Abstract
Summary: A left-handed patient with a grade II left frontal lobe astrocytoma had spontaneous seizures causing speech arrest and uncontrolled right upper extremity movements. Word-generation functional MR (fMR) imaging showed activity nearly exclusively in the right inferior frontal gyrus. The clinical history of the speech arrest and the intraoperative mapping proved left-hemisphere language dominance. Tumor involvement of the left inferior frontal gyrus caused uncoupling of the blood oxygenation level‐dependent (BOLD) and neuronal response, leading to the erroneous fMR imaging appearance of right-hemisphere language dominance. Discrepancies between BOLD and intraoperative mapping in areas near lesions illustrate the complementary nature of these techniques.
The preoperative use of functional MR (fMR) imaging to identify eloquent cortex near resectable lesions is quickly becoming a common clinical imaging scenario (1, 2). Yet, it is clear that blood oxygenation level‐dependent (BOLD) contrast can be significantly compromised near regional cerebral disease (3–5). Evidence suggests that cortical BOLD activation is reduced in glial tumors, both at the edge of the tumor and in normal vascular territories somewhat removed from the tumor (4). Loss of regional cerebral vasoactivity near these tumors is thought to be a major contributing factor (3, 4). Such effects may result in the underestimation of genuine neuronal function and influence the diagnostic accuracy of BOLD fMR imaging. The present case is intended to illustrate how lesion-induced neurovascular uncoupling can simulate functional cortical reorganization.
Case Report
Subject
A previously healthy 19-year-old left-handed man experienced sudden speech arrest lasting approximately 1 minute while he was at a fast food establishment. Subsequently, the speech arrest was associated with uncontrolled movements in his right upper extremity that lasted an additional 20 seconds. No loss of consciousness occurred, and the symptoms quickly subsided, leaving the patient with normal speech and motor function. Conventional MR imaging revealed a large nonenhancing mass within the left frontal lobe, centered in the left subcentral and inferior frontal gyri (IFG) (Fig 1). The pars opercularis of the left IFG was displaced superiorly and anteriorly and also stretched by the tumor. The pars triangularis and pars orbitalis were essentially uninvolved by direct tumor extension. Also, the tumor displaced the inferolateral portion of the pre- and postcentral gyri superiorly and posteriorly.
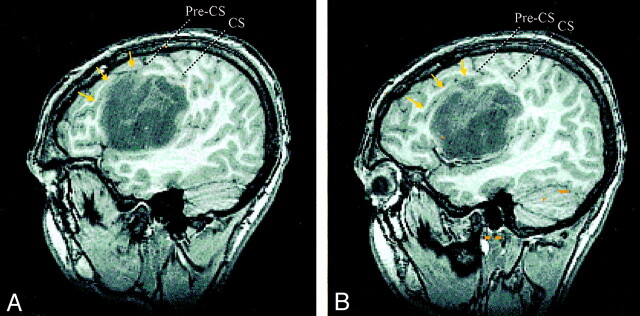
Fig 1.
Sagittal SPGR images of a left frontal astrocytoma centered in the left subcentral gyrus and IFG. The tumor displaces the pars opercularis of the left IFG superiorly and anteriorly, as demarcated by the displaced inferior frontal sulcus (arrows). The lower central sulcus (CS), precentral sulcus (Pre-CS), precentral gyrus, and postcentral gyrus are displaced superiorly and posteriorly.
Imaging Parameters
fMR images of the anterior language and regional motor functions were acquired by using a single-shot echo-planar T2*-weighted sequence with the following parameters: TR/TE, 3000/50; flip angle, 90°; field of view, 24 × 24 cm; section thickness, 5 mm; contiguous sections; and matrix; 64 × 64. The functional images were superimposed onto anatomic spoiled gradient-recalled acquisition in the steady state (SPGR) images and represented in three dimensions. A cross-correlation analysis was performed to construct activation maps by using a correlation coefficient threshold of 0.5; statistical significance was defined as P < .001.
Functional Tasks
Functional motor tasks included finger tapping, elbow flexion and extension, shoulder movement, bilateral tongue movement, and bilateral lip movement. A silent word-generation task and an overt word-generation task were used to generate cortical maps corresponding to anterior language function. Tasks were administered in three periods of 30 seconds each. On-off intervals that generated cortical signal intensity waveforms were correlated with an idealized reference wave form to construct cortical activation maps. Activation maps were represented on 3D anatomic SPGR images, and the location of activated areas in relation to the left frontal tumor was correlated with intraoperative mapping results.
