Abstract
BACKGROUND AND PURPOSE: Intractable epilepsy is a well-recognized complication following head trauma, and many factors have been implicated in its pathogenesis. This study was performed to determine the severity of tissue damage after severe head injury as assessed with magnetization transfer (MT) MR imaging and the relationship of this damage with seizure intractability.
METHODS: Forty-four patients, 13 without seizures (disease controls) and 31 with seizures, underwent T1-weighted MT MR imaging 1–10 years after head trauma. Phase-corrected gradient-echo (GRE) imaging was also performed in all patients to look for the presence of hemosiderin. All patients were evaluated for the presence of an MT abnormality beyond an abnormality seen on T2-weighted images, an MT abnormality within a T2 abnormality, and hemosiderin deposition.
RESULTS: Patients with an MT abnormality beyond a T2 abnormality had a significantly higher intractability of seizures compared with those with an MT abnormality within a T2 abnormality (P < .05). In addition, the mere presence of hemosiderin deposit was not associated with seizure intractability; however, gliosis around the hemosiderin as seen on T1-weighted MT images was associated with seizure intractability.
CONCLUSIONS: T1-weighted MT imaging may be of value in predicting the intractability of the seizure in delayed posttraumatic epilepsy.
Posttraumatic epilepsy (PTE) is an established consequence of head injury. It accounts for about 4% of cases of focal epilepsy in the general population and is the leading cause of epilepsy with onset in young adulthood (1, 2). PTE is classified into three groups: immediate seizures, which occur within 24 hours after the injury; delayed early seizures, which occur during the first week; and late posttraumatic seizures, which occur more than 1 week after the injury (3, 4). The first two groups are usually termed early, and these seizures are considered to be direct reactions to brain damage. Brain trauma is the cause of epilepsy in approximately 5% of the patients referred to specialized epilepsy centers, and these cases of epilepsy are often difficult to control with medical therapy (5, 6). Factors involved in the origin of posttraumatic seizures are still unclear. The commonly accepted risk factors for delayed posttraumatic seizures are those identified by Jennett (7): early posttraumatic seizures, depressed skull facture and intracranial hematoma, prolonged unconsciousness, low Glasgow Coma Scale score, and prolonged posttraumatic amnesia. According to another group (8), documented cortical-subcortical brain lesions represent the main risk factor for delayed PTE.
Many studies with MR imaging have demonstrated the diffuse axonal injury in patients with head injuries. Mittl et al (9) found evidence of hemorrhagic and nonhemorrhagic shearing injury on T2- and T2*-weighted MR images in patients with normal head CT scans who had sustained even minor head trauma. MR imaging has demonstrated increased sensitivity for the detection of traumatic white matter abnormalities, including diffuse axonal injury, when compared with CT (9). However, characterization of these lesions and prediction of patient outcome remains problematic (10). Recently, magnetization transfer (MT) MR imaging has been used for better detection and tissue characterization of diffuse axonal injury (11, 12) and perilesional gliosis in neurocysticercosis-related seizures (13, 14). Lesions of diffuse axonal injury are characterized by axonal disruption with subsequent development of Wallerian degeneration and gliosis. In the early phase of trauma in which there is reactive swelling of axons, collapse and fragmentation of myelin sheath occurs, whereas in the delayed posttraumatic period reactive gliosis predominates (15, 16). We hypothesized that the extent of gliosis, which is the major abnormality in delayed PTE besides focal encephalomalacia, may be better visualized on T1-weighted MT images than on conventional spin-echo (SE) images.
We evaluated the relationship between the extent of gliosis visualized on T1-weighted MT and conventional T2-weighted spin-echo MR images with seizure intractability in patients with delayed PTE.
