Abstract
Summary: We applied a single-shot echo-planar axial diffusion tensor sequence with parallel imaging for tractography of the cervical cord. Placing a large region of interest on a reconstructed sagittal image, the cervical cord was depicted as a tract bundle showing color-encoded cephalocaudally. By setting one region of interest in the cerebral peduncle and another in the entire cord on source images, tracts on each side were visualized. This method is promising for tractography of the cervical cord.
The parallel imaging technique uses arrays of multiple receiver coils to reduce imaging time by a factor equal to the number of coils used. By shortening the echo train length, we can obtain diffusion-weighted (DW) images less affected by susceptibility artifacts when parallel imaging is employed. Our purpose was to examine the feasibility of diffusion-tensor (DT) imaging by using the parallel imaging technique for tractography of the cervical cord.
Description of the Technique
We studied seven volunteers (five men and two women with an age range of 21–37 years and a mean age of 31.7 years) and 12 patients (seven men and five women; age range, 21–90 years; mean age, 46.7 years) between November 2003 and March 2004. The patients were prospectively included in this study after they provided written consent at the time of routine MR imaging of the cervical spine. The clinical diagnosis of the patients based on clinical and MR findings included degenerative spinal canal stenosis (10 patients) and disk herniation (two patients).
Images were obtained with a whole-body 1.5-T MR imaging system with a maximum gradient capability of 30 mT/m and a slew rate of 150 mT/m/ms by using a synergy head-neck receiver coil that had three channels (head, anterior neck, and posterior neck parts). A single-shot echo-planar sequence was used to acquire DW images. The fat signal was suppressed by using the spectral selective presaturation with the inversion recovery technique. The parallel imaging technique was used (reduction factor, 1.8) to reduce distortion inherent in the echo-planar imaging technique. A motion-probing gradient was applied in six directions (Dxx, Dyy, Dzz, Dxy, Dyz, and Dxz), and a b value of 1000 s/mm2 was used for each of these six directions. Other imaging parameters are shown in the Table. Imaging time was 4 minutes 50 seconds.
Scan parameters used for tractography
| Imaging plane | Axial |
| Phase encoding direction | Right-left |
| Echo time (msec) | 88 |
| Number of sections | 60 |
| Section thickness (mm) | 2.5 |
| Intersection gap (mm) | 0 |
| Field of view (mm) | 230 × 172.5 |
| Imaging matrix | 112 × 90 zero-filled to 128 × 128 |
| Reconstructed voxel size (mm) | 1.8 × 1.8 × 2.5 |
| Number of scan averaging | 4 |
Note.—“Scan averaging” means modulus averaging of obtained signal.
For postprocessing, we used a tractography program incorporated in research software provided by the manufacturer (Philips Medical Systems, Best, the Netherlands). On a reconstructed sagittal DW image, we drew an automatic 3D region of interest in the 19 subjects. This 3D region of interest was created by one click on a desired location, and an algorithm of a flood-fill type was used to check whether neighboring voxels in 3D should belong to the region of interest according to a threshold set by a user. It made it possible to generate a tractogram of the entire cervical cord. For the purpose of depicting tracts on each side, in the latter consecutive 10 of the 19 subjects we included the brain stem in the imaging area and set two regions of interest, one in the right or left cerebral peduncle and the other in an entire part of the cord on reconstructed DW images in the axial plane. The threshold of fractional anisotropy, which represents the directionality of water diffusion, to generate a tractogram was usually set at 0.2–0.3 as was similarly selected by Melhem et al (1) in fiber tracking of the brain.
Two radiologists (K.T., A.F.) reviewed the images obtained. They paid attention to how the cord was demonstrated in the one region of interest method and how the white matter tracts on each side were depicted in the two region of interest method.
Results
In our color-coded tractograms, fibers running in the cephalocaudal direction were shown in blue, those in the anteroposterior direction in green, and those in the right-left direction in red. In the one region of interest method, the cervical cord was depicted as a bundle of tracts showing mainly blue that was encoded in the cephalocaudal direction in all of the 19 subjects (Fig 1). More upper and lower parts of the cord, not included in the original region of interest, were demonstrated. Although the nerve roots were demonstrated variably in each subject, those of the lower cervical cord were better depicted in 15 of the 19 subjects (2). In the two region of interest method, tracts possibly representing the corticospinal or spinothalamic tracts were visualized. Fused tractograms of the two sides demonstrated the portion of the medullary decussation in all of the ten subjects (Fig 2). The tracts from two sides of the brain did not completely cross, however, but rather seemed to combine or go down on the same side within the cervical cord in each subject. It is interesting that there was asymmetry in the number of tracts on the two sides in all of the 10 patients in whom the two region of interest method was applied. More tracts connected to the left cerebral hemisphere were demonstrated in six subjects, whereas those connected to the right hemisphere were more visualized in four subjects. On interview after MR imaging study, all of the 10 subjects stated that they were right-handed. Image distortion was judged to be acceptable on both kinds of tractography.