Mapping Eloquent Cortex
With functional imaging, right upper extremity motor cortex was mapped in an expected location of the precentral gyrus superior and posterior to left frontal mass, whereas tongue and lip motor function were observed in the superiorly displaced lower precentral gyrus at the superior-posterior edge of the tumor (Fig 2). Anterior language activation was robust in the right IFG and inferior frontal sulcus and miniscule in the displaced left IFG. This finding implied strong right-hemisphere dominance in this left-handed individual (Fig 3). Talairach coordinates of the right IFG activity corresponded to Brodmann areas 44 and posterior 45, as well as to an adjacent inferior portion of area 9. The left IFG activity was seen at the anterior-superior edge of the tumor located in the displaced and stretched left pars opercularis.
Fig 2.
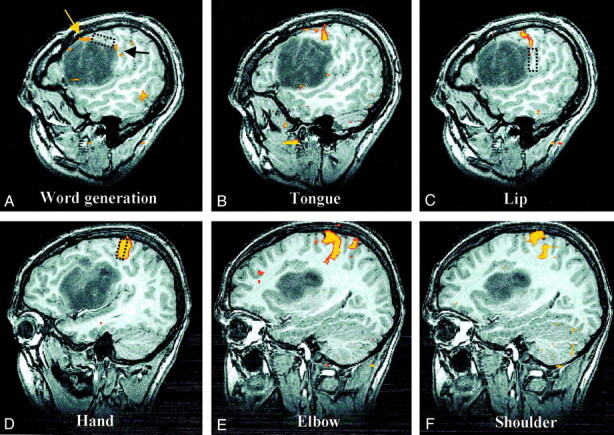
Sagittal spoiled gradient-echo and fMR images of the left hemisphere show cortical activation, compared with intraoperative mapping sites.
A, Language activation. BOLD language activation is displaced superiorly (yellow arrow) but located immediately anterior to language function mapped intraoperatively (rectangle). A small amount of additional activation associated with the word-generation task (black arrow) corresponds more closely to the intraoperative location of lip function (see image in C).
B, Tongue activation. BOLD tongue activation is situated between intraoperative maps of lip and language function, as would be expected, but intraoperative attempts to find tongue motor function were unsuccessful.
C, Lip activation. Corticonuclear (tongue and lip) BOLD activation is in superiorly displaced precentral gyrus at the superior-posterior edge of the tumor located immediately above the area of lip function mapped intraoperatively (rectangle).
D, Hand activation. The corticospinal (hand, elbow, and shoulder) cortical function is in the normal portion of the precentral gyrus superior and posterior to the tumor. With these three areas, only intraoperative mapping of hand function was attempted, and the result corresponded precisely with the location of BOLD activation (rectangle).
E, Elbow activation.
F, Shoulder activation.
Fig 3.
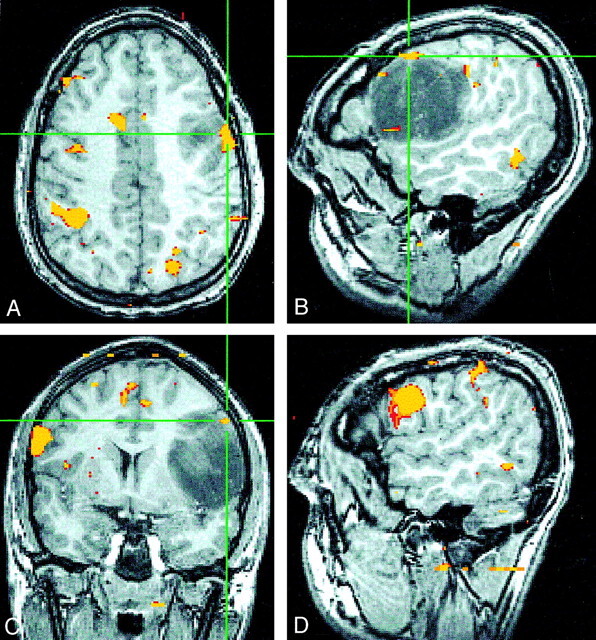
Images falsely suggesting strong right-hemisphere dominance.
A–C, Axial (A), sagittal (B), and coronal (C) SPGR fMR images show minimal left-frontal language activity (crosshairs) during a word-generation task.
C and D, Coronal (C) and sagittal (D) fMR images show robust activity in the right IFG and inferior frontal sulcus that falsely implies right-hemisphere language dominance in this individual.
