Abstract
Summary: A new, coated bioactive coil has been developed to improve the long-term results of endovascular treatment of intracranial aneurysms. The purpose of this preliminary study was to assess the feasibility and safety of selective embolization of intracranial aneurysms with Matrix coils in 20 consecutive patients.
Endovascular treatment of intracranial aneurysms by endosaccular coil placement has become an accepted alternative to surgical clip placement (1); however, the main disadvantage of this technique compared with surgery is aneurysm recurrence (2), especially for large aneurysmal sacs or wide-necked aneurysms. To circumvent this potential limitation, many investigations are currently evaluating new coils, including biologically active coils (3), radioactive coils (4), and coated coils with a swelling hydrogel (5). The aim of these innovations is to promote intra-aneurysmal clot organization and fibrosis (3, 4) or to increase packing density (5), which is associated with a higher long-term occlusion rate (6). The complete Matrix coils (Target Therapeutics, Fremont, CA) product line has recently become available, but no study has yet evaluated the feasibility of endovascular treatment with these coils. We report our experience of selective embolization with Matrix coils in 20 consecutive patients with an intracranial aneurysm.
Technique
Matrix detachable coils are platinum coils covered with a bioabsorbable polymer (90% polyglycolide, 10% polyactide) and attached to a stainless steel delivery wire. The coil consists of 70% polymer and 30% platinum. The details of coil characteristics were described elsewhere (7), and the detachment system is the same as that used with Guglielmi detachable coils (GDCs). The product line is similar to that of bare GDCs and includes 3D and 2D coils. Variable degrees of stiffness are available, including standard, soft, and ultrasoft coils.
Between February and March 2004, 20 consecutive patients with 20 cerebral aneurysms were treated by selective endovascular approach in our department. Fourteen patients presented with a subarachnoid hemorrhage (SAH) and were classified according to the Hunt and Hess scale (8): nine were grade I or II, one was grade III, and four were grade IV or V. Six patients were asymptomatic. Locations of aneurysms were as follows: posterior communicating artery in six, anterior communicating artery in five, basilar artery in three, middle cerebral artery bifurcation in three, internal carotid artery bifurcation in two, and posterior cerebral artery in one. All endovascular procedures were performed under general anesthesia and systemic heparinization. A bolus infusion of heparin (30–40 IU/kg body weight) was followed by a continuous drip (1000–1500 IU/h), with the purpose of doubling the baseline activated clotting time. No patient was placed on antiplatelet medication before treatment. All patients were treated by selective embolization with Matrix coils. Technique of endosaccular coil placement procedure has already been published in the literature (7). Aneurysm packing, by the use of an Excelsior 10 microcatheter (Target Therapeutics), was obtained by forming a basket with one or more 3D coils that were subsequently filled with smaller 2D coils. In all cases, we attempted to pack the aneurysm with Matrix coils alone. Soft, ultrasoft, or both soft and ultrasoft Matrix coils were used at the end of the procedure to complete aneurysm obliteration. To avoid friction inside the microcatheter, Matrix coils were placed in a saline solution for at least 60 seconds to hydrate the polymer completely. When packing difficulties (high frictions between coils, compartmentalization) with Matrix coils were encountered because of their stiffness, additional GDCs were used to achieve aneurysm occlusion. In three cases with unfavorable neck-to-sac ratios, the remodeling technique (9) was used to avoid coil protrusion into the parent artery. Patients were examined by angiography to document aneurysm obliteration. Angiographic results were classified into three groups: complete occlusion (no contrast medium filling of the aneurysmal sac), neck remnant (residual contrast medium filling the aneurysmal neck), and residual flow (residual contrast medium filling the aneurysmal body). A senior neurosurgeon (J.-P.L.) recorded the clinical course, including worsening of symptoms and death, at 3 months after the treatment. Clinical outcome was graded according to a modified Glasgow outcome scale (10) as follows: excellent (neurologically intact); good (mild hemiparesis, cranial nerve palsy, or other deficit that does not interfere with daily functioning or work); fair (significant hemiparesis, aphasia, confusion, or other deficit that interferes with daily activities or prevents a return to work); and poor (coma or severe neurologic deficit rendering the patient dependent on family or nursing staff).
Results
Selective embolization was successfully performed in all patients. Complete aneurysm occlusion by the use of Matrix coils alone, however, was achieved in only five patients. In these cases, cerebral aneurysms had a diameter greater than 7 mm or were treated with the remodeling technique. In the remaining 15 patients, additional GDCs were required to complete aneurysm obliteration. GDC coils—that are softer than Matrix coils—were used to fill the residual part of the aneurysm that could not be safely treated with stiffer coils because of a kickback effect on the microcatheter. A total of 68 Matrix coils were used and passed through the microcatheter without any friction. In all aneurysms, from two to 12 Matrix coils were delivered to obtain a good packing. During the delivery process, the first 3D Matrix coil was adapted smoothly to the aneurysm wall and automatically assumed its configuration upon deployment. Then, smaller 3D or 2D coils were added and packed the aneurysms. Because of the new and complete Matrix product line, soft and ultrasoft coils were used and few frictions were encountered between coils within the aneurysmal sac. When the maximal diameter of the aneurysm was less than 8 mm, however, there was a tendency of compartmentalization that required changing the position of the microcatheter to reach another part of the aneurysm. In most cases, the catheter has been repositioned only once. Detachment of coils occurred in less than 2 minutes in all cases, and no coil rupture or involuntary coil detachment was observed. No stretching of Matrix coils was encountered during their repositioning and no technical complication occurred in the present series.