Methods
Forty-four patients with a history of severe head injury with or without seizures who came to the Department of Neurology, Sanjay Gandhi Post-Graduate Institute of Medical Sciences and Department of Neurosurgery, KG’s Medical College, Lucknow, India, during September 1999 to October 2001 were prospectively examined with clinical evaluation, electroencephalography (EEG, n = 21), and MR imaging. Patients with a retained metallic foreign body or scalp plate were excluded from the study. There were 38 male and six female patients ranging in age from 11 to 50 years. Thirty-one patients had epilepsy, and 13 had no history of seizures. All these patients were imaged between 1 and 10 years after the head injury. The 13 patients (10 male and three female patients, aged 12–47 years) with no history of seizures were labeled as disease controls and will be referred to as disease controls in the remainder of the article. In addition, normative data were obtained for calculation of MT ratios from images acquired in 20 healthy volunteers with no history of neurologic illness (13 male and seven female subjects; age range, 14–48 years; mean age, 30.2 years). Informed consent was obtained from all patients, volunteers, and/or patient caregivers. The patients with head trauma who presented with seizures within 1 week of trauma were considered to have early PTE, whereas those who presented after 1 week were considered to have delayed PTE. Of the 31 patients who had seizures, two had early PTE and 29 had delayed PTE. These two patients with early PTE continued to have simple partial seizures at the time of MR imaging. Clinical data and topographic distribution of the injury are summarized in Table 1. The seizures were considered as intractable when they could not be controlled even after taking 2–3 antiepileptic drugs. EEG abnormalities were seen in 12 of 14 patients with epilepsy and in four of seven disease controls with no clinical epilepsy. The abnormalities included slow wave transients, sporadic spike waves, and continuous slow wave activity over the damaged brain.
TABLE 1:
Summary of the clinical data
| Characteristic |
Patients without Seizures (n = 13)* |
Patients with Seizures (n = 31) |
||||||
|---|---|---|---|---|---|---|---|---|
| Location of Injury | FL (n = 11) | TL (n = 2) | Early PTE (n = 2) |
Delayed PTE (n = 29) |
||||
| FL (n = 2) | PL (n = 5) | FL (n = 19) | FTL (n = 1) | TL (n = 2) | OL (n = 2) | |||
| Type of seizure | ||||||||
| Simple partial | — | — | 2 | — | 3 | — | — | — |
| Complex partial | — | — | — | — | 1 | 1 | 1 | — |
| Tonic | — | — | — | — | 2 | — | — | — |
| Generalized tonic clonic | — | — | — | 5 | 2 | — | 1 | 2 |
| SPS-GTC | — | — | — | — | 11 | — | — | — |
Note.—SPS-GTCS indicates simple partial seizure to generalized tonic clonic seizure; FTL, frontotemporal lobe; OL, occipital lobe; PL, parietal lobe; FL, frontal lobe; TL, temporal lobe.
These disease controls had no seizures at any time after head injury.
Cranial MR imaging was performed with a 1.5-T superconducting system (Magnetom SP; Siemens, Erlangen, Germany) by using a circularly polarized head coil. Conventional SE, proton density-weighted, T2-weighted (2200/12, 80/1 [TR/TE/excitations]), and T1-weighted (1000/15/2) sequences were performed in the axial plane by using a 256 × 256 matrix, 0.5 intersection gap, and 5-mm section thickness. T1-weighted MT SE MR imaging also was performed with the same parameters as those for T1-weighted imaging except for the off-resonance pulse. The pulse sequence used for MT contrast consisted of an off-resonance saturation pulse immediately before the 90° pulse (excitation pulse to saturate the magnetization of protons with restricted motion) (17). The bandwidth of the saturation pulse was 250 Hz with a frequency offset of 1.5 kHz. The TR and TE parameters were chosen to minimize T1 and T2 effects (18). In addition, phase-corrected gradient-echo (GRE) T2*- weighted imaging (800/15, 35/15°/2 [TR/TE/flip angle/excitations]) was performed to look for any susceptibility effect due to the presence of hemosiderin (19, 20). Contrast material-enhanced T1-weighted MT SE imaging also was performed in five patients by using gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) in a dose of 0.1 mmol per kilogram of body weight intravenously to look for any break in the blood-brain barrier. Serial imaging was performed in five patients with seizures and in five patients without seizures to look for any time-related changes on MR images.
All patients were evaluated independently by two authors (R.K.G., R.K.). All cases were evaluated for the presence of hemosiderin and encephalomalacia, as seen on T2- and T2*-weighted images with and without phase correction. An MT abnormality beyond the T2 abnormality was considered to be present when the hyperintensity on T1-weighted MT images extended beyond the abnormality seen on T2-weighted images. When the abnormality seen on T1-weighted MT images did not extend beyond the hyperintensity seen on T2-weighted images, it was termed an MT abnormality within the T2 abnormality.