Intraoperative Mapping of Eloquent Cortex
Intraoperative maps of cortical areas representing the right hand, lip, and tongue motor function were attempted while the patient was awake by using a bipolar electrode with a depolarizing diameter as large as 1 cm. As with the fMR imaging, hand cortical function was mapped at the expected location within the precentral gyrus above the lesion. This precisely corresponded to the observed BOLD activation (Fig 2D). Lip motor function was mapped at the posterior border of the mass, adjacent to but below the observed BOLD activation (Fig 2C). Attempts to map tongue function intraoperatively were unsuccessful despite robust BOLD activation seen with fMR imaging (Fig 2B). Speech arrest was caused by depolarizing cortical tissue at the superior edge of the left frontal neoplasm, which was located immediately posterior to the small region of BOLD activity seen on the fMR image, in the displaced pars opercularis (Fig 2A). The tumor was partially resected with care to avoid the dominant cortical language area intraoperatively mapped to the left hemisphere. The patient did not have a postoperative language deficit. Histologic analysis revealed a grade II astrocytoma with some gemistocytes and mini-gemistocytes.
Discussion
The physiologic basis of BOLD fMR imaging signal intensity is the cerebrovascular dilatation induced by neuronal firing. Cortical neuronal activity stimulates local vascular dilatation and increases regional cerebral blood flow, volume, and oxygen concentration, exceeding the metabolic demands of the activated neurons (6). The relationship between oxygen supply and neuronal demand is mediated by vasoactive mechanisms at the regional level that increase oxyhemoglobin levels and decrease deoxyhemoglobin concentration compared with those in the nonactivated state (7,8). This neurovascular coupling is likely due to chemical mediation (9, 10), requiring 2 seconds or longer to produce detectable changes in deoxyhemoglobin levels.
The cerebral vasculature is highly responsive to changes in blood O2 and CO2, but neurovascular coupling associated with neuronal activation per se is likely mediated by local ionic and metabolic factors (9,10). These mechanisms are not fully elucidated, but K+ locally released into the interstitial space by activated neurons is believed to cause relaxation of small resistance vessels. Adenosine and H+ are metabolic products of increased Na+/K+ ATPase activity that may also directly cause vasodilatation, particularly in conditions in which oxygen and glucose supplies cannot keep up with neuronal demand. Nitric oxide and arachidonic acid derivatives may have modulatory (facilitative) roles in supporting vasodilatation in response to neuronal activity or increased CO2 concentrations. Other ionic and metabolic factors may more globally affect cerebral perfusion and secondarily affect BOLD signal intensity via substances released by vascular nerves and mast cells. Autoregulation of perfusion pressures could also affect BOLD signal intensity. This may involve arterial myogenic and endothelial mechanisms.
Evidence is beginning to accumulate and suggests that the BOLD response near regional cerebral disease less accurately reflects genuine neuronal activity than that in normal brain (3–5), although systematic investigations of this phenomenon are lacking.
Recent data indicate that cortical BOLD activation can be reduced near glial tumors, both at the edge of the tumor and in normal vascular territories somewhat removed from the tumor (4). Loss of regional cerebral vasoactivity near these tumors has been suggested to be a contributing factor (3, 4). At the interface of tumors and normal brain, astrocytes and macrophages can release nitric oxide that can regionally increase CBF and decrease the oxygen extraction fraction (11), which may also decrease BOLD signal intensity differences (4). Tumor-induced changes in regional tissue pH and glucose, lactate, and adenosine triphosphate levels have been documented (12, 13), although such effects on BOLD-neuronal coupling are not clear. Glial tumors can induce abnormal vessel proliferation in adjacent brain (14–16), altering regional CBF, CBV, vasoactivity, and potentially, BOLD contrast. The degree of angiogenesis depends on the grade of the tumor, and it is regionally variable within a given glial neoplasm. Thus, subregions of an active cortical field may be affected. Also, it is well known that the infiltrative nature of glial tumors may leave functional neurons within the tumor bed (17, 18); this may compromise neuronal contacts with the capillary beds and astrocytes (19). Because astrocytes may act to redistribute K+ released by activated neurons to those vessels that control resistance (20), loss of astrocytic connections could contribute to the reduced BOLD response observed in this setting (4). The consequences of these phenomena to BOLD signal intensity are not fully characterized, but clinical experience suggests that one or more of these factors may reduce the BOLD signal intensity response to cortical activation.
Other factors, including vasogenic edema and tumoral hemorrhage, could contribute to the observed decrease in near-lesion BOLD contrast. Despite the theoretical consequences of vasogenic edema induced dilutional and tissue pressure changes on neurovascular coupling, evidence for a substantial impact on BOLD contrast is lacking in a small number of patients studied (3). The true impact of vasogenic edema awaits further investigation in larger patient populations with a range of tumor types. Microhemorrhages associated with intraparenchymal tumors could hinder the detection of changing susceptibility gradients that provide BOLD contrast, but confirmation of this effect requires verification with histologic correlation.