Endovascular treatment resulted in 16 excellent clinical outcomes, three good outcomes, and one death. Among the four patients with a grade IV or V SAH, one died because of a severe vasospasm and three kept a mild hemiparesis or an upper limb weakness. No permanent neurologic morbidity or mortality related to the procedure was observed in the present series. Three patients, however, presented transient ischemic attacks (TIA) a few hours after the endovascular treatment. These patients had wide-neck aneurysms that were treated with Matrix coils and by the use of the remodeling technique. Systemic heparinization was prolonged for 48–72 hours and clopidogrel (75 mg/day) was administered for one month. All patients made an excellent recovery.
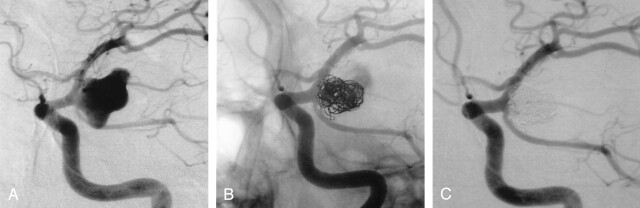
Angiographic results consisted of 15 complete occlusions and five neck remnants. Although the aneurysm occlusion was judged complete in 15 cases, the packing density of the aneurysmal sac appeared lower (Fig 1) than with bare coils.
Fig 1.
SAH in a 48-year-old man. Internal carotid artery angiogram (A) shows a 12-mm aneurysm of the posterior communicating artery. Selective embolization with Matrix coils only: 12 coils were delivered within the aneurysmal sac. Conventional non-substracted (B) and substracted (C) angiograms at the end of the procedure showed a complete aneurysm occlusion despite a moderate packing.
Discussion
This study shows that, despite their relative stiffness, Matrix coils may be used to treat intracranial aneurysms by selective embolization. Most patients showed excellent clinical and anatomic results, and there was no permanent morbidity or mortality related to the device or the procedure. However, three patients who were treated by Matrix coils with the remodeling technique experienced TIA that resolved after systemic heparinization. It is not clear whether these events were related to the use of the remodeling technique, which is known to increase thromboembolic complications (11), or to the Matrix coils that might be more thrombogenic than bare coils because of their coating.
The current limitation of Matrix coils is their stiffness that does not allow to completely treat small aneurysms with these coils alone. Indeed, these coils have a tendency to compartmentalize into the aneurysmal sac, and it requires repositioning of the catheter in order to complete aneurysm occlusion. This increases the duration and the risks of the procedure. There are, however, no formal data suggesting that aneurysms should exclusively be packed with Matrix coils to obtain a good healing of the neck. Moreover, these difficulties were observed in small aneurysms with a small neck that are known to show fewer recurrences than wide-necked or large aneurysms (12). In our department, the therapeutic strategy of selective embolization of intracranial aneurysms is to treat small aneurysms with a small neck with bare coils and to keep Matrix coils for large or wide-necked aneurysms. In these cases, we try to put one or more 3D Matrix coils to completely bridge the aneurysmal neck to put as much coated material as possible in the neck region. Then, softer 2D coils are delivered to complete aneurysm occlusion. In cases of wide-neck aneurysms, the use of a remodeling balloon is required to prevent coil protrusion into the parent artery. It also appeared helpful in the present series to completely occlude aneurysms with Matrix coils alone. Because of the inflated balloon at the neck, Matrix coils were conforming to the aneurysmal sac without any compartmentalization.
Finally, one must be aware of the final appearance of packing density of Matrix coils within the aneurysmal sac. Matrix coil consists of 70% polymer and 30% platinum and explains that packing density appears lower (Fig 1) than that with bare coils even if the treatment is complete.
Conclusion
This series shows that Matrix coils may be used for the endovascular treatment of intracranial aneurysms; however, and because of their stiffness, additional bare coils are needed to complete aneurysm occlusion in most cases of small aneurysms. Long-term follow-up is mandatory to establish the utility and potential advantages of this new coil.
References
- 1.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. ISAT of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–1403 [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Tateshima S, Gonzalez NR, Vinuela F. Matrix and bioabsorbable polymeric coils accelerate healing intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–2037 [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Leblanc P, desfaits AC, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke 2002;33:421–427 [DOI] [PubMed] [Google Scholar]
- 5.Cloft HJ, Kallmes DF. Aneurysm packing with Hydrocoil embolic system versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 6.Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol 2002;23:762–767 [PMC free article] [PubMed] [Google Scholar]
- 7.Gulielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2. Preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 8.Hunt W, Hess R. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 9.Moret J, Cognard C, Weill A, et al. The reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 10.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet 1975;1:480–484 [DOI] [PubMed] [Google Scholar]
- 11.Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003;24:127–132 [PMC free article] [PubMed] [Google Scholar]
- 12.Zubillaga A, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol 1994;15:815–820 [PMC free article] [PubMed] [Google Scholar]