Abstract
BACKGROUND AND PURPOSE: Diffusion-weighted (DW) imaging at b = 2000 s/mm2 offers theoretical advantages over DW imaging at b = 1000 s/mm2 for detection of hyperacute ischemic stroke. The purpose of this study was to determine whether b = 2000 images are better than b = 1000 images for detecting and estimating the extent of diffusion change within 6 hours after stroke onset.
METHODS: We compared DW images obtained with a b value of 1000 s/mm2 (TR/TE/NEX, 7500/71/1) with those obtained with a b value of 2000 s/mm2 (TR/TE/NEX, 7500/83/2) in 94 patients examined within 6 hours of clinically suspicious hyperacute ischemic stroke (57 men, 37 women; mean age ± SD, 62 years ± 8; age range, 47–80 years; mean time interval ± SD, 206 ± 90 min). Three observers performed qualitative analysis of DW images and reached a consensus about lesion conspicuity, lesion extent, and image artifact. In the quantitative analysis of 34 patients with lesions in the territory of the middle cerebral artery, the signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and volume of ischemic lesion were measured and findings of the b = 1000 and b = 2000 images were compared.
RESULTS: The sensitivity and specificity of b = 1000 and b = 2000 images were calculated as 94% (80/85)/ 100% (9/9) and 98% (83/85)/ 100% (9/9), respectively, relative to the presence or absence of infarction on the follow-up T2-weighted images. In three patients, hyperintense lesions were depicted only on b = 2000 images. On qualitative analysis, lesions were more conspicuous and larger on b = 2000 images in 23 and 11 patients, respectively. On quantitative analysis, as the b value increased, the mean lesion volume increased by 47% (22.1 ± 27.9 mL at b = 1000 s/mm2 versus 32.5 ± 36.5 mL at b = 2000 s/mm2, P < .001, n = 34). As the b value increased, mean SNR decreased both in the lesion and in the contralateral normal area by 17% and 28%, respectively, but the mean CNR increased by 23% (8.7 ± 6.4 at b = 1000 s/mm2 versus 10.7 ± 6.5 at b = 2000 s/mm2, P < .001, n = 34).
CONCLUSION: DW images acquired with a b value of 2000 s/mm2 were better than DW images acquired with a b value of 1000 s/mm2 for the detection and estimation of the extent of diffusion change in patients examined within 6 hours of ischemic stroke onset.
In patients with hyperacute brain ischemia, the clear demonstration of lesion location and extent by imaging studies is very important for therapeutic decision making. For detection and extent estimation of the ischemic lesion, diffusion-weighted (DW) imaging is currently superior to other imaging modalities. CT has been used for many years as the first-line imaging technique for patients with hyperacute ischemic stroke. However, CT has limitations not only for detecting small lesions, especially in the posterior fossa, but also for estimating the extent of the lesion. Although CT is able to show early signs of ischemic stroke, especially in patients with lesions in the middle cerebral artery (MCA) territory, it is frequently difficult to know the exact extent of the lesion on the basis of nonenhanced CT findings alone (1–5). Perfusion CT is a promising tool that provides quantitative hemodynamic information about cerebral blood volume, cerebral blood flow, and mean transit time, but it cannot image the whole brain in its current state of technological development (6). Conventional T2-weighted imaging or proton density–weighted imaging has 30–50% false-negative results during the first 3 to 6 hours after stroke onset (1, 7). DW imaging is much more sensitive than T2-weighted imaging or fluid attenuated inversion recovery (FLAIR) imaging for the detection of stroke during the first 6 hours after symptom onset, with a sensitivity as high as 94–100% (8–10). However, even diffusion MR imaging fails to detect ischemic lesions in some clinical situations such as mild ischemic change, early reperfusion and rapid normalization of diffusion change, or small lesions in the brain stem (11, 12).
Maximum b values applied in the stroke diffusion studies were usually in the range of 800–1500 s/mm2 (8–14), but recent progress in MR gradient technology permits higher b values and greater diffusion sensitivity on 1.5T MR systems. Given its greater diffusion weighting, DW imaging with a high b value (i.e., b = 2000 or 3000 s/mm2) does offer advantages over DW imaging at b = 1000 s/mm2 for detection of hyperacute ischemic lesions, especially for lesions with mild diffusion changes. Initial application of DW imaging with b values of 2500–3000 s/mm2 for acute or subacute infarction provided no apparent diagnostic advantages compared with those of b = 1000 s/mm2 images (15, 16). However, it is still unclear whether a high b value is better than a conventional b value of 1000 s/mm2 for the detection of ischemic lesions and their extent, especially during the first 6 hours after stroke onset. In this study, we compared DW images acquired at b = 2000 s/mm2 with those at b = 1000 s/mm2 in patients examined within the first 6 hours after stroke onset.