The potential for tumor-induced loss of BOLD contrast is particularly worrisome in mapping functional brain systems associated with asymmetric but bilateral hemispheric activation, such as the language and motor systems. The problem may arise from the assumption that the relationship between BOLD contrast and neuronal activity observed in normal brains can be extrapolated to diseased brains. Given the inherent variability in the BOLD activation area among individuals and among various techniques, it is appealing to scale activity in cortex involved by a lesion to uninvolved cortex as an indicator of cortical reorganization. A greater than expected proportion of the nonlesional BOLD activation compared with the perilesional BOLD activation (21, 22) may represent cortical reorganization. However, the present case illustrates that reorganization can be simulated by lesion-induced neurovascular uncoupling that causes reduced BOLD contrast despite the presence of genuinely functioning cortical neurons.
The pseudo-reorganization of language cortex in this case may have been potentiated by a genuine functional adaptive response to the presence of compromised left language cortex. Even in healthy individuals, increasing the relative difficulty of a unilateral motor task is associated with increased ipsilateral cortical activity (23). Additionally, functional motor cortical reorganization can occur without morphologic reorganization, through inhibitory-excitatory neuronal exchange (24). Tumor-induced compromise of cortical functions could cause the recruitment of homologous brain regions in the uninvolved hemisphere or in adjacent uninvolved brain, which is needed to carry out a particular task in the absence of genuine functional reorganization. The relatively robust activity observed in the right IFG and inferior frontal sulcus in this case caused the appearance of right-hemisphere language dominance. This finding may have been the result of some actual shared-hemisphere language function in this left-handed patient, recruitment of homologous brain regions, incomplete cortical reorganization, or a combination of factors. Despite the fMR imaging appearance, the patient presented here was shown to have had left-hemisphere language dominance.
Another interesting aspect of the case presented here is the discrepancy in locations determined by BOLD mapping versus those determined by intraoperative mapping near the tumor. Although the two techniques showed excellent agreement for hand motor function somewhat removed from the tumor, both language and lip motor function showed adjacency but little overlap between the techniques. Previous work demonstrated good agreement between fMR imaging and intraoperative mapping, but displacement of as much as 2 cm has been observed in a minority of individuals (25). This effect could be due to inaccuracies with the intraoperative mapping method or with the BOLD fMR imaging technique, particularly in brain adjacent to a tumor. The reproducible intraoperative results observed throughout the surgical procedure in this patient and the precise agreement in location between techniques for hand motor function suggests that fMR imaging was, at least in part, the culprit in this case.
The lip movement paradigm used in this case could have induced considerable tongue movement, causing BOLD activity in the cortical tongue area. This, in combination with tumor-induced neurovascular uncoupling in the lip cortical area, could account for the discrepancy in localized lip function between the BOLD method and the intraoperative mapping techniques. Also, tumor-induced neurovascular uncoupling could possibly have reduced BOLD contrast in language cortex, leaving only a small amount of BOLD signal intensity at the anterior edge of the activated cortical field. This effect may account for the more posterior location of language function, as identified intraoperatively, compared with the fMR imaging results. That is to say, cortical subfield neurovascular uncoupling may erroneously infer concurrence between the epicenters of BOLD and neuronal activity. In the case presented here, tongue activity was robust and reproducible at fMR imaging, and it was situated in the expected location between language and lip function proved with intraoperative mapping. However, it could not be found with the intraoperative method. This observation may have been due to limitations of the intraoperative technique in identifying eloquent cortex deep in a sulcus and potentiated by tumor-induced anatomic distortions or other logistical factors.
Conclusion
The consequences of misinterpreting tumor-induced BOLD effects as cortical reorganization are potentially serious. The fMR imaging appearance of lesion-induced cortical reorganization from one hemisphere to the other or from one region of brain to an adjacent site could indicate genuine reorganization, decreased BOLD-neuronal coupling in involved regions of brain compared with uninvolved regions, or a combination of factors. The preoperative appearance of glial tumor‐induced cortical reorganization generally warrants intraoperative confirmation before resection, as in this case. Until more research is performed, caution should be exercised in interpreting the fMR imaging appearance of cortical reorganization caused by other brain diseases. This case also suggests that the agreement between BOLD fMR imaging and intraoperative mapping may decrease in cortex closer to the lesion of interest compared with cortex removed from the lesion. This result is likely due to limitations of both techniques, and it requires elucidation with further studies. At this point, it is clear that fMR imaging and intraoperative mapping are complementary methods.
Acknowledgments
The authors would like to thank Marlyn Colegrove for her valuable assistance in preparing the manuscript.
