Abstract
Summary: Intracranial chondrosarcomas have a predilection for the skull base, for which CT and MR imaging findings have been described. We present a rare case of primary chondrosarcoma arising from the falx in a young woman with no history of radiation. The CT, conventional MR imaging, perfusion MR imaging, and digital subtraction angiography findings are described.
Intracranial chondrosarcomas are rare tumors of the CNS (1–5). Seventy-five percent of intracranial chondrosarcomas originate at the skull base. Rarely, however, these tumors can arise from the meninges along the falx, tentorium, and convexity (1, 4, 5). Three histologic variants have been described based on their cytoarchitecture: mesenchymal, myxoid, and classical chondrosarcomas. Imaging characteristics of various modalities, including CT, conventional MR imaging, perfusion MR imaging, and digital subtraction angiography, have been described in detail, along with a brief review of the literature.
Case Report
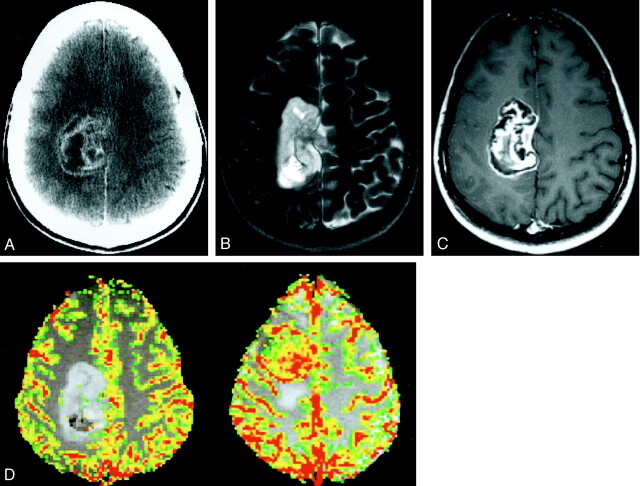
A 28-year-old woman was referred to our hospital with a 6-month history of headaches and left arm tingling and numbness that had gradually progressed to involve the left half of her face. An unenhanced CT scan obtained at an outside hospital at the time of presentation revealed a “large” right parietal tumor. The patient’s medical history was noncontributory, and she had no known medical problems. She had declined surgery at that time to get a second opinion; however, during the interim, she developed acute left foot drop, which prompted her to come to the emergency department at our institution. A neurologic examination revealed an alert, well-oriented patient with intact cranial nerves and normal reflexes. A decrease in proprioception in her left first toe was noted, but motor strength was normal in all extremities. All laboratory indices were within the normal range. A repeat contrast-enhanced CT scan was obtained and revealed a 6 × 5 × 4 cm lobulated heterogeneously enhancing mass predominantly in the right parafalcine parietal region, extending into the underlying brain parenchyma and corpus callosum (Fig 1A). The mass was hyperintense on T2-weighted MR images (Fig 1B). On contrast-enhanced T1-weighted images, the mass showed heterogeneous enhancement (Fig 1C). In addition, several linear areas of extremely low signal intensity were present, which, retrospectively, represented cartilaginous tissue. A minimal amount of surrounding vasogenic edema could be seen and, interestingly, showed no evidence of increased perfusion on perfusion MR images (Fig 1D, left). Digital subtraction angiography showed mass effect on the adjacent vascular structures without any evidence of a tumor blush or neovascularity.
Fig 1.
Images from the case of a 28-year-old woman with a 6-month history of headaches and left arm tingling and numbness that had gradually progressed to involve the left half of her face.
A, Axial contrast-enhanced CT scan shows a 6-cm, lobulated, heterogeneously enhancing mass in the right parafalcine parietal region, extending into the underlying brain parenchyma.
B, Axial T2-weighted image (3400/119/1 [TR/TE/number of excitations]) shows the mass to have high signal intensity relative to cortex, with scattered foci of low signal intensity. The surrounding brain parenchyma appears normal, with no evidence of vasogenic edema.
