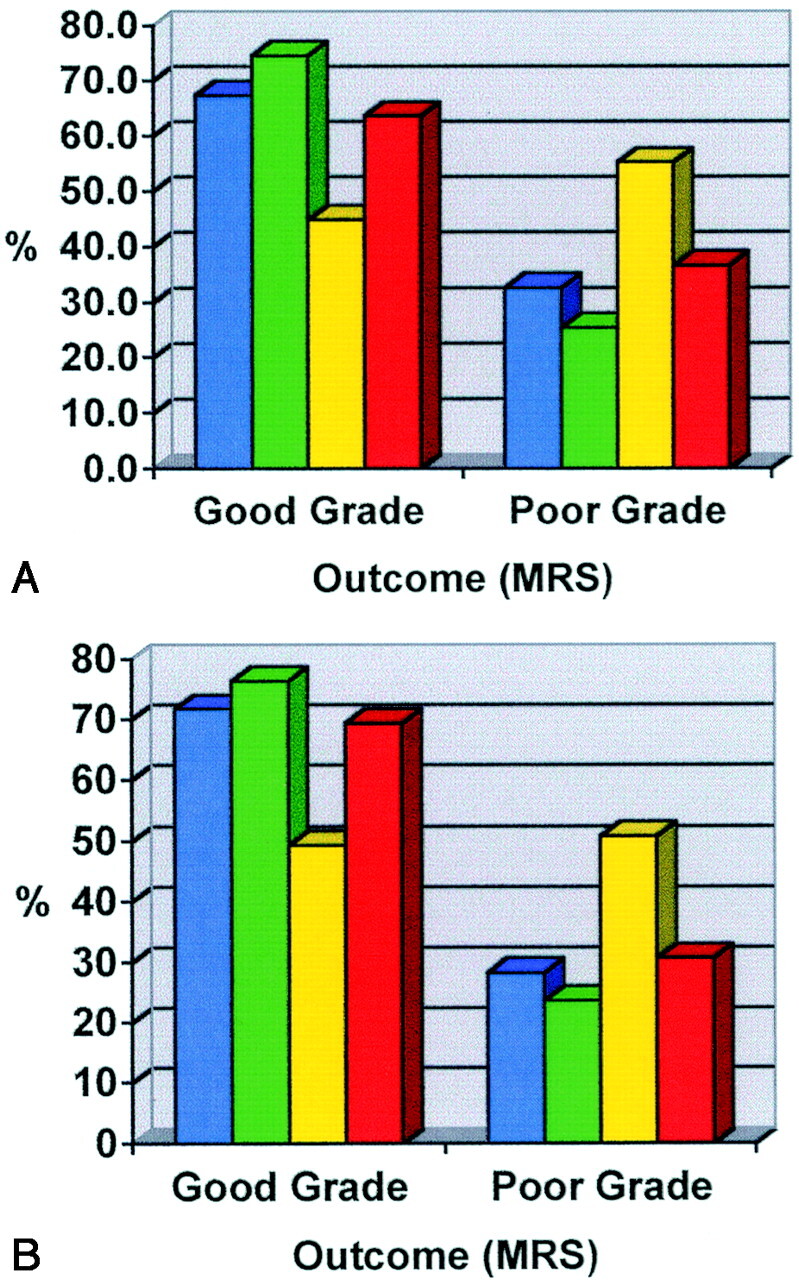
A and B, Outcome at 2 months (A) and at 1 year (B) shown as percentage of patients achieving a good grade (MRS 0–2) or poor grade (MRS 3–6) in our study and ISAT (international) for each treatment option of endovascular therapy and surgery. Blue indicates endovascular group in current study; green, endovascular group in ISAT; yellow, surgery group in current study; red, surgery group in ISAT.
After surgical clipping, two patients (3%) died before the 2-month follow-up and another three patients (4%) died between 2 months and 1 year in our study. This gave an overall mortality of 7% (5 patients) in our study, compared with ISAT which reported 10.1% (80 patients) mortality at 1 year (Fig 1). In this study, three (60%) of the five surgical patients who died following treatment had a poor clinical grade at presentation.
Although no significant difference in age with respect to outcome was noted in patients treated with endovascular therapy, those treated surgically who had a good outcome were significantly younger (mean age, 47 years) than those treated surgically who had a poor outcome (mean age, 59 years) using Mann Whitney (P = .01).