Footnotes
Supported by a grant from the Dana Foundation Clinical Hypotheses Program in Imaging.
References
- 1.Yetkin FZ, Ulmer JL, Mueller WM, Cox RW, Haughton VM. Functional magnetic resonance imaging assessment of the risk of post-operative hemiparesis after excision of cerebral tumors. Int J Neuroradiol 1998;4:253–257 [Google Scholar]
- 2.Laurito JT, Bryan RN, Mathews VP, Ulmer JL, Lowe MJ. Functional brain mapping. In: RSNA Categorical Course in Diagnostic Radiology: Neuroradiology. Oak Brook, IL: RSNA; 2000:79–104
- 3.Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000;21:1415–1422 [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol 2000;21:1055–1063 [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YJ, Chung TS, Soo Yoon Y., et al. The role of functional MR imaging in patients with ischemia in the visual cortex. AJNR Am J Neuroradiol 2001;22:1043–1049 [PMC free article] [PubMed] [Google Scholar]
- 6.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev 1995;7:240–276 [PubMed] [Google Scholar]
- 7.Belliveau JW, Kennedy DN Jr, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 1991;254:716–719 [DOI] [PubMed] [Google Scholar]
- 8.Kwong KK, Chesler DA, Weisskoff RM., et al. Dynamic magnetic resonance imaging of the human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 1992;89:5675–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 1996;272:551–554 [DOI] [PubMed] [Google Scholar]
- 10.Kuschinsky W. Regulation of cerebral blood flow. In: Moonen CTW, Bandettini PA, eds. Functional MRI. New York: Springer-Verlag; 1999:15–24
- 11.Whittle IR, Collins F, Kelly PA, Ritchie I, Ironside JW. Nitric oxide synthase is expressed in experimental malignant glioma and influences tumor blood flow. Acta Neurochir(Wien) 1996;138:870–875 [DOI] [PubMed] [Google Scholar]
- 12.Hossmann KA, Linn F, Okada. Bioluminescence and fluoroscopic imaging of tissue pH and metabolites in experimental brain tumors of cats. NMR Biomed 1992;5:259–264 [DOI] [PubMed] [Google Scholar]
- 13.Linn F, Seo K, Hossmann KA. Experimental transplantation gliomas in adult cats, III: regional biochemistry. Acta Neurochir (Wein) 1989;99:85–93 [DOI] [PubMed] [Google Scholar]
- 14.Liwincz BH, Wu SZ, Tew JM Jr. The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg 1987;66:536–541 [DOI] [PubMed] [Google Scholar]
- 15.Tyler JL, Diksic M, Vilemure JG, et al. Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med 1987;28:1123–1133 [PubMed] [Google Scholar]
- 16.Ji Y, Powers SK, Brown JT, Miner R. Characterization of the tumor invasion area in the rat intracerebral glioma. J Neurooncol 1996;30:189–197 [DOI] [PubMed] [Google Scholar]
- 17.Ojemann J, Miller J, Silbergeld D. Preserved function in brain invaded by tumor. Neurosurgery 1996;39:253–259 [DOI] [PubMed] [Google Scholar]
- 18.Skirboll SS, Ojemann GA, Berger MS, Lettich E, Winn HR. Functional cortex and subcortical white matter located within gliomas. Neurosurgery 1996;38:678–685 [PubMed] [Google Scholar]
- 19.Nagano N, Sasaki H, Aoyagi M, Hirakawa K. Invasion of experimental rat brain tumor: early morphological changes following microinjection of C6 glioma cells. Acta Neuropathol (Berl) 1993;86:117–125 [DOI] [PubMed] [Google Scholar]
- 20.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science 1987;237:896–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshiura T, Hasuo K, Mihara F, Masuda K, Morioka T, Fukui M. Increased activity of the ipsilateral motor cortex during a hand motor task in patients with brain tumor and paresis. AJNR Am J Neuroradiol 1997;18:865–869 [PMC free article] [PubMed] [Google Scholar]
- 22.Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving primary motor cortex. J Neurosurg 1999;91:238–250 [DOI] [PubMed] [Google Scholar]
- 23.Mattay VS, Weinberger DR. Organization of the human motor system as studied by functional magnetic resonance imaging. Eur J Radiol 1999;30:105–114 [DOI] [PubMed] [Google Scholar]
- 24.Sanes JN, Sunser S, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci USA 1988;85:2003–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yetkin ZF, Mueller WM, Morris GL, et al. Functional MR activation correlated with intra-operative cortical mapping. AJNR Am J Neuroradiol 1997;18:1311–1315 [PMC free article] [PubMed] [Google Scholar]