Methods
Patients
Our institutional review board approved a research protocol for high-b-value DW imaging performed in patients with suspected brain ischemia. We obtained informed consent from each patient before MR imaging examinations. We examined 446 consecutive patients with clinical suspicion of acute cerebral infarction between January 2002 and February 2003. Among them, 94 patients were recruited as they fulfilled our inclusion and exclusion criteria. We included the patients with (a) complete acute stroke MR imaging protocol (described later) performed within 6 hours after symptom onset, (b) DW images obtained with b values of 1000 and 2000 s/mm2 in the same imaging session, (c) follow-up MR imaging (described later) obtained within 1 week (mean, 3 days). We excluded those patients who already presented with an abnormality on T2-weighted images in the area corresponding to diffusion abnormality on DW images, as we wished to know the sensitivity of the two b values in the lesion with only diffusion abnormality. In all study patients, neurologists made the final diagnosis on the basis of their clinical assessment and the neuroimaging studies. Finally, we enrolled 94 patients who fulfilled the inclusion and exclusion criteria described above (57 men, 37 women; mean age ± SD, 62 years ± 8; age range, 47–80 years; mean time interval ± SD, 206 ± 90 min).
MR Imaging
We examined all patients by using a 1.5T whole-body MR imager (CVi; GE Medical Systems, Milwaukee, WI). The system had echo-planar imaging capability with maximum gradient strength in x, y, or z directions of 40 T/m/ms and a slew rate of 150 T/m/s. Our acute stroke MR protocol comprised T2-weighted imaging, DW imaging, 3D time-of-flight MR angiography (3D TOF MRA) covering the circle of Willis, and the first-pass perfusion study if necessary. Total examination time was approximately 10–15 minutes. DW imaging was performed by using a single-shot spin-echo echo-planar imaging sequence. The imaging parameters for b = 1000 images were as follows: TR, 7500 ms; TE, 71 ms; 20 transverse sections; 5-mm section thickness; 2-mm intersection gap; 128 × 128 matrix; 250 mm FOV; NEX, 1; and imaging time, 28 s. By averaging the images obtained with the three orthogonal directions, diffusion trace images were calculated for each section. Before the start of our study, we compared b = 2000 images (NEX, 1) with b = 1000 images (NEX, 1), especially in terms of signal-to-noise ratio and found that b = 2000 images had excessive noise. We decided to increase the NEX to 2 to compensate for the signal loss caused by a higher b value. As the NEX increased, the machine automatically increased both the echo time and the image acquisition time to 83 ms and 56 s, respectively, but all other imaging parameters of b = 2000 images were the same as those of b = 1000 images. In each patient, b = 2000 images were obtained immediately after the acquisition of b = 1000 images in the same session. Follow-up MR imaging was performed within 1 week (mean, 3 days) and comprised fast spin-echo T2-weighted imaging, DW imaging with a b value of 2000, 3D TOF MRA, and contrast-enhanced MR angiography from the aortic arch to the intracranial arteries.
Postprocessing and Image Analysis
All DW images were transferred to a workstation (Advantage Window 4.0; GE Medical Systems). An ADC map was produced from the diffusion trace images and b = 0 images.
Qualitative Analysis
Three observers (CGC, DHL, JHL) with experience in DW imaging for more than 3 years did qualitative analysis of the diffusion trace images in a nonblinded fashion. Before visual assessment, we adjusted the window level and width of our PACS view monitors to make the gray scale level of the two DW imaging sets similar. The observers assessed the DW images in terms of lesion conspicuity, lesion extent, and image artifacts. The observers compared the b = 2000 images with the b = 1000 images side-by-side and finally, by consensus, concluded that the b = 2000 images were better, the same as, or worse than b = 1000 images in terms of lesion conspicuity, lesion extent, and image artifacts. We did not use any scale or scoring system for each item of qualitative analysis.