The presence of focal hemosiderin deposit was confirmed by phase-corrected GRE imaging. Hemosiderin is seen as areas of hypointensity on T2-weighted images that show bloom effect on GRE images and hypointensity on phase-corrected GRE images (20). Gliosis was considered to be present around the hemosiderin deposit on T1-weighted MT images when the hyperintensity extended beyond the hemosiderin deposit seen as hypointensity on GRE phase images.
In all patients with MT abnormalities beyond the T2 abnormalities, the T2 abnormalities and MT abnormalities were segmented by using the maximum likelihood estimation algorithm (21) and were subtracted to confirm it. Similarly, in all patients with gliosis around the hemosiderin deposit in the brain parenchyma, hemosiderin was segmented by using phase-corrected GRE imaging and was subtracted from the segmented T1-weighted MT abnormality of the same patient and position, to confirm the extent of gliosis.
MT ratio (MTR) values were calculated from T2 and MT visible abnormal white matter regions, T2 and T2* normal regions, and MT visible abnormal white matter regions and corresponding contralateral normal regions. For MT ratio quantitation, MT ratio maps were generated from T1-weighed MT and T1-weighted images by using the equation MTR = [1 - (Mp/Mo)] × 100%, where Mo and Mp represent the signal intensity with the saturation pulse off and on, respectively (22). The MT ratio values of T2 and MT visible abnormal regions and normal contralateral regions were obtained through the region-of-interest analysis (22).
Data were analyzed by using SPSS (version 10) statistical software (SPSS, Inc., Chicago, IL). Association between seizure type and hemosiderin deposit, as well as between MT abnormality beyond T2 abnormality and MT abnormality within T2 abnormality, was studied by using the χ2 test with Yates correction. A P value of .05 or less was considered to indicate a statistically significant association.
Results
All 13 disease control patients with no seizure showed variable encephalomalacia in the frontal and temporal lobes. Six of these patients had evidence of hemosiderin demonstrated on phase-corrected GRE images. None of these patients showed hyperintensity on T1-weighted MT images beyond the hyperintensity seen on T2-weighted images (Table 2; Fig 1a–d).
TABLE 2:
Summary of results
| Seizure Characteristic | MT Abnormality within T2 Abnormality (n = 31) |
MT Abnormality beyond T2 Abnormality (n = 13) |
||||
|---|---|---|---|---|---|---|
| NH | FH | Total | NH | FH | Total | |
| No seizures (n = 13) | 7 | 6 | 13 | — | — | 0 |
| Controlled with AEDs (n = 15) | 6 | 8 | 14 | — | 1 | 1 |
| Intractable (n = 16) | 2 | 2 | 4 | 3 | 7 + 2* | 12 |
Note.—AED indicates antiepileptic drug; NH, no hemosiderin deposit; FH, focal hemosiderin deposit.
These two patients had gliosis around the hemosiderin.
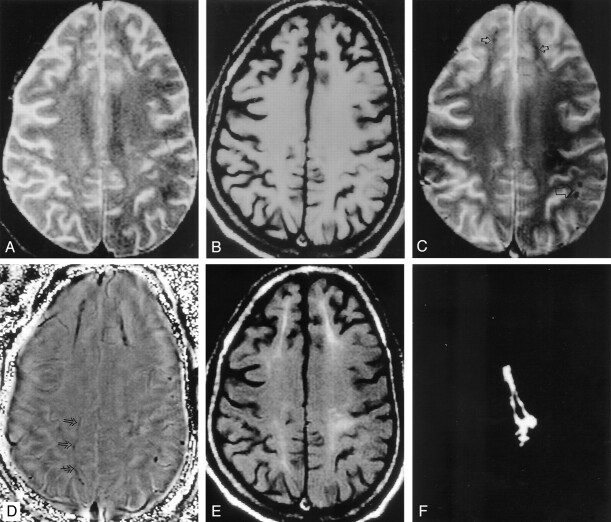
Fig 1.
Patient without seizures after head trauma (disease control).
A and B, Axial T2-weighted image (A) through the lateral ventricles shows a large hyperintensity involving the left frontal region; this area appears hypointense on the T1-weighted image (B).
C, T2*-weighted image does not show any bloom effect to suggest hemosiderin deposit.