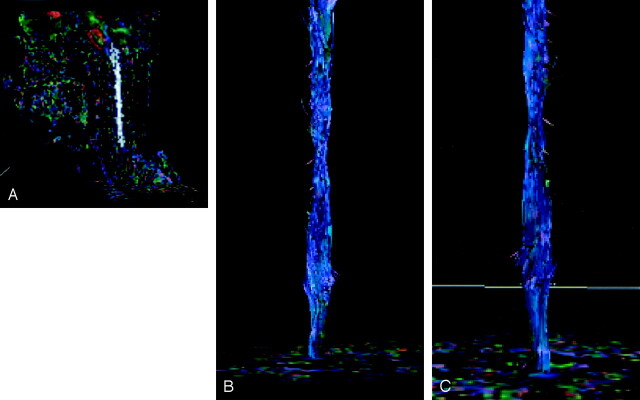
Fig 1.
Tractography by the one region-of-interest method.
A, Fractional anisotropy map reconstructed in the sagittal plane shows a single region of interest displayed in white to generate a tractogram of the entire cervical cord.
B, Corresponding tractogram viewed from the front shows the cervical cord mainly composed of tracts running in the cephalocaudal direction represented in blue.
C, Corresponding tractogram viewed from the back shows some dorsal nerve roots.
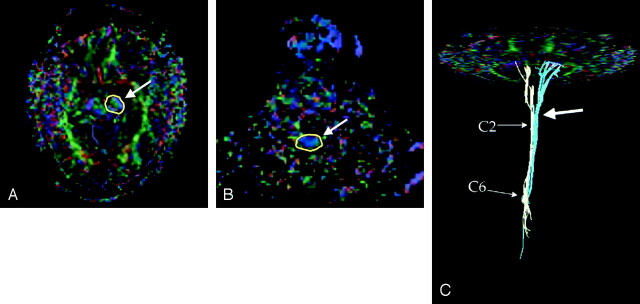
Fig 2.
Tractography by the two region-of-interest method.
A, One of source images shows a region of interest) in the left cerebral peduncle (arrow).
B, Another source image in the lower cervical level shows a region of interest encompassing the entire cord (arrow).
C, Combined image of the tracts passing the cerebral peduncles on each side shows the medullary decussation (arrow). Tracts coming through the right cerebral peduncle are shown in light yellow and those coming through the left cerebral peduncle are shown in light blue. Below the medullary decussation, the tracts from each side seem to be mixed.
Discussion
Several groups have successfully performed DT imaging of the cervical spinal cord. Quantitative analysis of some anisotropy parameters of the rat cervical cord has been reported in vitro or in vivo by using an experimental imager operating at up to 14T (2–8). In the human cervical cord, DT imaging at 1.5T has been accomplished by use of cardiac-gated and navigated and multi-shot echo-planar techniques (9, 10). A recent study performed DT imaging by using parallel imaging that is similar to ours and calculated the fractional anisotropy and mean diffusivity (11). These human studies were limited to measurement of anisotropy parameters like the rat studies.
As in the brain, the clinical value of DT tractography of the spinal cord is anticipated. There are already preliminary reports describing tractography of the cervical cord by using line scan (12) or single-shot echo-planar imaging (13). In contradistinction to these reports, our technique could always generate tractograms of the entire cervical cord. In each case, the cervical cord appeared as a bundle of tracts giving a color that indicated anisotropy in the cephalocaudal direction. In this regard, we attribute our results to reduction in image distortion and susceptibility artifacts—despite use of single-shot echo-planar imaging—by using parallel imaging, which shortens the echo train length. The use of single-shot echo-planar imaging was presumably effective in providing images less affected by respiratory and cardiac motion. Meanwhile, the nerve roots were not sufficiently demonstrated on tractograms generated by both one region of interest and two region of interest methods. We believe that the limited spatial resolution was the main reason why our methods failed to demonstrate the nerve roots well.