Quantitative Analysis
A single investigator (HJK) performed quantitative analysis for 34 patients with measurable infarcts in the MCA territory (18 men, 16 women; mean age 63 years ± 11; age range 39–88 years; mean time interval ± SD, 179 ± 90 minutes). The investigator measured the MR signal intensity (SI), signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), ADC, and volume of the ischemic lesion from trace images and ADC maps by drawing a region of interest (ROI) on a workstation. The ROI measurement of SI was made in three areas: the ischemic lesion, corresponding contralateral normal area, and the background region in the air-filled space outside of the patient’s head in the phase-encoding direction. Image noise was defined as the standard deviation of background SI (16). For the SI measurement of the ischemic lesion, we selected the section of DW images with the largest amount of the lesion and carefully located a round or elliptical region of interest of 200–600 mm3 at the appropriate portion of the ischemic lesion with special attention given to avoid CSF contamination. If the ischemic lesion was inhomogeneous, we located the region of interest in the area with mild SI change. We first obtained data from the b = 2000 DW image and then from the b = 1000 DW image at the corresponding location. From these SI measurements, the SNR and CNR of the ischemic lesion were calculated by using following equations, respectively (16):
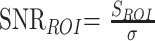 |
1 |
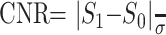 |
2 |
where SROI = SI at region of interest, S1 = SI at the ischemic lesion, S0 = SI in the corresponding contralateral normal area, and σ = SD of background SI.
The mean ADC values of the ischemic lesion and the corresponding contralateral normal area were automatically obtained from the ADC map after positioning the region of interest at the trace images. The relative ADC of the ischemic lesion was calculated as a percentage over the corresponding contralateral normal area. We also measured the volume of the ischemic lesion on the initial and follow-up trace images by using volume analysis software (Advantage Window 4.0). For the volume measurement, we used a semiautomatic threshold-based segmentation technique. The 3D segmentation of the ischemic lesion was automatically performed after applying a threshold value of SI. We measured the lesion volume from the initial b = 1000, b = 2000 images, and follow-up b = 2000 images, respectively, with a time interval of at least 1 week for each measurement to avoid single observer bias.
Statistical Analysis
The paired samples t test was used to compare SI, SNR, CNR, ADC, and lesion volume between the b = 1000 and b = 2000 images. A P value <.05 was considered statistically significant.
Results
In 11 of the 94 study patients, no definite hyperintense lesions were observed on the initial DW images obtained with a b value of either 1000 or 2000. In two of these 11 patients, acute small infarcts in the basal ganglia were confirmed on follow-up T2-weighted images. The other nine patients showed no definite abnormality on follow-up T2-weighted images. Their final clinical diagnoses were transient ischemic attack in five patients, factitious disorder in three, and spinal cord lesion with brown squared syndrome in one. In the other three of the 94 patients, hyperintense lesions were detected on the b = 2000 images but not on the b = 1000 images. In these three patients, two had lesions in the left MCA territory (cortical and subcortical area, respectively), and the other patient had a small lesion at posterior limb of the internal capsule that were confirmed on follow-up T2-weighted image (Fig 1, 2). In the remaining 80 of the 94 patients, hyperintense lesions were detected on both b = 1000 and 2000 images. In 83 patients with hyperintense lesions on the initial b = 1000 or 2000 images, follow-up T2-weighted imaging revealed infarcts with variable size changes depending on the progression or partial normalization of the initial lesions. Partial normalization of the initial lesions was observed in five patients, but no patients showed complete normalization of the initial lesions on follow-up T2-weighted images. Therefore, the sensitivity/specificity of the b = 1000 and 2000 images were calculated as 94% (80/85)/100% (9/9) and 98% (83/85)/100% (9/9), respectively, relative to the presence or absence of infarction on the follow-up T2-weighted images. In the 83 patients with hyperintense lesions on initial b = 1000 or 2000 images, the time interval between symptom onset and MR imaging was within 3 hours in 42 patients or within 3 to 6 hours in 41 patients. The infarction patterns in those 83 patients are summarized in Table 1. Territorial infarction was the most common type of stroke (57/83, 69%) and most of these were MCA infarcts (52/57). The causes of MCA infarction were cardiogenic embolism in 23 patients, proximal carotid atherosclerosis in 14, MCA atherosclerosis in seven and unknown causes in eight patients.
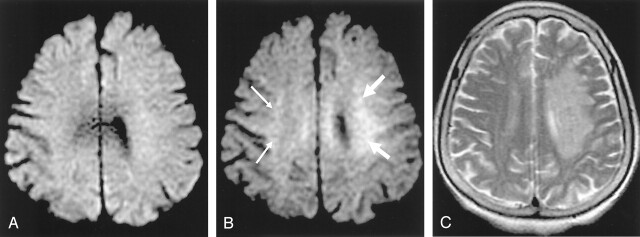
Fig 1.