In the subgroup of patients aged 60 years or older, 60% (6/10) of endovascular patients had a good outcome at discharge.
To model the relationship of predictor variables (age and clinical grade) to outcome at 1 year for all patients, logistic regression was used. The model was found to be a good fit (Hosner-Lemeshow statistic of 9.25, P = .322) and explained 74.3% of the data. The odds ratios, Exp (B), showed that, with every increasing year of age, a patient is 1.071 times more likely to have a poor outcome regardless of treatment option. With each decline in WFNS grade, a patient is more likely to have a poor outcome. Results showed that whichever treatment option is chosen, a patient with WFNS grade 2 compared with a patient with grade 1 is 2.5 times more likely to have a poor outcome; a patient with grade 5 is 43.9 times more likely to have a poor outcome at 1 year.
Whereas the analysis presented above describes the outcomes for the groups, individuals within each group displayed some variability. For example, when comparing WFNS grade at admission to outcome grade at 1 year, seven of 14 patients with a poor clinical condition at presentation achieved a good outcome when treated with endovascular therapy (Table 4).
TABLE 4:
Comparison of MRS outcome grade at 1 year with WFNS grade at admission
| WFNS Grade at Admission | Endovascular 1-year Outcome |
Surgery 1-year Outcome |
||
|---|---|---|---|---|
| Good | Poor | Good | Poor | |
| Good | ||||
| 1 | 15 | 3 | 20 | 7 |
| 2 | 11 | 3 | 13 | 10 |
| Poor | ||||
| 3 | 3 | 3 | 0 | 6 |
| 4 | 2 | 1 | 0 | 1 |
| 5 | 2 | 1 | 0 | 9 |
| 6 (not accessible) | 0 | 2 | 0 | 1 |
Note.—Data are number of patients.
These results can be explored further in relation to data on the procedures, adverse events, and additional procedures performed. The length of the procedure was longer for surgery than endovascular therapy (medians, 4.2 and 2.2 hours, respectively).
Documented procedural complications included aneurysm rupture during endovascular procedure in four patients (9%) and during surgical procedures in six patients (9%); parent artery occlusion after endovascular procedure in two (4%) and after surgery in six (9%); thromboembolic complications after embolization in four (9%) and after surgery in zero (0%); and neurologic deterioration at the end of endovascular procedure in five (11%) and after surgery in 12 (18%).
Two patients in the endovascular group reported epilepsy, one from childhood and the other experienced seizures after insertion of a ventriculoperitoneal shunt to treat hydrocephalus. In the surgical group, 10 patients developed epilepsy following treatment; one had an ictal seizure followed by further seizures after surgery.
A low frequency of nonprocedural re-bleeding has been observed to date. Two patients re-bled after discharge: one following endovascular therapy who required further treatment by surgical clipping; and one patient who died following surgical clipping.
All patients who undergo endovascular therapy have a routine follow-up angiography at 6 months and at 2 years after the procedure. This is not the case for patients who undergo surgical clipping; they will have further angiography only when it is clinically indicated. The results at 1 year are shown in Table 5.
TABLE 5:
Occlusion rates at 1 year for endovascular and surgical treatment groups
| Occlusion at 1 year | Endovascular (n = 46) | Surgical (n = 67) |
|---|---|---|
| Complete occlusion with coils | 35 (76) | |
| Partial occlusion with coils (neck remnant) | 11 (24) | |
| Presumed complete occlusion with clip (not checked with imaging) | 62 (92) | |
| Clip not occluding aneurysm neck | 4 (6) | |
| Aneurysm wrapped only | 1 (1) |
Discussion
The ISAT study undertook a meticulous randomized trial to address the question of safety and efficacy of endovascular coiling as opposed to neurosurgical clipping (9). Although a clear benefit of endovascular coiling was demonstrated, that study was limited to the subgroup of predominantly patients with a good WFNS grade who had small ruptured saccular aneurysms in the anterior circulation (10). Whereas in our view the ISAT results are important, further questions have been raised in the literature about sample bias with respect to sample size, randomization, and intention to treat in ISAT (11, 12). In the current study, we addressed the remaining patients who came to our center with aneurysmal SAH. A team decision rather than the study design determined the management strategy. Clinical decisions were made in the absence of clear guidelines and were dependent on experience rather than definitive evidence.