We assume that the limited spatial resolution also made it difficult to identify in what part of the cord the tracts demonstrated by the two region of interest method were running. On the basis of the position of the upper region of interest, our tractograms by the two region of interest technique possibly visualized in a mixed manner descending tracts that include the lateral and ventral corticospinal tracts, the rubospinal tract, as well as ascending tracts that include the dorsal, lateral, and ventral spinocerebellar tracts and dorsal and ventral spinothalamic tracts. In any case, our two region of interest method could visualize fine nerve tracts by using a commercially available coil system. We consider that DT imaging with single-shot echo-planar imaging, in combination with the parallel imaging technique, was also effective in this regard. In contrast to the use of a b value of 700 s/mm2 in previous studies of preliminary tractography of the cervical cord (12, 13), we employed that of 1000 s/mm2 that is widely used in DT imaging of the brain. In our experience, in three of the seven volunteers whose tractograms of b values of 500 and 1000 s/mm2 were compared, those of 1000 s/mm2 were better in demonstrating tracts.
Although at first we supposed that, below the medullary decussation, tracts passing each side of the cerebral peduncle would cross to the other side, most tracts on the two sides were mixed or went down on the same side. This is probably owing to the present DT imaging software that depicts crossing fibers as they are connected because tracts are essentially visualized by means of the largest eigenvalue. Further developments would solve this problem and demonstrate crossing of tracts at the medullary decussation correctly. It is interesting to note that there was asymmetry in the number of tracts on the two sides in the two region of interest method. At present, we are unaware of the exact reason. As this study included a small number of subjects, we are planning to examine whether this finding is true and clarify the reason in a larger series. Another limitation of our technique was the threshold of fractional anisotropy. Although we followed a previous brain fiber tracking method in this regard, application in a more quantitative way may be effective to track fine white matter fibers in the spinal cord.
Conclusion
DT imaging using parallel imaging is a promising method to perform tractography of the cervical spinal cord. We would like to emphasize that our technique can be performed in a routine clinical examination because the imaging time is acceptable. Although some issues remain to be solved or clarified, DT tractography facilitated by the parallel imaging cord may improve evaluation of the normal and affected anatomical structures of the cervical cord.
References
- 1.Melhem ER, Mori S, et al. Diffusion tensor MR imaging of the brain and white matter tractography. AJR Am J Roentgenol 2002;178:3–16 [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa H, Kwee IL, Nakada T. Magnetic resonance axonography of the rat spinal cord: postmortem effects. J Neurosurg 1995;83:1023–1028 [DOI] [PubMed] [Google Scholar]
- 3.Inglis BA, Yang L, Wirth ED 3rd, et al. Diffusion anisotropy in excised normal rat spinal cord measured by NMR microscopy. Magn Reson Imaging 1997;15:441–450 [DOI] [PubMed] [Google Scholar]
- 4.Fenyes DA, Narayana PA. In vivo echo-planar imaging of rat spinal cord. Magn Reson Imaging 1998;16:1249–1255 [DOI] [PubMed] [Google Scholar]
- 5.Fenyes DA, Narayana PA. In vivo diffusion characteristics of rat spinal cord. Magn Reson Imaging 1999;17:717–722 [DOI] [PubMed] [Google Scholar]
- 6.Inglis BA, Neubauer D, Yang L, et al. Diffusion tensor MR imaging and comparative histology of glioma engrafted in the rat spinal cord. AJNR Am J Neuroradiol 1999;20:713–716 [PMC free article] [PubMed] [Google Scholar]
- 7.Fenyes DA, Narayana PA. In vivo diffusion tensor imaging of rat spinal cord with echo planar imaging. Magn Reson Med 1999;42:300–306 [DOI] [PubMed] [Google Scholar]
- 8.Elshafiey I, Bilgen M, He R, Narayana PA. In vivo diffusion tensor imaging of rat spinal cord at 7 T. Magn Reson Imaging 2002;20:243–247 [DOI] [PubMed] [Google Scholar]
- 9.Clark CA, Werring DJ, Miller DH. Diffusion imaging of the spinal cord in vivo: estimation of the principal diffusivities and application to multiple sclerosis. Magn Reson Med 2000;43:133–138 [DOI] [PubMed] [Google Scholar]
- 10.Ries M, Jones RA, Dousset V, Moonen CT. Diffusion tensor MRI of the spinal cord. Magn Reson Med 2000;44:884–892 [DOI] [PubMed] [Google Scholar]
- 11.Cercignani M, Horsfield MA, Agosta F, Filippi M. Sensitivity-encoded diffusion tensor MR imaging of the cervical cord. AJNR Am J Neuroradiol 2003;24:1254–1256 [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy BP, Zientara GP, Huppi PS, et al. Line scan diffusion tensor MRI of the cervical spinal cord in preterm infants. J Magn Reson Imaging 2001;13:949–953 [DOI] [PubMed] [Google Scholar]
- 13.Wheeler-Kingshott CA, Hickman SJ, Parker GJ, et al. Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage 2002;16:93–102 [DOI] [PubMed] [Google Scholar]