A–C, An 88-year-old woman who had right-side weakness for 1 hour 50 minutes. Left MCA was not visible on initial 3D TOF MRA images (not presented).
Abnormal hyperintensity is noted at left corona radiata on b = 2000 image (B, white arrows) but it is not definite on b = 1000 image (A). Note relative hyperintensity of right corona radiata on b = 2000 image compared with b = 1000 image (B, small white arrows). This is normal and therefore should not be regarded as an ischemic lesion. Follow-up T2-weighted images obtained 3 days later shows progression of the infarction in the corresponding left corona radiata area (C).
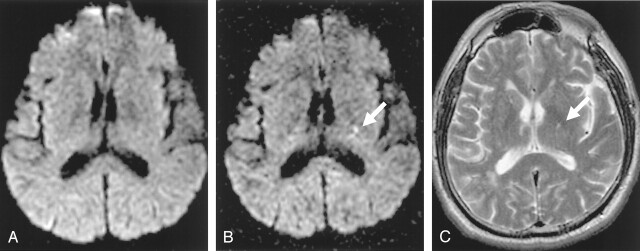
Fig 2.
A–C, A 63-year-old man who had right hemiparesis for 6 hours. Abnormal hyperintensity is noted at left posterior limb of internal capsule on b = 2000 image (B, white arrow) but it is not definite on b = 1000 image (A). Follow-up T2-weighted image obtained 2 days later shows a small hyper-intense lesion in the same location (C, white arrow).
TABLE 1:
Patterns of infarction in 83 patients who had lesions on initial DW images
| Pattern of Infarction | Patients (No.) |
|---|---|
| ACA | 4 |
| MCA (1 or 2 lesions) | 39 |
| Scattered MCA (>3 lesions) | 13 |
| ACA + MCA | 1 |
| Brain stem or cerebellar lesion | 11 |
| Small lesion in deep gray matter or adjacent white matter | 12 |
| Border zone lesion | 3 |
| Total | 83 |
Note.—DW images, diffusion-weighted images; ACA, anterior cerebral artery; MCA, middle cerebral artery.
The results of qualitative analysis for lesion conspicuity, lesion extent and artifact are summarized in Table 2. In 23 of 83 patients, the ischemic lesions were more conspicuous on b = 2000 images than on b = 1000 images. In 11 of those 23 patients, the ischemic lesions were larger on b = 2000 images than on b = 1000 images. These patients were more often in the patient group examined within 3 hours after stroke than in the group examined between 3 and 6 hours after stroke (9/42 versus 2/41, respectively). The locations of the ischemic lesions in those 11 patients were the MCA territory (7), anterior cerebral artery territory (1), internal capsule (1,) and brain stem (2) (Fig 3, 4). As far as the artifacts were concerned, there was no significant difference between the b = 2000 and the b = 1000 images. In two patients, prominent SI decrease was observed around the third and lateral ventricles on the b = 1000 images. This artifact may be caused by the CSF pulsation within the ventricles. In the other three patients, motion artifact and some artifacts of gradient instability or eddy current were observed on the b = 2000 images.
TABLE 2:
Results of qualitative analysis in 83 patients who had lesions on initial DW images
| Item of Analysis | Type of Consensus | Time Window |
Total | |
|---|---|---|---|---|
| 0 ∼ 3 hours | 3 ∼ 6 hours | |||
| Lesion conspicuity | 2000 > 1000 | 15 | 8 | 23 |
| 2000 ∼ 1000 | 27 | 33 | 60 | |
| 1000 > 2000 | 0 | 0 | 0 | |
| Total | 42 | 41 | 83 | |
| Lesion extent | 2000 > 1000 | 9 | 2 | 11 |
| 2000 ∼ 1000 | 33 | 39 | 72 | |
| 1000 > 2000 | 0 | 0 | 0 | |
| Total | 42 | 41 | 83 | |
| Artifacts | 2000 > 1000 | 2 | 0 | 2 |
| 2000 ∼ 1000 | 38 | 40 | 78 | |
| 1000 > 2000 | 2 | 1 | 3 | |
| Total | 42 | 41 | 83 | |
Note.—2000 > 1000, b = 2000 images are superior to b = 1000 images; 2000 ∼ 1000, b = 2000 images are the same as b = 1000 images; 1000 > 2000, b = 2000 images are inferior to b = 1000 images in each item of qualitative analysis.
