Abstract
BACKGROUND AND PURPOSE: Cerebral hyperperfusion syndrome is a rare but serious complication of carotid endarterectomy (CEA). The aim of the present study was to determine whether intraoperative blood flow velocity (BFV) monitoring in the middle cerebral artery (MCA) by using transcranial Doppler ultrasonography (TCD) could be used as a reliable technique to detect cerebral hyperperfusion following CEA by comparing findings with those of brain single photon emission CT (SPECT).
METHODS: Intraoperative BFV monitoring was attempted in 67 patients undergoing CEA for treatment of ipsilateral internal carotid artery (ICA) stenosis (≥70%). Cerebral blood flow (CBF) was also assessed using SPECT, which was performed before and immediately after CEA.
RESULTS: Intraoperative BFV monitoring was achieved in 60 patients. Of the 60 patients, post-CEA hyperperfusion (CBF increase ≥100%, compared with preoperative values) was observed in six patients. The sensitivity, specificity, and positive predictive value of the BFV increases immediately after declamping of the ICA for detecting post-CEA hyperperfusion was 100%, 94% and 67%, respectively, with a cut-off point 2.0-fold that of preclamping BFV. The sensitivity and specificity of the BFV increases at the end of the procedure for detecting post-CEA hyperperfusion were 100% for both parameters, with cut-off points of 2.0- to 2.2-fold BFV of preclamping value. Hyperperfusion syndrome developed in two patients with post-CEA hyperperfusion, but intracerebral hemorrhage did not occur. In one of these two patients, BFV monitoring was not possible because of failure to obtain an adequate bone window.
CONCLUSION: Intraoperative MCA BFV monitoring by using TCD is a less reliable method to detect cerebral hyperperfusion following CEA than postoperative MCA BFV monitoring, provided adequate monitoring can be achieved.
Complications of carotid endarterectomy (CEA) may include cerebral embolism, cerebral hypoperfusion during carotid clamping, or cerebral hyperperfusion after declamping. Cerebral hyperperfusion after CEA is defined as a major increase in ipsilateral cerebral blood flow (CBF) following surgical repair of carotid stenosis that is well above the metabolic demands of the brain tissue (1, 2). A rapid restoration of normal perfusion pressure following CEA may result in regional hyperperfusion secondary to impaired autoregulation that occurs in the context of chronic ischemia (2). The clinical symptoms of cerebral hyperperfusion syndrome include unilateral headache, face and eye pain, seizure, and focal symptoms that occur secondary to cerebral edema or intracerebral hemorrhage (1–3). Intracerebral hemorrhage due to cerebral hyperperfusion is uncommon, but the prognosis for patients with this condition is poor (1, 3–8). Most authors suggest that early and strict control of postoperative blood pressure may be effective in preventing cerebral hyperperfusion syndrome (1–3, 9–12). Therefore, early recognition of cerebral hyperperfusion is important to minimize perioperative risks associated with CEA.
Transcranial Doppler ultrasonography (TCD) can be used to assess blood flow velocities in the major cerebral arteries of the circle of Willis. Use of TCD in this manner can characterize pathogenic hemodynamic mechanisms associated with various complications of CEA (13). As a result, several investigators have advocated the use of intraoperative TCD monitoring to predict the risk of postoperative cerebral hyperperfusion syndrome (9, 14). However, the accuracy of intraoperative TCD monitoring for detection of cerebral hyperperfusion itself remains unknown.
The aim of the present study was to determine whether intraoperative TCD monitoring could be used as a reliable technique to detect cerebral hyperperfusion following CEA. Changes in blood flow velocity in the middle cerebral artery (MCA) were compared with CBF changes measured quantitatively by single photon emission CT (SPECT) in characterizing hyperperfusion states following declamping of the internal carotid artery (ICA).
Methods
Patients
Between January 1999 and January 2002, 67 patients with ipsilateral ICA stenosis (≥70%) and useful residual function (modified Rankin disability scale 0, 1, or 2) underwent CEA.
Fifty-nine of the 67 patients were men, and eight were women. Mean age of the population was 68.6 ± 5.7 years (mean ± SD), ranging from 50 to 78 years. Thirty-nine patients showed ischemic symptoms in the ipsilateral carotid territory, and 28 patients exhibited asymptomatic ICA stenosis. All patients underwent preoperative angiography with arterial catheterization and MR imaging. Overall average degree of ICA stenosis was 84.0 ± 9.1%, with a range of 70% to 95%, as per the North American Symptomatic Carotid Endarterectomy Trial (15). The contralateral ICA was completely occluded in five patients, and 20 patients had 60% to 95% stenosis. No patient had stenosis >50% in the MCA ipsilateral to the ICA stenosis.