The results presented herein are important as they complement the ISAT results; they confirm the trend reported by ISAT for patients treated with endovascular therapy in the wider nonrandomized population. Surgical outcomes appear poorer than those reported by ISAT, but it must be recognized that our study included patients with poor WFNS grades.
We acknowledge that our study is based in a single center with a relatively small sample owing to the early cessation of ISAT. This unit is a regional neuroscience center serving a population of 2.2 million and admitting approximately 200 patients with SAH per annum. The unit has actively participated in many international studies including ISAT (9) and the International Study of Unruptured Intracranial Aneurysms (13).
In the entire group of patients treated (surgical and endovascular), age and clinical grade were found by logistic regression to be independently predictive of clinical outcome. The effect that clinical grade had on the patient’s subsequent outcome in our study was similar to the effects reported in the literature. Prospective (14, 15) and retrospective (16, 17) studies concur that the outcome of management is strongly related to a patient’s clinical grade on admission. Of patients who were alert on admission, a mean of 75% had a good recovery and 13% died, whereas of the patients admitted in a comatose state, a mean of only 11% made a good recovery and 72% died. Our study showed that patient outcome was significantly related to clinical grade at both admission (P < .01) and at the time of treatment (P < .01) by using χ2 analysis. The overall mortality rate in the study was 14.8%. In the actively treated groups, mortality rates increased from 6.1% for patients with grade 1 or 2 (good) to 11.7% for patients with grades 3–6 (poor).
Results obtained in the conservatively managed group were comparable to those seen in the literature: eight (89%) of nine patients died. This poses the question as to whether all patients, regardless of age, should receive some form of active treatment.
The difference in frequency of epilepsy is of note, with the endovascular group demonstrating no occurrences after intervention. The consequences of this observation on an individual’s life may be wide ranging and need to be considered not just at the level of impairment, but also in terms of function (i.e., limiting their activity and participation in normal life, return to work, driving, and normal daily activities).
Of those patients whose aneurysms were treated with surgical clipping, only four (6%) had any form of angiographic follow-up either intraoperative or after treatment. These angiograms were obtained after treatment when clinical condition indicated a requirement for angiography. The results showed that in two cases there was a significant neck remnant and in another case the clip was partially occluding the parent artery. In the fourth case, the clip was not occluding the aneurysm at all; this patient required a second craniotomy and clip repositioning. In those patients whose aneurysms were treated surgically, in whom complete occlusion was documented in case notes (94%), no follow-up angiography had been performed; complete occlusion was an assumption made on surgical results and the lack of clinical symptoms in the postoperative period, which would indicate a potential problem in clip positioning. It is not routine protocol in this center to perform either intraoperative or postoperative angiography for surgical patients, unless there has been a complication during the procedure or postoperative symptoms justify it.
In a study (18) that evaluated the need for late angiographic follow-up of surgically treated aneurysms, the authors found that while most aneurysm rests appear to remain stable, a small subset enlarge or even rupture. In addition, the authors found a small but significant risk of de novo aneurysm formation, particularly in patients with multiple aneurysms. These findings support the rationale for late follow-up angiography. Anatomic factors that predict the usefulness of intraoperative angiography were evaluated in a study (19) in which the authors found that intraoperative angiograms resulted in clip repositioning in 46 (16.8%) of 273 studies. However, the rate of clip repositioning in aneurysms of the posterior communicating or anterior choroidal arteries was less than that of other locations (P = .05). They concluded that intraoperative angiography might not be necessary when aneurysms are in these two locations.
There is concern as to the long-term occlusion rates of aneurysms treated with endovascular coiling. In our center, results of follow-up angiography performed before 1-year follow-up showed that 11 (24%) of 46 aneurysms demonstrated partial occlusion; of these, 10 aneurysms (91%) showed 90–95% occlusion with small neck remnants. One patient required further treatment with endovascular coiling. However, only long-term follow-up will eventually determine the rate of aneurysm recanalization and repeat bleeding in this patient group.