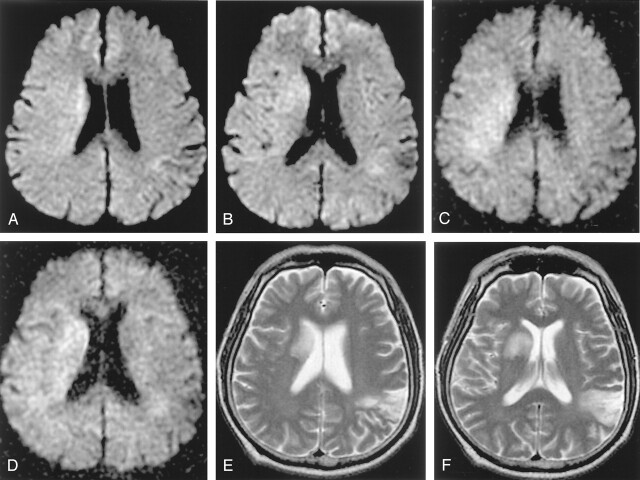
Fig 3.
A–F, A 64-year-old man who had left hemiparesis for 1 hour 20 minutes. Initial b = 1000 images reveal subtle hyperintensity in the right corona radiata and basal ganglia (A, B). The lesion is more conspicuous and extensive on b = 2000 images (C, D). Follow-up T2-weighted image obtained 3 days later shows progression to infarction at the basal ganglia (E, F). Initial diffusion change is partly normalized in the posterior portion of the initial lesion. An old infarction is noted in the left posterior temporal cortex on the T2-weighted image.
Fig 4.
A–C, A 57-year-old man who had left hemparesis for 4 hours 40 minutes. Initial b = 1000 image reveals subtle hyperintensity in the right pons (A). The lesion is more conspicuous on the b = 2000 image (B). Follow-up T2-weighted image obtained 2 days later shows the infarction at the corresponding location.
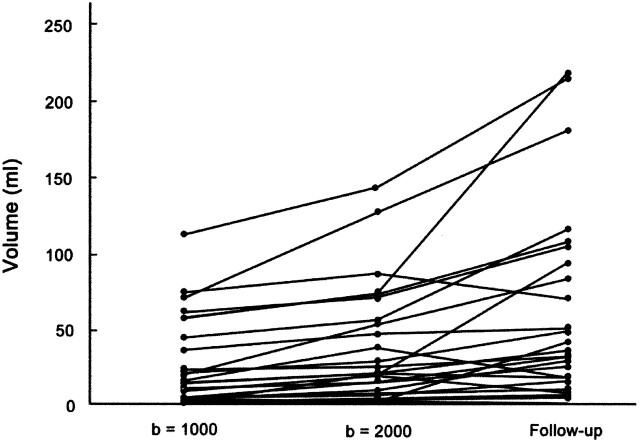
The results of the quantitative analysis are summarized in Table 3. As the b value increased, the mean lesion volume increased by 47% (22.1 ± 27.9 mL on b = 1000 images versus 32.5 ± 36.5 mL on b = 2000 images, P < .001, n = 34). The measured lesion volumes are presented graphically in Figure 5. As the b value increased, the mean SI of brain tissue decreased by 35% and 45%, respectively, in both the lesion and the contralateral normal area. The background noise level was lower in b = 2000 images than in b = 1000 images, probably owing to the increased NEX (9.0 ± 1.9 on b = 2000 images versus 11.6 ± 2.1 on b = 1000 images, P < .001, n = 34). As the b value increased, the mean SNR decreased by 17% and 28%, respectively, in both the lesions and in the contralateral normal areas (P < .001). This decrease of SNR on b = 2000 images was caused by the prominent decrease of tissue SI despite the decrease of background noise. However, as the b value increased, the mean CNR between the lesion and contralateral normal area increased by 23% (8.7 ± 6.4 on b = 1000 images versus 10.7 ± 6.5 on b = 2000 images, P < .001, n = 34). This increase of CNR on b = 2000 images seems to have been caused mainly by the decrease of background noise level because the mean SI difference between the lesion and contralateral normal tissue was almost the same in b = 1000 and b = 2000 images (Table 3). As the b value increased, the mean ADC decreased in both the lesion and the contralateral normal area by 23% and 20%, respectively. The relative ADC of the ischemic lesions was 79 ± 13% and 75 ± 13% on b = 1000 and 2000 images, respectively.