This protocol was reviewed and approved by the institutional ethics committee, and informed consent was obtained from all patients or their next of kin.
TCD Method
TCD ultrasonography was performed to measure blood flow velocity (BFV) in the MCA ipsilateral to CEA throughout the procedure. The TCD method consisted of a pulsed Doppler that operated at two MHz (TC2020, PIONEER, Uberlingen, Germany) and employed a transtemporal approach, permitting insonation of the M1 segment of the MCA. Cranial depths of 40 to 66 mm were sampled at 2-mm steps. The power output of the transducer was set at 100 mV/cm2, allowing for optimal insonation via the transtemporal window.
Systolic BFV in the MCA was used for this analysis. The following data points were gathered and recorded: the value 1 minute before ICA clamping (BFV-0), the value 3 minutes after declamping of the ICA (BFV-1), and the value at the end of the procedure (BFV-2). The BFV-1 and BFV-2 for each patient was calculated as a percentage of the BFV-0.
SPECT method
CBF was assessed by using [123I]N-isopropyl-p-iodoamphetamine (IMP) and SPECT before and immediately after CEA. In addition, patients with post-CEA hyperperfusion underwent a third CBF measurement in the same manner, 3 days after CEA. Preoperative SPECT study was performed more than 1 month after the last ischemic event and 7 to 10 days before CEA.
SPECT studies were performed by using a ring-type SPECT scanner (Headtome-SET080, Shimadzu Corp., Kyoto, Japan), which provided 31 tomographic images simultaneously. The spatial resolution of the scanner with a low-energy, all-purpose collimator was 13 mm FWHM at the center of the field of view, and the section thickness was 25 mm FWHM at the field of view center. Image sections were taken at 5 mm center-to-center spacing, parallel to the orbitomeatal (OM) line. The images were reconstructed by using the weighted-filtered back projection technique, in which the attenuation correction was made by detecting the edge of the object. An attenuation coefficient of 0.065 cm-1, Butterworth filter (cutoff = 0.45 cycle/cm; order = 3) and ramp filter were used for image reconstruction.
The IMP SPECT study was performed as described previously, and the CBF images were calculated according to the IMP-autoradiography method (16, 17). In each image section obtained immediately after CEA, an irregular region of interest (region of interest) of 16 cm2 or more was manually and bilaterally drawn in the cerebral cortex perfused by the MCA, following the atlas developed by Kretschmann and Weinrich (18). These ROIs were placed in regions where infarction was not present, as confirmed by MR imaging. After the CBF was determined in each region of interest, the ratio of ipsilateral regional CBF (I) to contralateral regional CBF (C) (I/C ratio) was calculated in each image section. The tomographic plane with the highest I/C ratio was selected and analyzed for each patient. Next, the tomographic plane that was determined in image sections obtained immediately after CEA was visually selected in image sections obtained preoperatively, and a region of interest was set in the cerebral cortex perfused by the ipsilateral MCA in the same manner. CT and MR imaging-SPECT imaging co-registration was not used.
Post-CEA hyperperfusion was defined as CBF increase of ≥100% (i.e., a doubling) compared with preoperative values, according to Piepgras et al (1).
Intraoperative and Postoperative Management
All patients underwent surgery under general anesthesia more than 1 month after the last ischemic event. Patients were premedicated with midazolam (7.5 mg PO). Anesthesia was induced with fentanyl (2 to 3 μg/Kg IV), propofol (1.5 to 3 mg/Kg IV), vecuronium (0.1 mg/Kg IV) and maintained by repeated boluses of fentanyl (1 to 2 μg/Kg IV), vecuronium, and 0.4% to 1.0% inspired isoflurane. All patients were artificially ventilated with an air-oxygen mixture (inspired fraction of oxygen ∼0.30). Analysis of intermittent drawn arterial blood gas samples ensured normal ventilation (4.7 to 5.2 kPa). Routine monitoring during anesthesia included standard electrocardiography, intraarterial catheter for direct arterial blood pressure measurement, pulse oximetry, and capnography. Blood pressure was kept stable in a range ±20% of the preoperative level throughout the procedure by adjusting the depth of anesthesia or, if needed, by intravenous administration of a vasodilator (nitroglycerin) or a vasoconstrictor (theoadrenalin).
