Abstract
Summary: We report CT findings in seven patients with diffuse cerebral edema and increased attenuation in the basilar cisterns resembling subarachnoid hemorrhage. On the basis of autopsy (three cases) and lumbar puncture (four cases) findings, true subarachnoid hemorrhage was reasonably excluded. Pathophysiologic changes that occur with diffuse cerebral edema are explored, with proposed explanations for the appearance of a pseudo-subarachnoid hemorrhage.
Increased attenuation of the basal cisterns and subarachnoid spaces on CT scans is a characteristic finding of acute subarachnoid hemorrhage (SAH). Several radiographic mimics of SAH have been reported, including pyogenic leptomeningitis, CT appearance of intrathecally administered contrast material, and leakage of high-dose intravenous contrast medium into the subarachnoid spaces (1–6). Together, these CT mimics of SAH have been called pseudo-SAH (5–7).
The pseudo-SAH appearance may also be seen in patients with acute neurologic deficits in whom evidence of diffuse cerebral edema is present at CT examination. In this report, we describe seven patients with pseudo-SAH hemorrhage associated with diffuse cerebral edema. We also review the literature, investigate the pathophysiology behind its appearance, and discuss imaging findings that aid in its recognition and its distinction from true SAH.
Case Reports
We retrospectively reviewed the clinical, laboratory, and pathology-autopsy reports in seven patients without trauma (Table). Diffuse cerebral edema and increased attenuation of the subarachnoid spaces was noted on CT scans. Nonenhanced head CT scans were obtained in all cases, and one of the patients (case 1) was also examined with a contrast-enhanced study. In each case, the attenuation within the basal cisterns mimicked that of SAH (Figs 1A, 2A). The patients were aged 8 months to 37 years and included four male and three female patients. The time from their clinical deterioration (usually during intubation) to the CT examination varied widely, ranging from approximately 2 hours before deterioration (case 4) to approximately 33 hours after resuscitation (case 2). Attenuation values within the basal cisterns were obtainable in three of the patients (cases 5–7). Two of the patients (cases 6 and 7) had undergone prior examinations, with images available for comparison. These permitted quantification of the observed decrease in attenuation within the deep gray matter structures. True SAH was excluded by means of either autopsy (three cases) or lumbar puncture (four cases).
Summary of clinical and imaging findings
| Case | Patient Age/Sex | Signs of Edema* | Attenuation Value (HU)† | Proof‡ | Diagnosis at Death or Discharge§ |
|---|---|---|---|---|---|
| 1 | 5 y/M | C, E | NA | Autopsy result | Diffuse cerebral edema secondary to hyponatremia |
| 2 | 37 y/F | C, E, GW | NA | Autopsy result | Sudden cardiac death, anoxic injury |
| 3 | 22 y/M | C, E | NA | Negative LP result | Pseudo-tumor cerebri |
| 4 | 22 y/F | C, E | NA | Autopsy result | DKA with hypoxic and metabolic encephalopathy |
| 5 | 8 mo/F | E, GW | 29 | Negative LP result | Diffuse cerebral edema, SIDS |
| 6 | 5 y/M | C, E, GW | 33 | Negative LP result | Cardiopulmonary failure secondary to unknown metabolic disorder (possibly Leigh disease) |
| 7 | 6 y/M | C, E, GW | 29 | Negative LP result | Septic Shock |
C indicates compression and/or mass effect on the fourth ventricle; E, effacement of the basal cisterns and cortical sulci; GW, decreased gray matter–white matter differentiation.
Mean attenuation levels were obtained with a region of interest drawn in the basal cisterns. NA indicates not available.
LP indicates lumbar puncture.
DKA indicates diabetic ketoacidosis; SIDS, sudden infant death syndrome.
