Abstract
Summary: We report three cases of callosal dysgenesis that were evaluated by diffusion tensor imaging and fiber tractography. In partial agenesis of corpus callosum, fiber tracts from all regions of brain converged to a partially developed small genu portion and connected to the contralateral side. In complete callosal agenesis, fibers from the hemispheres failed to cross the midline and formed thick bundles running anteroposteriorly (eg, Probst bundle). The thickness of the anterior commissure was enlarged or smaller than normal brain, and other white matter tracts were not markedly different from normal brain tissue.
Callosal dysgenesis, either complete or partial agenesis of corpus callosum (ACC), is a not an uncommon congenital anomaly and various associated anomalies can occur. Aberrant fiber connections are present because of the failure of midline crossing of white matter tracts from cortex. The longitudinal callosal fasicle (LCF; Probst bundle) is found in both cerebral hemispheres of ACC and gives rise to some aberrant fibers toward the midline. It was firstly described by Probst (1) and is known to be a typical sign of ACC. It consists of heterotopic myelinated callosal fibers and results from a migration disorder of callosal fibers. Conventional MR findings of ACC have been well documented in the literature (2, 3). This article describes a novel technique, diffusion tensor MR imaging and fiber tractography (FT), which can show white matter fiber orientation in vivo and neuroanatomic configuration of aberrant hemispheric fiber connections in ACC.
Case Reports
The first patient was a 22-month-old male infant who visited pediatric psychiatry for evaluation of mental retardation. He did not have any significant perinatal history and showed normal motor functions. On routine anatomic images, the corpus callosum was not fully developed and only a small genu portion was seen.
The second patient was a 7-day-old male neonate delivered at full term who was found to have callosal abnormality at prenatal sonography. No specific perinatal history accounted for the callosal abnormality. Anatomic T2-weighted imaging revealed complete ACC, parallel configuration of lateral ventricles, and colpocephaly.
The third patient was an 18-month-old male infant presenting with spasticity of the right arm. He did not have any history of perinatal asphyxia or prematurity. Physical examination revealed right spastic hemiplegia with grade I spasticity in the right upper extremity and heel cord muscles. He was able to walk independently from the 17th month. T2-weighted images demonstrated complete ACC and complex migration anomaly of the left hemisphere.
Six- and 18-month-old babies who visited our institute for the evaluation of simple febrile convulsion were also studied by diffusion tensor imaging as control subjects, because age- and sex-matched controls were not available. They were found to have no specific CNS diseases or abnormalities at routine brain MR imaging. Informed consent was provided by all the participants’ parents or legal guardians, and all procedures were performed under the approval of the institutional review board for clinical studies.
Diffusion tensor imaging was performed by using a 1.5-T system (Intera; Philips Medical Systems, Best, the Netherlands) with synergy-L sensitivity encoding (SENSE) head coil. Diffusion weighting was performed by using single-shot spin-echo echo planar imaging and navigator echo phase correction. Parameters of diffusion tensor imaging were as follows: a data matrix of 128 over a 23-cm field of view, zero-filled to 256 matrices, 3-mm section thickness without a gap, TE = 88 ms, TR = 6000 ms, SENSE factor = 2; number of acquisitions = 4, b = 600 s/mm2 with six different directions, and total imaging time less than 3 minutes.
Anisotropy was calculated by using the orientation-independent fractional anisotropy (FA) and diffusion tensor color maps were created from FA values and the three vector elements. Vector maps were assigned to red (x element, left-right), green (y, anteroposterior), and blue (z, superior-inferior) with proportional intensity scale according to the FA. Three-dimensional FT was obtained by using PRIDE software (Philips Medical Systems) based on FACT methods described by Mori et al (4). Anisotropy threshold for termination of the tracking was 0.2, and direction threshold was 0.75 (0 = full deflection, 1 = no deflection). Fiber bundles were coded as 2D color maps (eg, red [x], green [y], and blue [z]).