D, T1-weighted MT image does not show abnormality beyond the abnormality seen on the T2-weighted (A) and T1-weighted (B) images.
In the remaining 31 patients, 15 had seizures that were controlled with antiepileptic drugs and 16 had intractable seizures (Table 2). Of the 15 patients with controlled seizures, 14 had an MT abnormality within the abnormality seen on T2-weighted images, and one patient had an MT abnormality beyond the abnormality seen on T2-weighted images. Eight of these 14 patients and the one patient with MT abnormality beyond the T2 abnormality showed focal hemosiderin deposit.
In the 16 patients with intractable seizures, four showed MT abnormality within the T2 abnormal region, and two of them showed focal hemosiderin deposit. The remaining 12 patients showed an MT abnormality beyond the T2 abnormal region (Fig 2f), and nine of them showed focal hemosiderin deposit. Two of these nine patients with hemosiderin deposit showed hyperintensity around the hemosiderin suggestive of gliosis (Fig 3f). No significant difference was noted with respect to focal hemosiderin deposition among the different groups with or without seizures (P > .05).
Fig 2.
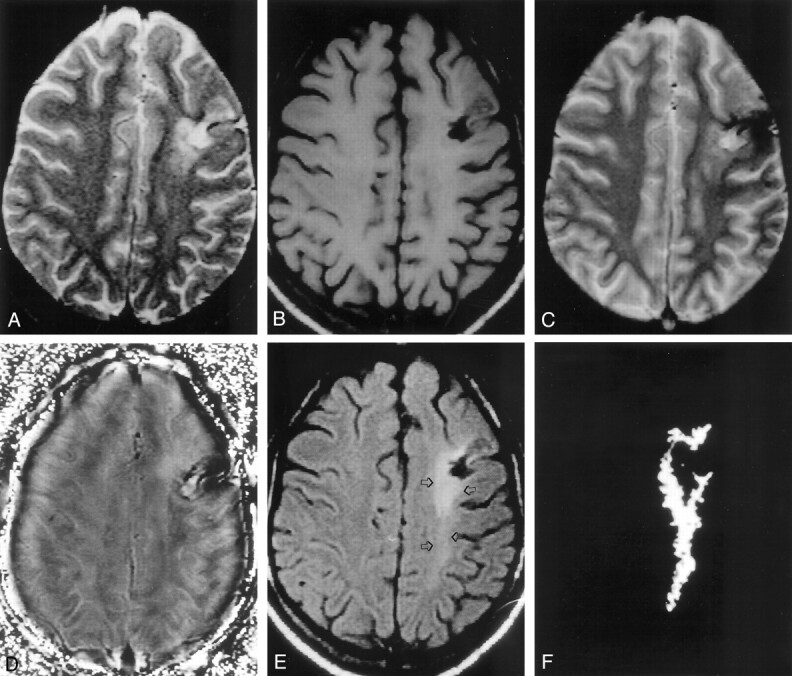
MT abnormality beyond the abnormality seen on the T2-weighted image, in a patient with intractable PTE.
A, Axial T2-weighted image through the supraventricular region shows mixed hyperintensity in the left frontal region.
B, Corresponding T1-weighted image shows variable hypointensity in the left frontal region.
C, T2*-weighted image shows hypointensity associated with blooming, which confirms hemosiderin deposit in that region.
D, Corresponding phase-corrected GRE image shows negative signal intensity around the lesion, consistent with focal hemosiderin deposit.
E, T1-weighted MT image shows a hypointense cavity surrounded by an area of hyperintensity that extends to the left parietal region (arrows), suggestive of gliosis.
F, Difference of segmentation of the abnormality seen in A and E confirms the extension of hyperintensity beyond the T2 abnormality.
Fig 3.
Gliosis around the hemosiderin deposit in the brain parenchyma of a patient with intractable PTE.
A and B, Axial T2-weighted image (A) through the supraventricular region shows subtle hyperintensity in the white matter involving both sides of the frontal lobe and the left parietal region; this is not visible on the corresponding T1-weighted image (B).
C, T2*-weighted image shows multiple hypointense focal round lesions (large arrow) in the left parieto-occipital region and linear hypointensities (small arrows) in both frontal regions and the left parietal region.