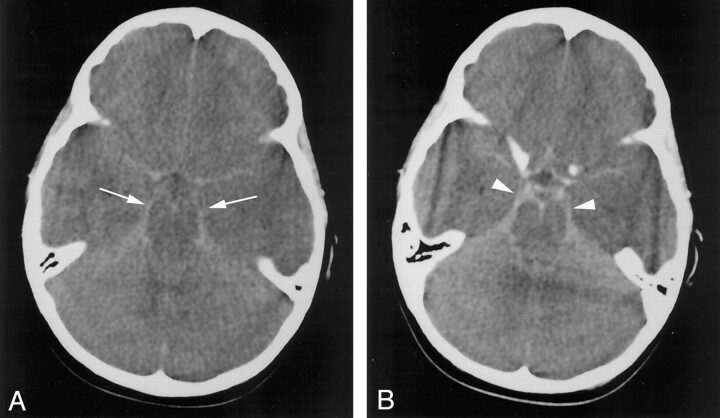
Fig 1.
Case 1. Axial nonenhanced CT images through the basal cisterns.
A, Image obtained before contrast enhancement reveals abnormally increased attenuation within the basal cisterns (arrows).
B, The basal cisterns (arrowheads) are enhancing after the intravenous administration of contrast material.
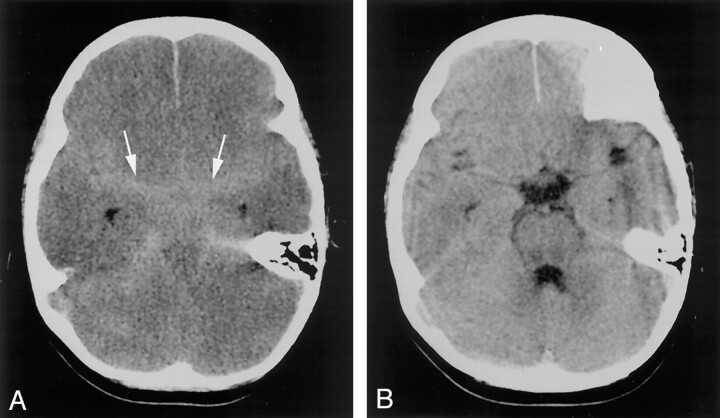
Fig 2.
Case 6. Axial nonenhanced CT images through the basal cisterns.
A, Image reveals effacement of and increased attenuation within the basal cisterns (arrowheads).
B, The findings were not present on the prior image obtained approximately 3 days earlier.
CT scans in each of the seven patients displayed increased attenuation of the subarachnoid spaces in the basal cisterns (mimicking SAH), as well as signs of diffuse cerebral edema (Figs 1 and 2). Signs of cerebral edema included the following: effacement of the basal cisterns and cortical sulci (seven patients), compression or mass effect or both on the fourth ventricle (six patients), and poor gray matter—white matter differentiation (four patients). One patient (case 1) had an additional finding of abnormal enhancement within the basal cisterns after the intravenous administration of contrast material (Fig 1B). None of the patients had evidence of hemorrhage or increased attenuation within the ventricular system or the superficial sulci. When obtainable, the attenuation values within the basal cisterns ranged from 21 to 40 HU, with mean values of 29–33 HU (Table). In the two cases with prior CT examinations (cases 6 and 7), the mean attenuation value of the deep gray matter (thalami) decreased by 12.5% and 6.5%, respectively, with the development of the pseudo-SAH appearance. The CT findings were prospectively interpreted as demonstrating pseudo-SAH in five of the cases, with aneurysmal-type SAH suggested in the remaining two cases. Six of the patients subsequently died, and one patient (case 3) fully recovered after surgical intervention.
Discussion
The pseudo-SAH appearance within the basal cisterns, in conjunction with diffuse cerebral edema, has received only limited attention in the medical literature. Avrahami et al (7) noted pseudo-SAH in a case series of comatose patients (without trauma), and pseudo-SAH was the subject of a case report by Al-Yamany et al (6). Others (3, 4) have noted increased attenuation of the falx and tentorium in association with cerebral edema, but they made no mention of increased attenuation within the basal cisterns.