An intraluminal shunt was not used in these procedures. The mean duration of ICA clamping was 32 minutes, ranging from 18 to 45 minutes. A bolus of heparin (5000 U) was given before ICA clamping, and protamine was administered at the conclusion of CEA.
All patients underwent CT imaging on the first postoperative day and MR imaging on the third postoperative day to confirm presence or absence of additional ischemic lesions.
In all patients with post-CEA hyperperfusion, intensive control of arterial blood pressure between 100 and 140 mm Hg was instituted by using intravenous administration of antihypertensive drugs immediately after SPECT. When CBF decreased and hyperperfusion resolved on the third postoperative day, pharmacologic control of blood pressure was discontinued. However, when hyperperfusion persisted, systolic arterial blood pressure was maintained below 140 mm Hg. When hyperperfusion syndrome developed, the patient was placed in a barbiturate coma. A diagnosis of hyperperfusion syndrome required: 1) seizure, deterioration of consciousness level or development of focal neurologic signs such as motor weakness, and 2) hyperperfusion on the SPECT performed after CEA without findings of any additional ischemic lesion on postoperative CT scan or MR imaging.
Statistical Analyses
Correlations between BFV increases [(BFV calculated as a percentage of the preclamping value) − 100%] and CBF increases [(CBF calculated as a percentage of the preoperative value) − 100%] were determined by using linear and polynomial regression analyses and by computing regression equations and correlation coefficients, and the function of better fit was determined. Statistical significance was set at the P < .05 level. The sensitivity and specificity of BFV increases for detecting post-CEA hyperperfusion was evaluated by using ROC curves.
Results
All patients recovered from surgery without new major neurologic deficits, and postoperative CT or MR imaging did not detect additional ischemic lesions. Mean arterial blood pressure fluctuated over a range +16% to −13% between the time points bounded by BFV-0 and BFV-2. The interval between ICA declamping and BFV-2 determination ranged from 30 to 48 minutes. The interval between BFV-2 determination and postoperative CBF measurement by SPECT ranged from 39 to 73 minutes.
Measurement of CBF was achieved in all patients, and intraoperative TCD monitoring was achieved in 60 patients (90% of all patients). In the remaining seven patients, TCD monitoring was not possible because of failure to obtain an adequate bone window. Of the 67 patients, seven patients (10%) met CBF criteria for post-CEA hyperperfusion in the entire ipsilateral hemisphere on the SPECT images. In one of these seven patients with post-CEA hyperperfusion, TCD monitoring was not achieved.
The BFV increased following declamping of the ICA in six patients with post-CEA hyperperfusion (Fig 1). Post-declamping BFV was >200% of preclamp values and increased to more than 220% of preclamp values by the end of the procedure. Intraoperative changes in BFV in the 54 patients without post-CEA hyperperfusion are illustrated in Figure 2. In three of the patients without post-CEA hyperperfusion, BFV increased to >200% of preclamp values immediately after declamping, but BFV was decreased at the end of the procedure. Patients that did not ultimately experience post-CEA hyperperfusion did not exhibit BFV >200% of preclamp values at the end of the procedure.
Fig 1.
Intraoperative BFV after ICA declamping, expressed as percentages of preclamping BFV values, for patients with post-CEA hyperperfusion (n = 6). Arrow indicates patient that developed hyperperfusion syndrome.
Fig 2.
Intraoperative BFV after ICA declamping, expressed as percentages of preclamping BFV values, for patients without post-CEA hyperperfusion (n = 54).