C, Axial contrast-enhanced T1-weighted image (600/14/1) shows enhancement with linear areas of extremely low signal intensity, consistent with islands of cartilaginous tissue.
D, Axial color overlay perfusion MR imaging map (1000/54). The image is a perfusion color overlay of a chondrosarcoma (left) that shows hypoperfusion in the region of the mass relative to normal white matter. The image on the right is a perfusion color overlay of a meningioma (right), which in comparison shows increased perfusion.
Preoperative diagnosis was that of a parafalcine meningioma. A stereotactic head frame was applied, and the patient underwent computer-assisted stereotactic volumetric excision the next day. At the time of surgery, the tumor was extra-axial in origin and was attached to the falx by a small stalk. The patient experienced an uneventful postoperative course and was discharged while receiving Dilantin and a tapered steroid regimen.
Histopathology
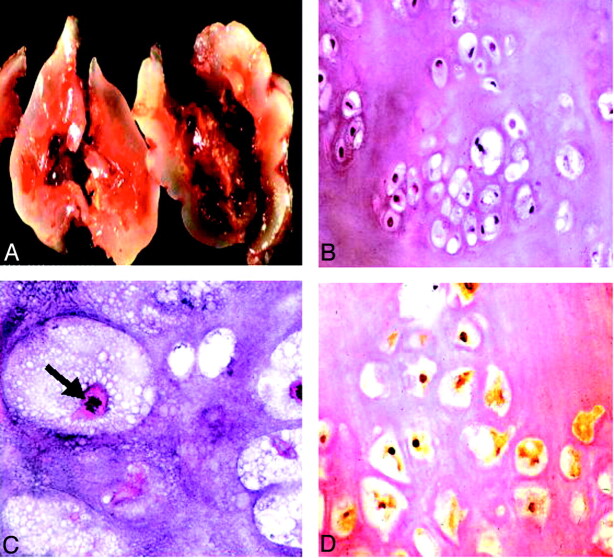
Macroscopically, the tumor had a bluish white glistening external surface made up of homogenous tan cartilaginous tissue (Fig 2A). Microscopic examination revealed a lobulated appearance with variable low to moderate cellularity, composed of chondrocytes embedded in a hyaline matrix. Nuclear pleomorphism and mitotic figures were detected, and necrosis was present (Fig 2B and C). Few focal areas of myxoid and osteoid tissue could be seen. The mass, however, had an overall appearance of classical chondrosarcoma. Immunostaining revealed strong nuclear reactivity for S-100 protein, indicative of a cartilaginous tumor (Fig 2D).
Fig 2.
Histopathologic findings.
A, Gross specimen shows a bluish white, pearly, glistening lobulated tumor.
B, Low power slide shows a hyaline tumor of low to moderate cellularity with mild variation in size and shape of the tumor cells with mostly small and dark nuclei, showing mild nuclear pleomorphism (hematoxylin and eosin; original magnification, ×40).
C, High power slide reveals mitotic figures (arrow). Findings consistent with the appearance of a conventional chondrosarcoma (hematoxylin and eosin; original magnification, ×200).
D, Nuclear and, to a lesser degree, cytoplasmic immunoreactivity for S-100 protein are present (immunoperoxidase; original magnification, ×100), indicative of a cartilaginous tumor.
Discussion
Chondroid tumors are predominantly lesions of the axial skeleton but can infrequently have an extraskeletal origin. Primary intracranial chondrosarcomas constitute <0.16% of all brain tumors, an overwhelming majority of which are skull base tumors (1, 2). They are thought to arise from undifferentiated cells from cartilaginous synchondroses (1–3, 6, 7). Much less common sites include the falx, choroid plexus, and convexity. The histogenesis at this site is uncertain but is thought to arise from the pluripotent cells of the meninges.