D, In addition, phase-corrected GRE image shows subtle hypointensity (arrows) in the right parasagittal region that was not visible in C. All these hypointensities that are seen as negative signal intensity suggest hemosiderin deposition.
E, Corresponding T1-weighted MT image shows high signal intensity around the linear hemosiderin deposits, which is suggestive of gliosis.
F, Difference of the segmentation of the abnormality seen on the phase-corrected GRE image and T1-weighted MT image in the left frontal region confirms that the MT abnormality is beyond the hemosiderin deposit. The central black region represents the hemosiderin seen on the phase image.
Patients with a T1-weighted MT abnormality beyond the abnormality seen on T2-weighted images showed significantly higher (P < .05) intractable epilepsy (12 of 13 patients) compared with the group with T1-weighted MT abnormality within the abnormality seen on T2-weighted images (four of 31 patients).
Two of the 31 patients had early PTE that continued over a period of time. One had seizures well controlled with antiepileptic drugs, whereas the other had intractable seizures. The former had an MT abnormality within the T2 abnormality, and the latter had an MT abnormality beyond the T2 abnormality along with gliosis around the focal hemosiderin deposit (Fig 3).
In all 10 patients with or without seizures who underwent serial studies, no regression or progression of the abnormality was seen with any of the pulse sequences used in this study. There was no evidence of abnormal enhancing regions on postcontrast T1-weighted MT images in five patients with seizures.
Mean (± SD) MT ratios obtained from various locations in white matter and gray matter in 20 healthy volunteers were 36.12 ± 1.45 and 28.61 ± 1.26, respectively. In patients with normal T2-weighted images and visible abnormality in white matter on MR images, the MT ratio was 24.24 ± 2.54, whereas in patients with T2 visible abnormality in white matter the MT ratio was 20.12 ± 2.31. However, the MT ratio in normal contralateral white matter was 35.32 ± 2.01.
Discussion
Delayed PTE occurs in more than 30% of patients with penetrating head injuries, intracerebral and subdural hematoma, depressed skull fracture, or early seizures after injury (6, 23). Seizures following trauma are related to severity of brain injury, and dural penetration increases seizure occurrence (24). The injury variables significantly associated with delayed PTE are loss of consciousness, focal neurologic signs at first examination, missile injuries, frontal lesions, intracerebral hemorrhage, diffuse cerebral contusions, prolonged ( 3 days) posttraumatic amnesia, cortical-subcortical lesions, and depressed skull fracture (25).
MT MR imaging is known to show a larger region of axonopathy in head injury compared with conventional MR imaging (12). The MT ratio in these cases is found to be lower than the normal gray matter-white matter MT ratio (11, 12). Follow-up of these patients shows residual T2 hyperintensity that is considered gliosis (26). In 13 patients in the present study, we observed an abnormal area on the T1-weighted MT images that was beyond the abnormality seen on the T2-weighted images and that had an MT ratio of 22–26%. All these patients had head trauma at least 1 year before MR imaging. This suggests that the brain area affected by the injury is actually more than what is observed on T2-weighted images. The low MT ratio region (ie, the hyperintense region on a T1-weighted MT image) has been shown to be due to gliosis in an experimental model with chronic head trauma (11).
Severity of injury is directly responsible for the increased delayed PTE (7). In the present series, the gliosis as seen with a combination of T2-weighted and T1-weighted MT imaging was more than what was observed with either sequence alone and depicted a more severe parenchymal injury. The patients with greater injury than what was observed on T2-weighted images had significantly higher occurrence of intractable epilepsy than did the patients with MT abnormality within T2 abnormality. This suggests that T1-weighted MT imaging, by demonstrating a much larger gliosis, may predict intractability in delayed PTE.
Surgery has been used in patients with PTE as a means to have either a postoperative seizure-free or a reduced-seizure period in patients with involvement of either the temporal lobe or the neocortex (2, 27, 28). The surgical results are much better in posttraumatic mesial temporal sclerosis than neocortical epilepsy (27). The surgical results depend on the excision of the gliotic region as suggested by T2- and T1-weighted images and the localization of the abnormal region on intraoperative mapping with electrodes (28). It is believed that incomplete identification of the gliotic region is responsible for the failure of surgery more in neocortical PTE than in the temporal lobe epilepsy (27). We believe that this combined approach of mapping the gliotic region may be of value in planning appropriate therapy and may yield better results in these cases with neocortical PTE.