TABLE 3:
Results of quantitative analysis in 34 patients who had lesions in the MCA territory (mean ± SD)
| b = 1000 (1NEX) |
b = 2000 (2NEX) |
|||
|---|---|---|---|---|
| Lesion | Contralateral | Lesion | Contralateral | |
| Mean Vol. (ml) | 22.1 ± 27.9 | NA | 32.5 ± 36.5* | NA |
| Signal Intensity | 440.2 ± 96.8 | 342.3 ± 62.3 | 286.4 ± 87.5* | 189.6 ± 42.5* |
| Noise | 11.6 ± 2.1 | 9.0 ± 1.9* | ||
| SNR | 38.7 ± 9.8 | 30.0 ± 6.4 | 32.3 ± 8.1* | 21.6 ± 4.8* |
| CNR | 8.7 ± 6.4 | 10.7 ± 6.5* | ||
| ADC (10−4 × mm2/s) | 6.0 ± 1.3 | 7.6 ± 1.5 | 4.6 ± 0.9* | 6.1 ± 0.7* |
Abbreviations—NEX; number of excitation, Contralateral; contralateral normal area corresponding to the ischemic lesion. Vol.; volume, NA; not available, SNR; signal to noise ratio defined as SROI/σ (SROI = signal intensity at the region of interest, σ = standard deviation of background signal intensity), CNR; contrast to noise ratio defined as the difference of SNR between the lesion and corresponding contra-lateral normal area, ADC; apparent diffusion coefficient,
statistical difference between b = 1000 and b = 2000 images (paired samples t test, P < 0.001).
Fig 5.
Mean volume of the lesions from initial b = 1000, b = 2000, and follow-up b = 2000 images. As the b value increases, the mean volume of the lesion increases by 47% (22.1 ± 27.9 mL on b = 1000 image versus 32.5 ± 36.5 mL on b = 2000 image, P < .001, n = 34). The mean volume of the lesion measured on follow-up b = 2000 images (52.3 ± 61.6 mL) is significantly larger than those of the initial trace images of b = 1000 or 2000 because of progression or edematous swelling of the infarction in most of the patients.
Discussion
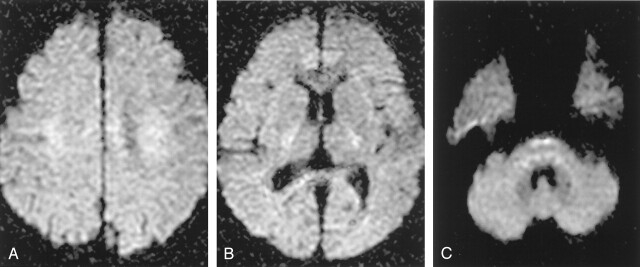
As the b value increases, the SI of normal gray matter decreases progressively relative to normal white matter; at b values greater than 2000, the gray-white matter pattern reverses relative to the usual b value of 1000 (17). In this study, the b = 2000 images also revealed relative hyperintensities to cortical gray matter, especially in the corona radiata, the posterior limb of internal capsule, and the lower brain stem (Fig 6). When analyzing b = 2000 images, this relative hyperintensity of normal white matter structures should not be erroneously diagnosed as ischemic lesions. Comparison of DW images with ADC maps may easily solve this type of problem. Meyer et al (15) calculated the theoretical SI difference between acute infarct and normal gray matter on DW images acquired with various b values and found the optimal b values to be between 1500 and 2000. Burdette et al (16) reported that the average SNR for b = 3000 images decreased to 45% of the b = 1000 images and the NEX should be increased by a factor of 4.7 to maintain equivalent SNR between the b = 1000 and the b = 3000 images. Therefore, it seems that a b value greater than 2000, for example b = 3000, would not be beneficial for patients with acute brain ischemia because of too much SI loss, long imaging time to compensate for the SI loss, or prominent relative hyperintensity of normal white matter.
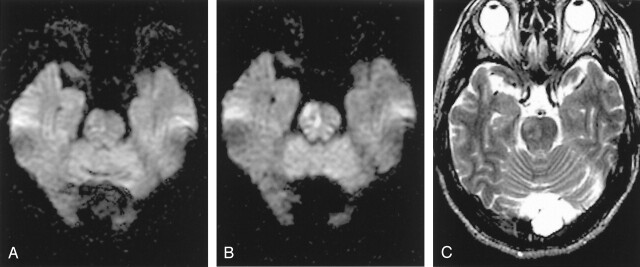
Fig 6.