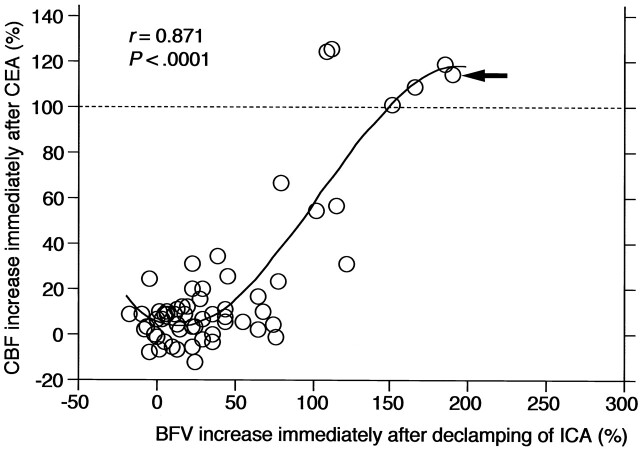
A strong cubic correlation was observed between the BFV increases immediately after declamping of the ICA and the CBF increases immediately after CEA (r = 0.871 and P < .0001) (Fig 3). Sensitivity and specificity of the BFV increases for detecting post-CEA hyperperfusion was 100% and 94%, respectively, in the cut-off point lying closest to the left upper corner of the ROC curve (cut-off point = 100%). In that cut-off point, the BFV increase achieved a positive predictive value of 67% (e.g., 33% incidence of false-positive results) and a negative predictive value of 100% (e.g., 0% incidence of false-negative results) for detecting post-CEA hyperperfusion.
Fig 3.
Correlation between BFV increases immediately after ICA declamping and CBF increases immediately after CEA. Curved line indicates cubic function of the best fit; arrow, patient that developed hyperperfusion syndrome; horizontal dashed line, CBF increase of 100% (the definition of hyperperfusion).
A strong quartic correlation was also observed between the BFV increases at the end of the procedure and the CBF increases immediately after CEA (r = 0.920 and P < .0001) (Fig 4). The sensitivity and specificity of the BFV increases for detecting post-CEA hyperperfusion were 100% in the cut-off points lying closest to the left upper corner of the ROC curve (cut-off points = 100–120%).
Fig 4.
Correlation between BFV increases at the end of the procedure and CBF increases immediately after CEA. Curved line indicates quartic function of the best fit; arrow, patient that developed hyperperfusion syndrome; horizontal dashed line, CBF increase of 100% (the definition of hyperperfusion).
In five of seven patients with post-CEA hyperperfusion, hyperperfusion had resolved on the SPECT performed on the third postoperative day, and these five patients had uneventful postoperative courses. However, the remaining two patients with post-CEA hyperperfusion experienced a progressive increase in CBF on the third postoperative day and developed hyperperfusion syndrome. One of these two patients experienced a focal seizure, as evidenced by motor disturbances of the right upper extremity 6 days after surgery. The TCD recordings and SPECT images for this patient is illustrated in Figure 5. The other patient, in whom TCD monitoring was not possible, experienced confusion and right motor weakness on the sixth postoperative day. Barbiturate coma was induced in both patients. Following termination of the barbiturate coma, both patients eventually experienced full recovery.
Fig 5.
A 75-year-old man with symptomatic lacunar infarcts in the right cerebral hemisphere and asymptomatic left ICA stenosis (90%) that exhibited hyperperfusion syndrome 6 days after CEA.
A, SPECT scans obtained preoperatively (left) and immediately after CEA (right) show postoperative hyperperfusion in the entire ipsilateral cerebral hemisphere.
B, TCD monitoring of the left middle cerebral artery during CEA reveals systolic blood flow velocity of 2.9-fold of preclamp values immediately after declamping. The value increased to 3.6-fold at the end of the procedure.
Discussion
The present study demonstrated that intraoperative MCA BFV monitoring by using TCD is a less reliable method to detect cerebral hyperperfusion following CEA than postoperative MCA BFV monitoring.
Piepgras et al (1) measured CBF quantitatively during CEA by using intracarotid injection of xenon-133 and showed that the risk of intracerebral hemorrhage in patients with post-CEA hyperperfusion, which was defined as CBF increase of ≥100% (i.e., a doubling) compared with preoperative values, was >10 times that of patients without post-CEA hyperperfusion. Further, several investigators have demonstrated the utility of SPECT in establishing the diagnosis of post-CEA hyperperfusion (12, 19–21) by demonstrating that all patients who developed hyperperfusion syndrome had postoperative CBF increase of ≥100% compared with preoperative values and patients without postoperative CBF increase of ≥100% compared with preoperative values did not experience hyperperfusion syndrome. Thus, we employed SPECT imaging as the criterion standard for diagnosing cerebral hyperperfusion and evaluated the data as a percentage of the preoperative value. The incidence of post-CEA hyperperfusion by Piepgras’ definition was 8–12% (1, 19), which is consistent with the present results.