There is a close association of peritraumatic hemorrhage with delayed PTE, and the prominent histopathologic features of hemosiderin deposit in posttraumatic brain tissue strongly implicate iron as an important causative factor in the complex process of posttraumatic epileptogenesis (29). Cortical iron injections in rats induced an epileptogenic focus with occurrence of seizures, which was explained by the peroxidation and destabilization of lipophil neuronal membranes with formation of free radicals. In head trauma, blood extravasation causes deposition of ferrous compounds into neural tissue and the Haber-Weiss iron catalyzed reaction, with hyperproduction of hydroxyl radicals. These reactive oxygen-derived species trigger subsequent formation of peroxidative agents, which in turn cause a self-sustained lipid peroxidation of phospholipid membranes and disruption of the cell wall leading to cell death (30). The induction of an epileptic focus by iron deposition is also related to a decreased nitric oxide synthase activity (31). In addition, there is an impaired glutamate entry into the astrocytes, with a consequent excessive accumulation of this excitatory amino acid in the extracellular space (30).
We have observed that the mere presence of hemosiderin, which is best demonstrated with T2*-weighted imaging, is not a significant causative factor for seizure occurrence. This was seen in all three groups irrespective of seizure activity. However, we observed two cases in which gliosis was seen on T1-weighted MT images around the hemosiderin deposit, and both these patients had intractable seizures. These observations indicate that mere demonstration of hemosiderin may not be strongly related to seizure activity unless there is formation of the gliotic scar around the hemosiderin. However, this needs further confirmation in a larger group of patients with similar abnormalities.
Conclusion
We conclude that T1-weighted MT imaging supplements T2-weighted imaging in depicting the severity of gliosis in delayed PTE and may be of value in predicting the intractability of the seizure in these cases. This information may be of value in better surgical treatment of patients with neocortical PTE by extended resection of MT-visible gliotic scar.
Footnotes
D.K.V. and S.C. received financial assistance from the Council of Scientific and Industrial Research, New Delhi, India.
Presented in part at the meeting of the International Society of Magnetic Resonance in Medicine, Honolulu, Hawaii, May 18–24, 2002.
References
- 1.Annegers JF. The epidemiology of epilepsy. In: Wyllie E, ed. The treatment of epilepsy: principles and practice, 2nd ed. Baltimore, Md: Williams & Wilkins;1996. :165–172
- 2.Diaz-Arrastia R, Agostini MA, Frol AB, et al. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol 2000;57:1611–1616 [DOI] [PubMed] [Google Scholar]
- 3.Dunn LT, Foy PM. Anticonvulsant and antibiotic prophylaxis in head injury. Ann R Coll Surg Engl 1994;76:147–149 [PMC free article] [PubMed] [Google Scholar]
- 4.Lee ST, Lui TN, Wong CW, Yeh YS, Tzaan WC. Early seizures after moderate closed head injury. Acta Neurochir (Wien) 1995;137:151–154 [DOI] [PubMed] [Google Scholar]
- 5.Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 1998;51:1256–1262 [DOI] [PubMed] [Google Scholar]
- 6.Annegers JF, Grabow JD, Groover RV, Laws ER Jr, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology 1980;30:683–689 [DOI] [PubMed] [Google Scholar]
- 7.Jennet WB. Epilepsy after non-missile head injuries, 2nd ed. London: William Heinemann Medical Books;1975
- 8.De Santis A, Sganzerla E, Spagnoli D, Bello L, Tiberio F. Risk factors for late post-traumatic epilepsy.Acta Neurochir 1992;55(suppl):64–67 [DOI] [PubMed] [Google Scholar]
- 9.Mittl RL Jr, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 1994;15:1583–1589 [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks DA. A method to quantitate axonal injury. Neuropathol Appl Neurobiol 1991;17:421–424 [DOI] [PubMed] [Google Scholar]