To explain the origin of pseudo-SAH in association with cerebral edema, we considered the proposed causes of pseudo-SAH in other diseases. In cases of meningitis, toxins elaborated by the offending organism lead to breakdown of the blood-brain barrier (BBB) (2). The resultant BBB disruption allows proteinaceous material to leak into the subarachnoid space (2, 8). However, CSF protein concentrations are sufficiently elevated to cause appreciable changes in CSF attenuation only in the most severe cases of meningitis (2, 9). Breakdown of the BBB and vasogenic edema usually accompany diffuse cerebral edema (10–12). During the acute phases of cerebral injury, vasogenic edema is predominately cleared by means of CSF resorption (10, 13, 14). While the CSF protein content may be elevated in cases of cerebral edema (and other pathologic entities), this elevation has a negligible effect on the CSF attenuation value at CT examination (9). Any increase in the CSF protein content would not contribute significantly to the pseudo-SAH appearance observed in cases of cerebral edema. Some have postulated that other factors, such as edema within the adjacent cortex, elevated intracranial pressure with venous distention, or purulent material within the subarachnoid spaces contribute to the pseudo-SAH appearance (6, 7).
The increase in intracranial pressure and the brain swelling that accompany cerebral edema narrow the subarachnoid spaces and displace CSF. The increased intracranial pressure causes engorgement and dilatation of the superficial (pial) venous structures (15–18). The resultant subarachnoid spaces become relatively devoid of the hypoattenuated CSF and fill with a larger fraction of meninges and blood vessels than in the normal state, potentially increasing their CT attenuation. The pooling of contrast agent within these distended venous structures may have accounted for the abnormal enhancement observed within the basal cisterns in one of our cases (Fig 1B). Avrahami et al (7) observed a similar pattern of contrast enhancement within the basal cisterns in patients with cerebral edema.
With development of cerebral edema, the attenuation of the brain parenchyma decreases concurrently (12, 19). The attenuation value of normal gray matter varies with patient age. Normal values of around 33 HU are observed in adults (20, 21). Prior CT scans were available in two of our cases. These permitted us to eliminate age-associated variation and to calculate the decrease in attenuation within the parenchyma in these cases of pseudo-SAH. In cases 6 and 7, attenuation within the thalami decreased by 12.5% and 6.5%, respectively, with development of edema and the pseudo-SAH appearance. This decrease in attenuation is indicative of cerebral edema and likely contributes to the pseudo-SAH appearance by increasing the conspicuity of the distended vasculature within the basal cisterns.
We were able to measure attenuation values within the basal cisterns in three of our patients (cases 5–7), with mean values ranging from 29 to 33 HU (Table). These values were well below the expected attenuation of clot within the basal cisterns in patients with SAH, in whom attenuation values typically range from 60 to70 HU (22–24). These lower attenuation values, in association with signs of diffuse cerebral edema, should permit the prospective recognition of pseudo-SAH and obviate more invasive testing.
Conclusion
A false appearance of SAH, or pseudo-SAH, may be seen on CT scans in cases of marked cerebral edema. A plausible explanation for this appearance includes a combination of displacement of hypoattenuated CSF, distention of the superficial vasculature, and edema within the adjacent cortex. Although an increase in the CSF protein content may be seen in cases of cerebral edema, this is unlikely to contribute to the pseudo-SAH appearance. Attenuation values within the basal cisterns in cases of pseudo-SAH are much lower than those observed in cases of true acute SAH. Radiologists should be aware of this potential mimic of SAH when evaluating patients with diffuse cerebral edema.
Footnotes
Presented at the 39th Annual Meeting of the American Society of Neuroradiology, Boston, MA, April 23–27, 2001.