Primary chondrosarcomas can be classified as mesenchymal, myxoid, or classical based on their cytoarchitecture. In the series published by Korten et al (2), which included 15 case reports plus 177 cases from a review of the literature, no sex dominance was shown. Ages varied from 3 months to 76 years, with a mean age of 37 years. Considering the rarity of occurrence of intracranial chondrosarcomas, it was difficult to establish a clear demographic pattern; however, it did seem that the mesenchymal variant had a predilection for a younger age group (ie, 20–30 years) (2, 3) and that the classic variant had a predominance in the 6th to 7th decades (2, 8, 9).
Mesenchymal chondrosarcomas microscopically show a distinctive appearance and histologically are malignant tumors with a densely cellular stroma of anaplastic mesenchymal cells, punctuated by nodules of neoplastic chondrocytes and hyaline cartilage. The presence of excessive collagen and cytoplasmic glycogen deposits are the mainstay of ultrastructural findings of these tumors (1, 3, 5). The mesenchymal chondrosarcomas are the most malignant of the three (mesenchymal, myxoid, and classical), illustrated by a strong tendency for intradural and cerebral growth, and occasionally distant metastases (2, 3, 6, 10). Myxoid chondrosarcomas, although common in soft tissues, are extremely rare within the cranium. Histologically, these represent an intermediate form, with ribbons of chondrocytes in a myxoid matrix (1, 3, 5, 11). These tumors have been described in the literature in association with entities such as Ollier disease and Mafucci syndrome (1, 5, 12). Classical chondrosarcomas, in contrast to the mesenchymal variant, are well differentiated with little if any mesenchymal tissue. The chondrosarcoma described in this report is of the classical type. They are composed of atypical chondrocytes with rare anaplastic figures in a well-developed cartilaginous matrix. Ultrastructural analysis reveals abundant mature cartilage production with significant cellular vacuolization by glycogen deposits (3, 11, 13). Immunohistochemistry patterns include strong reactivity to S-100 protein and vimentin but negative response to epithelial markers like epithelial membrane antigen and cytokeratin that help to differentiate chondrosarcomas from meningiomas and chordomas (1, 5, 14).
On images, extra-axial classical chondrosarcomas display the characteristics of a slow growing extra-axial mass, including good demarcation, cortical and white matter buckling, and possibly a CSF cleft (10, 15, 16). Even with parenchymal invasion, a small attachment to the falx is usually present. On CT scans, they are usually isoattenuated to hyperattenuated, with variable degrees of heterogeneous enhancement (1, 4, 15, 17). These tumors show varying amounts of histologic and radiologic calcifications (1, 3, 7, 10, 14); however, when compared with their skull base equivalent, this is found to a lesser extent in the classical variant (16). Calcifications, when present, may have a stippled or a “rings and arcs” appearance, characteristic of cartilaginous tumors (10, 15). Classical chondrosarcomas are slow growing and may show signs of pressure erosion on the adjacent bone, unlike the hyperostosis seen with meningiomas or the bone destruction seen with more aggressive tumors (16). MR imaging offers better demarcation of the lesion and tumor characterization (18). These tumors are frequently hypointense on T1-weighted images and extremely hyperintense on T2-weighted images (1). Even in the absence of gross calcification, the lesion may show areas of intense low signal intensity on the T1-weighted images that would reflect the presence of cartilage islands (1, 9, 10, 18). Contrast enhancement may be mild or moderate and has been described as a “honeycomb” pattern (1, 16, 18). Of note, surrounding vasogenic edema is usually mild in chondrosarcoma and the presence of a dural tail is extremely rare (1). Angiography shows varying degrees of tumor vascularity depending on the type of histologic variant, with the classical chondrosarcoma and 50% of mesenchymal chondrosarcomas showing avascularity (1, 3, 4, 9, 15, 17). Some of the mesenchymal and myxoid variants, however, can exhibit intense hypervascularity similar to vascular malformations and hemangiopericytomas (3, 9, 15).