- 11.Kimura H, Meaney DF, McGowan JC, et al. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: characterization by magnetization transfer ratio with histopathologic correlation. J Comput Assist Tomogr 1996;20:540–546 [DOI] [PubMed] [Google Scholar]
- 12.Bagley LJ, McGowan JC, Grossman RI, et al. Magnetization transfer imaging of traumatic brain injury. J Magn Reson Imaging 2000;11:1–8 [DOI] [PubMed] [Google Scholar]
- 13.Gupta RK, Kathuria MK, Pradhan S. Magnetization transfer magnetic resonance imaging demonstration of perilesional gliosis-relation with epilepsy in treated or healed neurocysticercosis. Lancet 1999;354:44–45 [DOI] [PubMed] [Google Scholar]
- 14.Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol 2000;48:181–187 [PubMed] [Google Scholar]
- 15.Crooks DA. The pathological concept of diffuse axonal injury: its pathogenesis and the assessment of severity. J Pathol 1991;165:5–10 [DOI] [PubMed] [Google Scholar]
- 16.Povlishock JT, Becker DP. Fate of reactive axonal swellings induced by head injury. Lab Invest 1985;52:540–552 [PubMed] [Google Scholar]
- 17.Wolf SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989;10:135–144 [DOI] [PubMed] [Google Scholar]
- 18.Niemi PT, Komu MES, Koskinen SK. Tissue specificity of low field strength magnetization transfer contrast imaging. J Magn Reson Imaging 1992;2:197–201 [DOI] [PubMed] [Google Scholar]
- 19.Yamada N, Imakita S, Sakuma T, Takamiya M. Intracranial calcification on gradient echo phase image: depiction of diamagnetic susceptibility. Radiology 1996;198:171–178 [DOI] [PubMed] [Google Scholar]
- 20.Gupta RK, Rao SB, Jain R, et al. Differentiation of calcification from chronic hemorrhage with corrected gradient echo phase imaging. J Comput Assist Tomogr 2001;25:698–704 [DOI] [PubMed] [Google Scholar]
- 21.Rathore RKS, Datta S, Gupta RK, Rao SB, Verma R. An MLE based segmentation method for quantitation in MR images (abstr). In: Proceedings of the Ninth Meeting of the International Society for Magnetic Resonance in Medicine 2001. Berkeley, CA: International Society for Magnetic Resonance in Medicine,2001;794
- 22.Kumar R, Gupta RK, Rathore RKS, Rao SB, Chawla S, Pradhan S. Multiparametric quantitation of the perilesional region in patients with healed or healing solitary cysticercus granuloma. Neuroimage 2002;15:1015–1020 [DOI] [PubMed] [Google Scholar]
- 23.Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury; I: clinical correlates: a report of the Vietnam head injury study. Neurology 1985;35:1406–1414 [DOI] [PubMed] [Google Scholar]
- 24.Caveness WF. Epilepsy, a product of trauma in our time. Epilepsia 1976;17:207–215 [DOI] [PubMed] [Google Scholar]
- 25.Iudice A, Murri L. Pharmacological prophylaxis of post-traumatic epilepsy. Drugs 2000;59:1091–1099 [DOI] [PubMed] [Google Scholar]
- 26.Parizel PM, Ozsarlak O, Van Goethem JW, et al. Imaging findings in diffuse axonal injury after closed head trauma. Euro Radiol 1998;6:960–965 [DOI] [PubMed] [Google Scholar]
- 27.Marks DA, Kim J, Spencer DD, Spencer SS. Seizure localization and pathology following head injury in patients with uncontrolled epilepsy. Neurology 1995;45:2051–2057 [DOI] [PubMed] [Google Scholar]
- 28.Kazemi NJ, So EL, Mosewich RK, et al. Resection of frontal encephalomalacias for intractable epilepsy: outcome and prognostic factors. Epilepsia 1997;38:670–677 [DOI] [PubMed] [Google Scholar]
- 29.Willmore LJ, Sypert GW, Munson JB. Recurrent seizures induced by cortical iron injection: a model of posttraumatic epilepsy. Ann Neurol 1978;4:329–336 [DOI] [PubMed] [Google Scholar]
- 30.Rubin JJ, Willmore LJ. Prevention of iron induced epileptiform discharges in rats by treatment with antiperoxidants. Exp Neurol 1980;67:472–480 [DOI] [PubMed] [Google Scholar]
- 31.Kabuto H, Yokoi I, Habu H, et al. Reduction in nitric oxide synthase activity with development of an epileptogenic focus induced by ferric chloride in the rat brain. Epilepsy Res 1996;25:65–68 [DOI] [PubMed] [Google Scholar]