References
- 1.Eckel T, Breiter SN, Lee HM. Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol 1998;170:503–505 [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn D, Moss ML, Chason DP, Muphree S, Casey S. Acute purulent leptomeningitis mimicking subarachnoid hemorrhage on CT. J Comput Assist Tomogr 1994;18:126–128 [DOI] [PubMed] [Google Scholar]
- 3.Osborn AG, Anderson RE, Wing DS. The false falx sign. Radiology 1980;134:421–425 [DOI] [PubMed] [Google Scholar]
- 4.Spiegel M, Fox AJ, Vinuela F, Pelz DM. Increased density of tentorium and falx: a false positive CT sign of subarachnoid hemorrhage. Can Assoc Radiol J 1986;37:243–247 [PubMed] [Google Scholar]
- 5.Osborn AG. Diagnostic Neuroradiology. St Louis, Mo: Mosby-Year Book, Inc.;1994. :212
- 6.Al-Yamany M, Deck J, Bernstein M. Pseudo-subarachnoid hemorrhage: a rare neuroimaging pitfall. Can J Neurol Sci 1999;26:57–59 [PubMed] [Google Scholar]
- 7.Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid hemorrhage in patients with brain edema. Eur J Radiol 1998;28:222–225 [DOI] [PubMed] [Google Scholar]
- 8.Prockop LD, Fishman RA. Experimental pneumococcal meningitis. Arch Neurol 19:449–463 [DOI] [PubMed] [Google Scholar]
- 9.Norman D, Price D, Boyd D, Fishman R, Newton TH. Quantitative aspects of computed tomography of the blood and cerebrospinal fluid. Radiology 1997;123:335–338 [DOI] [PubMed] [Google Scholar]
- 10.Klatzo I. Disturbances of the blood-brain barrier in cerebrovascular disorders. Acta Neuropathol (Berl) 1983. :suppl 8:81–88 [DOI] [PubMed] [Google Scholar]
- 11.Weisberg L, Greenberg J, Stazio A. Computed tomographic findings in brain swelling. Comput Med Imaging Graph 1990;14:263–268 [DOI] [PubMed] [Google Scholar]
- 12.Rieth KG, Fujiwara K, Di Chiro G, et al. Serial measurements of CT attenuation and specific gravity in experimental cerebral edema. Radiology 1980;135:343–348 [DOI] [PubMed] [Google Scholar]
- 13.Marmarou A, Hochwald G, Nakamura T, Tanaka K, Weaver J, Dunbar J. Brain edema resolution by CSF pathways and brain vasculature in cats. Am J Physiol 1994;267(2 pt 2):H514–H520 [DOI] [PubMed] [Google Scholar]
- 14.Mei Liu H, Sturner WQ. Extravasation of plasma proteins in brain trauma. Forensic Sci Int 1988;38:285–295 [DOI] [PubMed] [Google Scholar]
- 15.Wolff HG, Forbes HS. The cerebral circulation: observations of the pial circulation during changes in intracranial pressure. Arch Neurol Psychiatr 1928;20:1035–1047 [Google Scholar]
- 16.Yada K, Nakagawa Y, Tsuru M. Circulatory disturbance of the venous system during experimental intracranial hypertension. J Neurosurg 1973;39:723–729 [DOI] [PubMed] [Google Scholar]
- 17.Schmidek HH, Ludwig MA, Kapp JP. The cerebral venous system. Neurosurgery 1985;17:663–678 [DOI] [PubMed] [Google Scholar]
- 18.Osterholm JL. Reaction of the cerebral venous sinus system to acute intracranial hypertension. J Neurosurg 1970;32:654–659 [DOI] [PubMed] [Google Scholar]
- 19.Clasen RA, Huckman MS, Von Roenn KA, Pandolfi S, Laing I, Lobick J A correlative study of computed tomography and histology in human and experimental vasogenic cerebral edema. J Comput Assist Tomogr 1981;5:313–327 [DOI] [PubMed] [Google Scholar]
- 20.Cala LA, Thickbroom GW, Black JL, Collins DW, Mastaglia FL. Brain density and cerebrospinal fluid space size: CT of normal volunteers. AJNR Am J Neuroradiol 1981;2:41–47 [PMC free article] [PubMed] [Google Scholar]
- 21.Boris P, Bundgaard F, Olsen A. The CT (Hounsfield unit) number of brain tissue in healthy infants: a new reliable method for detection of possible degenerative disease. Childs Nerv Syst 1987;3:175–177 [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa M, Masatada T, Toyama Y, et al. CT angiography with helical CT in assessment of acute stage of subarachnoid hemorrhage. Radiat Med 1998;16:91–97 [PubMed] [Google Scholar]
- 23.Mizoi K, Yoshimoto T, Fujiwara S, Sugawara T, Takahashi A, Koshu K. Prevention of vasospasm by clot removal and intrathecal bolus injection of tissue-type plasminogen activator: preliminary report. Neurosurgery 1991;28:807–812 [DOI] [PubMed] [Google Scholar]
- 24.Fujita S. Computed tomographic grading with Hounsfield number related to delayed vasospasm in cases of ruptured cerebral aneurysm. Neurosurgery 1985;17:609–612 [DOI] [PubMed] [Google Scholar]