In b = 2000 images, densely packed white matter tracts normally shows relative hyperintensities compared with gray matter, especially at the corona radiata (A), internal capsule (B), and the lower pons level (C). These normal relative hyperintensities of white matter tracts should not be erroneously diagnosed as ischemic lesions.
Diffusion studies of human ischemic stroke performed with a b value higher than 1000 were rare (13, 15, 16). Warach et al (13) indicated that a higher b value (b = 1463) was helpful in identifying lesions with certainty, especially in the smallest lesions or those with a transitional zone between normal and abnormal diffusion. Meyer et al (15) compared the DW imaging findings of three different b values (b = 1000, 2500, and 3000) in 12 patients with acute infarction. They reported that although lesion contrast improved as the b values increased, the higher b value images had no impact on the diagnosis of acute stroke. In their study, most of the 12 patients seem to be examined after the hyperacute period, and the lesion extent was not evaluated. Burdette et al (16) compared b = 3000 images with b = 1000 images in 26 patients diagnosed with acute or subacute infarction in the time range of 10 hours to 14 days (median 2.5 days). They concluded that compared with b = 1000 images, b = 3000 images did not offer a diagnostic advantage for the evaluation of late acute or subacute cerebral infarctions, and that b = 3000 images are significantly inferior in terms of SNR and CNR, which were defined in the same way as in our study. In Burdette’s study, all 26 patients were examined after the hyper-acute period, and the lesion extent was not evaluated in detail.
As the b value increases, the amount of diffusion weighting increases (18). Therefore, higher b value DW imaging would be more advantageous in the diagnosis of hyperacute ischemic lesions in which mild diffusion changes can be present within the lesions, than in the diagnosis of acute or subacute lesions. In this study, we compared b = 2000 images with b = 1000 images in 94 patients who were examined within 6 hours after stroke onset. In the qualitative analysis, the lesion was larger on b = 2000 images than on b = 1000 images obtained in some patients, especially those examined within 3 hours after stroke onset. In the quantitative analysis, the mean lesion volumes on b = 2000 images were larger than those on b = 1000 images by 47% in 34 patients with MCA territorial lesions with mean relative ADC of 75–79%. These quantitative data support our somewhat subjective observation on qualitative analysis and suggest that b = 2000 images may detect SI change caused by mild ADC decrease, especially in the peripheral or adjacent portion of the ischemic core that cannot be seen clearly on b = 1000 images. Desmond et al (19) reported that ischemic penumbra defined by perfusion–diffusion mismatch showed a relative ADC of 75–90% and this area of mild ADC decrease was not prospectively identified on b = 1000 images but revealed hazy, subtle SI change on retrospective review. In practical situations, this area of mild ADC decrease can be easily misinterpreted as normal on the rapid review of DW images or interpreted as a portion of diffusion-perfusion mismatch. We think that b = 2000 images can reveal this mild decrease of ADC of the ischemic penumbra more conspicuously than b = 1000 images in patients with hyperacute territorial lesions (Fig 3).
Another diagnostic advantage of b = 2000 images is the detection of small lesions in the capsule or brain stem area, which are not clearly demonstrated on b = 1000 images. A small lesion in the vertebrobasilar territory may not be detected at initial DW imaging, especially in its early stage (10, 12). This may be owing to the small lesion size beyond the spatial resolution of DW imaging or to mild diffusion change within the lesion. According to Oppenheim et al (12), 31% of their patients with vertebrobasilar ischemic stroke had a false-negative initial DW image during the first 24 hours. This relatively high false-negative rate seems to have been caused by lower spatial resolution (2.9 × 3.3 × 7.9 mm) and lower b value (b = 800 in 50 of 139 patients) in their study. In our study, b = 2000 images showed the lesion more clearly than b = 1000 images in two of the 11 patients with vertebrobasilar ischemic stroke (Fig 4). This enhanced lesion conspicuity may be caused by increased CNR on b = 2000 images. Although the number of our study patient is small, our results suggest the potential benefit of b = 2000 images for the detection of small ischemic lesions, especially in the brain stem, in the deep gray matter, or in the adjacent white matter.