Recent advances in neuroimaging have allowed the acquisition of physiological information that complements the structural detail provided by traditional techniques. One such method is dynamic contrast-enhanced perfusion MR imaging, which provides qualitative and relative quantitative estimates of cerebral blood volume and reflects the underlying microvasculature and angiogenesis. Preliminary data on perfusion imaging of extra-axial lesions such as noncalcified meningiomas, which are highly vascular, seems to indicate higher relative cerebral blood volume and perfusion (19). In contrast, a poorly vascularized chondrosarcoma, as illustrated in our case, shows hypoperfusion and is essentially “cold” (Fig 1D), a feature that may help differentiate it from meningiomas, glioblastomas multiforme, and metastases. Islands of cartilaginous tissue, which are extremely hypointense on conventional images, show low relative cerebral blood volume, which we think may be due to their relative avascularity. Perfusion patterns in the other two variants (ie, mesenchymal and myxoid), have not been studied and cannot be commented on. Preliminary experience with perfusion MR imaging findings in calcified meningiomas at our institution reveals these lesions to have reduced relative cerebral blood volume or perfusion. However, the findings of a calcified meningioma on conventional CT scans or MR images make this diagnosis less problematic.
The imaging findings of a classic chondrosarcoma may superfluously resemble that of other neoplasms, and the differential diagnosis includes meningioma, hemangiopericytoma, metastases, and vascular malformations at that site (14, 18). However, we think that certain features can be used prospectively to differentiate a classic chondrosarcoma from a meningioma or a hemangiopericytoma. These include higher signal intensity on the T2-weighted images than that of a meningioma, islands of low signal intensity despite lack of gross calcification on all sequences due to well-differentiated cartilage, frequent preservation of the pial barrier, “honeycomb” enhancement, and, most importantly, lack of perfusion. In addition to these features, the relative lack of edema can also be used to distinguish it from tumors such as glioblastomas multiforme and metastases. Lack of vascularity and absence of flow voids differentiate it from a vascular malformation or the mesenchymal variety (17).
Radical excision is thought to be the treatment of choice by most neurosurgeons, and the extent of resection seems to be a significant prognostic factor (1–3, 8, 9, 18). Depending on their histology, these tumors, especially the high grade mesenchymal variants, may have a tendency for local recurrence and occasionally may even metastasize to distant organs (6, 17, 18). Some debate exists regarding optimal management and various treatment adjuncts, including radiation therapy, stereotactic surgery, and brachytherapy (1, 2, 20); however, a standard protocol has not been established (5).
Conclusion
Intracranial chondrosarcomas arising at the convexity or the falx are rare. However, we think an attempt to diagnose the classical variant can be made preoperatively. Presence of subtle clues, such as cartilage islands that exhibit very low signal intensity on T1- and T2-weighted images, relative lack of edema, and lack of perfusion on perfusion MR images, can help distinguish it from other similar appearing tumors, such as metastases and meningiomas. At our institution, some meningiomas that are found to be very vascular (on digital subtraction angiograms) undergo preoperative embolization to reduce tumor vascularity and bleeding at the time of surgery. In this patient, although the findings on digital subtraction angiograms showed an avascular lesion, the conventional MR imaging and CT findings were still suggestive of a meningioma. Therefore, the perfusion MR imaging helped to provide confirmatory evidence to the neurosurgeon that this was in fact an avascular mass. Because of its midline parafalcine location, resection of a lesion such as this could lead to high morbidity and potential mortality in the setting of significant bleeding.