When the MCA is the primary route of blood supply to the MCA territory, the rate of blood movement within the MCA trunk generally reflects changes in CBF (22). Indeed, we demonstrated a cubic correlation between the BFV increases immediately after ICA declamping and the CBF increases immediately after CEA in patients with stenosis <50% in the ipsilateral MCA. However, use of the BFV increases immediately after ICA declamping yielded a 33% incidence of false-positive results when used to detect post-CEA hyperperfusion. These false-positive results occurred particularly in patients in which the BFV increased immediately after ICA declamping but decreased at the end of the procedure. This phenomenon has been previously reported (23) and may reflect transient reactive hyperemia that occurs before restoration of cerebrovascular autoregulation which was impaired during ICA clamping.
The sensitivity and specificity of the end-procedure increases in BFV for detecting post-CEA hyperperfusion was 100% for both parameters. This finding suggests that postoperative BFV monitoring with measurement before ICA clamping as baseline can reliably detect cerebral hyperperfusion following CEA. In contrast, there may be no point in monitoring BFV intraoperatively because this method yields some false-positive findings and offers no practical gain. Since strict control of blood pressure in the postoperative period may be effective in preventing hyperperfusion syndrome (1–3, 9–12), early recognition of cerebral hyperperfusion is important to further minimize the perioperative risk of CEA. While two patients with post-CEA hyperperfusion exhibited cerebral hyperperfusion syndrome despite early and aggressive control of blood pressure, both patients eventually experienced full recovery. Dalman et al (9) reported that 11% of patients with post-CEA hyperperfusion were symptomatic with strict control of blood pressure, but none experienced intracerebral hemorrhage. This stands in contrast to the 2% incidence of intracerebral hemorrhage in patients undergoing CEA without strict postoperative blood pressure control.
Although several investigators using intraoperative TCD monitoring defined cerebral hyperperfusion as increases in BFV of >100% of preclamping values (9, 14), the evidence was lacking. According to the ROC analysis in the present study, the cut-off point of the BFV increases with the highest sensitivity and specificity for detecting post-CEA hyperperfusion was 100%, which corresponds to the value used in previous reports of intraoperative TCD monitoring. Further, this cut-off point is consistent with the value of post-CEA hyperperfusion determined by using CBF measurements (1, 19–21).
The TCD method still poses considerable technical difficulties. Approximately 10% of studies using TCD fail to detect MCA blood flow signals due to poor insonation of the cranial window (9, 22, 24, 25). In the present study, TCD monitoring could not be achieved in one of seven patients that were diagnosed with post-CEA hyperperfusion on SPECT imaging with subsequent development of cerebral hyperperfusion syndrome. In addition, in the event that there is occlusion or hemodynamically limiting stenosis in the ipsilateral MCA, TCD monitoring would not be useful for this purpose. Thus, alternative methods, such as SPECT or transcranial regional cerebral oxygen saturation monitoring by using near-infrared spectroscopy, are still necessary for early detection of post-CEA hyperperfusion (20, 21).
Conclusion
Although our patient cohort is limited, we demonstrated that intraoperative MCA BFV monitoring by using TCD is a less reliable method to detect cerebral hyperperfusion following CEA than postoperative MCA BFV monitoring, provided adequate monitoring can be achieved.
Footnotes
Supported in part by Grants-in-Aid for Science Research by the Ministry of Science, Education, Sports and Culture (No. 15591541), Japan and by the Mitsubishi Pharma Research Foundation.