For DW imaging parameters, we increased the NEX to 2 in b = 2000 images to compensate for the decreased SNR with a higher b value. By SI averaging, SNR of the b = 2000 images reached 83% and 72% of the b = 1000 images in the lesion and contralateral normal area, respectively (Table 3). As the b value increased from 1000 to 2000, the CNR increased by 23% in our study. The observed diagnostic advantage of b = 2000 images over b = 1000 images in this study seems to have been caused by this increase of CNR. We speculate that the SNR problem of b = 2000 images can be partly overcome in the near future by applying parallel imaging techniques combined with a multi-channel head coil, by using a 3T system, or by combining both strategies. However, the actual behavior of image contrast or SNR in those new DW imaging techniques may need further investigation.
Conclusion
DW imaging at b = 2000 s/mm2 was better than that at b = 1000 s/mm2 for the detection and estimation of extent of ischemia in patients with hyperacute ischemic stroke. The diagnostic advantage of b = 2000 images was observed not only in territorial lesions but also in small lesions in the brain stem or striatocapsular area. In some patients, mild diffusion change of the lesion was detected only by b = 2000 images. The increased CNR between the lesion and normal brain tissue on b = 2000 images was the main factor in this advantage.
Footnotes
Supported by grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (03-PJ1-PG1-CH06-0001).
References
- 1.Mohr JP, Biller J, Hilal SK, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke 1995;26:807–812 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 3.von Kummer R, Bourquain H, Bastianello S, et al. Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 2001;219:95–100 [DOI] [PubMed] [Google Scholar]
- 4.Aronovich BD, Reider-Groswasser II, Segev Y, Bornstein NM. Early CT changes and outcome of ischemic stroke. Eur J Neurol 2004;11:63–65 [DOI] [PubMed] [Google Scholar]
- 5.Lev MH, Farkas J, Gemmete JJ, et al. Acute stroke: improved nonenhanced CT detection –Benefits of soft-copy interpretation by using variable window width and center level settings. Radiology 1999;213:150–155 [DOI] [PubMed] [Google Scholar]
- 6.Eastwood JD, Lev MH, Wintermark M, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. Am J Neuroradiol 2003;24:1869–1875 [PMC free article] [PubMed] [Google Scholar]
- 7.Shimosegawa E, Inugami A, Okudera T, et al. Embolic cerebral infarction: MR findings in the first 3 hours after onset. Am J Roentgenol 1993;160:1077–1082 [DOI] [PubMed] [Google Scholar]
- 8.van Everdingen KJ, van der Grond J, Kappelle LJ, Lamos LMP, Mali WPTM. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke 1998;29:1783–1790 [DOI] [PubMed] [Google Scholar]
- 9.Perkins CJ, Kahya E, Roque CT, Roche PE, Newman GC. Fluid-attenuated inversion recovery and diffusion- and perfusion-weighted MRI abnormalities in 117 consecutive patients with stroke symptoms. Stroke 2001;32:2774–2781 [DOI] [PubMed] [Google Scholar]
- 10.Lövblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. Am J Neuroradiol 1998;19:1061–1066 [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PYK, Barker PB, Wityk RJ, Ulŭg AM, van Zijl PCM, Beauchamp, Jr., NJ. Diffusion-negative stroke: a report of two cases. Am J Neuroradiol 1999;20:1876–1880 [PMC free article] [PubMed] [Google Scholar]
- 12.Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. Am J Neuroradiol 2000;21:1434–1440 [PMC free article] [PubMed] [Google Scholar]
- 13.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231–241 [DOI] [PubMed] [Google Scholar]
- 14.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically-weighted echo planar MR imaging. Radiology 1996;199:391–401 [DOI] [PubMed] [Google Scholar]
- 15.Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, Homer D. High-b-value diffusion-weighted MR imaging of suspected brain infarction. Am J Neuroradiol 2000;21:1821–1829 [PMC free article] [PubMed] [Google Scholar]
- 16.Burdette JH, Elster AD. Diffusion-weighted imaging of cerebral infarctions: are higher B values better? J Comut Assist Tomogr 2002;26(4):622–627 [DOI] [PubMed] [Google Scholar]
- 17.DeLano MC, Cooper TG, Siebert JE, Potchen MJ, Kuppusamy K. High-b-value diffusion-weighted MR imaging of adult brain: Image contrast and apparent diffusion coefficient map features. Am J Neuroradiol 2000;21:1830–1836 [PMC free article] [PubMed] [Google Scholar]
- 18.Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 1965;42:288–292 [Google Scholar]
- 19.Desmond PM, Lovell AC, Rawlinson AA, et al. The value of apparent diffusion coefficient maps in early cerebral ischemia. Am J Neuroradiol 2001;22:1260–1267 [PMC free article] [PubMed] [Google Scholar]