Since the completion of this study, there have been further developments in the technology of endovascular therapy. These include a wider range of coils with variation in terms of morphologic shape and size. New techniques include the use of balloon remodeling or cerebral stents to treat a wider anatomic range of aneurysms. Current studies with newly developed coils give early indications of improvement in the durability of this technique.
To summarize, it should be acknowledged that endovascular therapy is both a feasible and efficacious treatment for cerebral aneurysms, particularly in patients of a poor clinical grade and those aged 60 years or older.
Conclusion
This study addresses a number of concerns that have been expressed about ISAT, specifically that ISAT results cannot be extrapolated to more than a subgroup of ruptured aneurysms (10). Albeit that our study is small and carried out at a single center, the results to date explore the experience of the remainder of the SAH population presenting to this center. Despite the inherent differences between the two treatment populations, the outcomes at both 2 months and 1 year for those subjects treated with endovascular therapy were superior to the outcomes in those treated with surgical clipping. Given the combination of ISAT and our results, endovascular therapy is now the first option for treatment with all patients who come to our center with aneurysmal SAH.
Acknowledgments
We would like to thank Janet Irwin for her support and assistance with data collection in this study.
References
- 1.Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography—a meta-analysis. Stroke 1996;27:625–629 [DOI] [PubMed] [Google Scholar]
- 2.Auer LM. Unfavorable outcome following early surgical repair of ruptured cerebral aneurysms: a critical review of 238 patients. Surg Neurol 1991;35:152–158 [DOI] [PubMed] [Google Scholar]
- 3.Edner G, Kagstrom E, Wallstedt L. Total overall management and surgical outcome after aneurysmal subarachnoid hemorrhage in a defined population. Br J Neurosurg 1992;6:409–420 [DOI] [PubMed] [Google Scholar]
- 4.Proust F, Hannequin D, Langlois O, Freger P, Creissard P. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients: the importance of control angiography. Stroke 1995;26:1553–1557 [DOI] [PubMed] [Google Scholar]
- 5.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. II. Clinical aspects and recommendations. Neurosurgery 2000;46:1360–1375; discussion 1375–1376 [DOI] [PubMed] [Google Scholar]
- 7.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery 1998;43:1016–1025 [DOI] [PubMed] [Google Scholar]
- 8.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol 1998;19:157–165 [PMC free article] [PubMed] [Google Scholar]
- 9.Molyneux A. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 10.Nichols DA, Brown J, Robert D, Meyer FB. Coils or clips in subarachnoid haemorrhage? Lancet 2002;360:1262–1263 [DOI] [PubMed] [Google Scholar]
- 11.Britz GW, Newell DW, West GA, Lam A. The ISAT trial [letter]. Lancet 2003;361:431–432; author reply 432 [DOI] [PubMed] [Google Scholar]
- 12.Harbaugh RE, Heros RC, Hadley MN. More on ISAT [letter]. Lancet 2003;361:783–784; author reply 784 [DOI] [PubMed] [Google Scholar]
- 13.Wiebers DO, Whinant J, Forbes G, et al. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 1998;339:1725–1733 [DOI] [PubMed] [Google Scholar]
- 14.Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. I. Overall management results. J Neurosurg 1990;73:18–36 [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Wolf PA, Bharucha NE, et al. Subarachnoid and intracerebral hemorrhage: natural history, prognosis, and precursive factors in the Framingham study. Neurology 1984;34:847–854 [DOI] [PubMed] [Google Scholar]
- 16.Gumprecht H, Winkler R, Gerstner W, Lumenta CB. Therapeutic management of grade IV aneurysm patients. Surg Neurol 1997;47:54–58; discussion 58–59 [DOI] [PubMed] [Google Scholar]
- 17.Deruty R, Pelissou-Guyotat I, Mottolese C, Amat D, Bognar L. Level of consciousness and age as prognostic factors in aneurysmal SAH. Acta Neurochir 1995;132:1–8 [DOI] [PubMed] [Google Scholar]
- 18.David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg 1999;91:396–401 [DOI] [PubMed] [Google Scholar]
- 19.Derdeyn CP, Moran CJ, Cross DT 3rd, Sherburn EW, Dacey RG Jr. Intracranial aneurysm: anatomic factors that predict the usefulness of intraoperative angiography. Radiology 1997;205:335–339 [DOI] [PubMed] [Google Scholar]