References
- 1.Piepgras DG, Morgan MK, Sundt TM Jr., Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 1988;68:532–536 [DOI] [PubMed] [Google Scholar]
- 2.Sundt TM Jr., Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr., O’Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy, with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 1981;56:533–543 [PubMed] [Google Scholar]
- 3.Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg 1986;64:29–34 [DOI] [PubMed] [Google Scholar]
- 4.Jansen C, Sprengers AM, Moll FL, et al. Prediction of intracerebral haemorrhage after carotid endarterectomy by clinical criteria and intraoperative transcranial Doppler monitoring: results of 233 operations. Eur J Vasc Surg 1994;8:220–225 [DOI] [PubMed] [Google Scholar]
- 5.Ouriel K, Shortell CK, Illig KA, Greenberg RK, Green RM. Intracerebral hemorrhage after carotid endarterectomy: Incidence, contribution to neurologic morbidity, and predictive factors. J Vasc Surg 1999;29:82–89 [DOI] [PubMed] [Google Scholar]
- 6.Pomposelli FB, Lamparello PJ, Riles TS, Craighead CC, Giangola G, Imparato AM. Intracranial hemorrhage after carotid endarterectomy. J Vasc Surg 1988;7:248–255 [PubMed] [Google Scholar]
- 7.Riles TS, Imparato AM, Jacobowitz GR, et al. The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg 1994;19:206–216 [DOI] [PubMed] [Google Scholar]
- 8.Schroeder T, Sillesen H, Boesen J, Laursen H, Sorensen P. Intracerebral haemorrhage after carotid endarterectomy. Eur J Vasc Surg 1987;1:51–60 [DOI] [PubMed] [Google Scholar]
- 9.Dalman JE, Beenakkers IC, Moll FL, Leusink JA, Ackerstaff RG. Transcranial Doppler monitoring during carotid endarterectomy helps to identify patients at risk of postoperative hyperperfusion. Eur J Vasc Endovasc Surg 1999;18:222–227 [DOI] [PubMed] [Google Scholar]
- 10.Mansoor GA, White WB, Grunnet M, Ruby ST. Intracerebral hemorrhage after carotid endarterectomy associated with ipsilateral fibrinoid necrosis: a consequence of the hyperperfusion syndrome? J Vasc Surg 1996;23:147–151 [DOI] [PubMed] [Google Scholar]
- 11.Reigel MM, Hollier LH, Sundt TM Jr., Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg 1987;5:628–634 [PubMed] [Google Scholar]
- 12.Yoshimoto T, Houkin K, Kuroda S, Abe H, Kashiwaba T. Low cerebral blood flow and perfusion reserve induce hyperperfusion after surgical revascularization: case reports and analysis of cerebral hemodynamics. Surg Neurol 1997;48:132–139 [DOI] [PubMed] [Google Scholar]
- 13.Spencer MP. Transcranial Doppler monitoring and causes of stroke from carotid endarterectomy. Stroke 1997;28:685–691 [DOI] [PubMed] [Google Scholar]
- 14.Keunen R, Nijmeijer HW, Tavy D, et al. An observational study of pre-operative transcranial Doppler examinations to predict cerebral hyperperfusion following carotid endarterectomies. Neurol Res 2001;23:593–598 [DOI] [PubMed] [Google Scholar]
- 15.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 16.Iida H, Itoh H, Nakazawa M, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med 1994;35:2019–2030 [PubMed] [Google Scholar]
- 17.Ogasawara K, Ito H, Sasoh M, et al. Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med 2003;44:520–525 [PubMed] [Google Scholar]
- 18.Kretschmann HJ, Weinrich W. Neuroanatomy and cranial computed tomography. New York: Thieme Inc;1986;70–74
- 19.Hosoda K, Kawaguchi T, Shibata Y, et al. Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 2001;32:1567–1573 [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara K, Konno H, Yukawa H, Endo H, Inoue T, Ogawa A. Transcranial regional cerebral oxygen saturation monitoring during carotid endarterectomy as a predictor of postoperative hyperperfusion. Neurosurgery 2003;53:309–314 [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara K, Yukawa H, Kobayashi M, et al. Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 2003;99:504–510 [DOI] [PubMed] [Google Scholar]
- 22.Pindzola RR, Balzer JR, Nemoto EM, Goldstein S, Yonas H. Cerebrovascular reserve in patients with carotid occlusive disease assessed by stable xenon-enhanced CT cerebral blood flow and transcranial Doppler. Stroke 2001;32:1811–1817 [DOI] [PubMed] [Google Scholar]
- 23.Schaafsma A, Veen L, Vos JP. Three cases of hyperperfusion syndrome identified by daily transcranial Doppler investigation after carotid surgery. Eur J Vasc Endovasc Surg 2002;23:17–22 [DOI] [PubMed] [Google Scholar]
- 24.Levi CR, O’Malley HM, Fell G, et al. Transcranial Doppler detected cerebral microembolism following carotid endarterectomy: High microembolic signal loads predict postoperative cerebral ischaemia. Brain 1997;120:621–629 [DOI] [PubMed] [Google Scholar]
- 25.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 1999;30:593–598 [DOI] [PubMed] [Google Scholar]