References
- 1.Oruckaptan HH, Berker M, Soylemezoglu F, et al. Parafalcine chondrosarcoma: an unusual localization for a classical variant: case report and review of the literature. Surg Neurol 2001;55:174–179 [DOI] [PubMed] [Google Scholar]
- 2.Korten AG, ter Berg HJ, Spincemaille GH, et al. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry 1998;65:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassounah M, Al-Mefty O, Akhtar M, et al. Primary cranial and intracranial chondrosarcoma: a survey. Acta Neurochir (Wien) 1985;78:123–132 [DOI] [PubMed] [Google Scholar]
- 4.Cianfriglia F, Pompili A, Occhipinti E. Intracranial malignant cartilaginous tumours: report of two cases and review of literature. Acta Neurochir (Wien) 1978;45:163–175 [DOI] [PubMed] [Google Scholar]
- 5.Gerszten PC, Pollack IF, Hamilton RL. Primary parafalcine chondrosarcoma in a child. Acta Neuropathol (Berl) 1998;95:111–114 [DOI] [PubMed] [Google Scholar]
- 6.Scheithauer BW, Rubinstein LJ. Meningeal mesenchymal chondrosarcoma: report of 8 cases with review of the literature. Cancer 1978;42:2744–2752 [DOI] [PubMed] [Google Scholar]
- 7.Rapidis AD, Archondakis G, Anteriotis D, et al. Chondrosarcomas of the skull base: review of the literature and report of two cases. J Craniomaxillofac Surg 1997;25:322–327 [DOI] [PubMed] [Google Scholar]
- 8.Gay E, Sekhar LN, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 1995;36:887–897 [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Sawada K, Kitamura K. Chondrosarcoma arising from the falx cerebri. Surg Neurol 1986;25:505–509 [DOI] [PubMed] [Google Scholar]
- 10.Cybulski GR, Russell EJ, D’Angelo CM, et al. Falcine chondrosarcoma: case report and literature review. Neurosurgery 1985;16:412–415 [DOI] [PubMed] [Google Scholar]
- 11.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 1977;40:818–831 [DOI] [PubMed] [Google Scholar]
- 12.Bushe KA, Naumann M, Warmuth-Metz M, et al. Maffucci’s syndrome with bilateral cartilaginous tumors of the cerebellopontine angle. Neurosurgery 1990;27:625–628 [DOI] [PubMed] [Google Scholar]
- 13.Fu YS, Kay S. A comparative ultrastructural study of mesenchymal chondrosarcoma and myxoid chondrosarcoma. Cancer 1974;33:1531–1542 [DOI] [PubMed] [Google Scholar]
- 14.Bourgouin PM, Tampieri D, Robitaille Y, et al. Low-grade myxoid chondrosarcoma of the base of the skull: CT, MR, and histopathology. J Comput Assist Tomogr 1992;16:268–273 [DOI] [PubMed] [Google Scholar]
- 15.Bahr AL, Gayler BW. Cranial chondrosarcomas: report of four cases and review of the literature. Radiology 1977;124:151–156 [DOI] [PubMed] [Google Scholar]
- 16.Lee YY, Van Tassel P, Raymond AK. Intracranial dural chondrosarcoma. AJNR Am J Neuroradiol 1988;9:1189–1193 [PMC free article] [PubMed] [Google Scholar]
- 17.Cho BK, Chi JG, Wang KC, et al. Intracranial mesenchymal chondrosarcoma: a case report and literature review. Childs Nerv Syst 1993;9:295–299 [DOI] [PubMed] [Google Scholar]
- 18.Bingaman KD, Alleyne CH, Jr., Olson JJ. Intracranial extraskeletal mesenchymal chondrosarcoma: case report. Neurosurgery 2000;46:207–212 [PubMed] [Google Scholar]
- 19.Cha S, Knopp EA, Johnson G, et al. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 2002;223:11–29 [DOI] [PubMed] [Google Scholar]
- 20.Kondziolka D, Lunsford LD, Flickinger JC. The role of radiosurgery in the management of chordoma and chondrosarcoma of the cranial base. Neurosurgery 1991;29:38–46 [DOI] [PubMed] [Google Scholar]