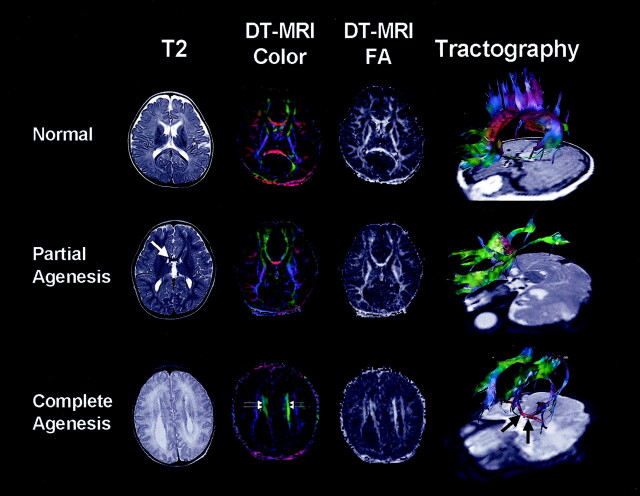
In partial form of callosal agenesis, small genu portion was demonstrated on anatomic images. High FA was measured at the midgenu portion and strong right-to-left (or vice versa) oriented fibers were seen as bright red color on diffusion tensor-based color map. White matter fibers from the parieto-occipital lobe formed back-to-front bundle and entered into the remaining genu portion. Fibers from frontal lobe also demonstrated interconnection to contralateral hemisphere through partially developed corpus callosum (Fig 1).
Fig 1.
Anatomic T2-weighted images, diffusion tensor-based color maps, FA maps, and FT of callosal dysgenesis. Upper row depicts normal findings of corpus callosum; i.e., clear red fibers and high FA bundles crossing midline at diffusion tensor MR imaging. Tractography shows interhemispheric fiber connections through corpus callosum; i.e., red-colored fibers in the midline. Middle row shows partially developed corpus callosum in genu portion (white arrow). Tractography demonstrates fibers from parieto-occipital regions converging into small red-colored genu as well as fibers from frontal lobes. Fibers from the posterior part run anteriorly and form longitudinal green-colored fibers, the Probst bundle. In complete agenesis of corpus callosum, the Probst bundle is more apparent as thick, green, high-FA fibers medial to lateral ventricle (double arrows). Tractography shows thick bundles of green color consist of various fibers from ipsilateral hemisphere, Probst bundle, and thick red-colored AC (black arrows).
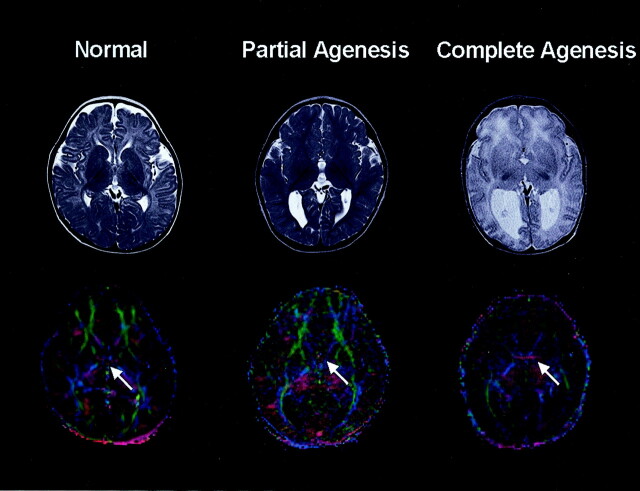
In the case of complete agenesis of corpus callosum, fibers from the hemispheres failed to cross the midline and formed a thick bundle running anteroposteriorly (eg, Probst bundle), which showed as a dark white matter tract on T2-weighted images and as a thick bundle of clear green color on diffusion tensor-based color map. An FA map showed a relatively high signal intensity of LCF than other white matter tracts; the measured value from two patients was 0.68 ± 0.18. Three-dimensional FT showed formation of thick green fiber bundles by various white matter tracts from many quarters of ipsilateral cortex (Fig 1). Anterior commissure (AC) fiber was clearly identified in the second patient as thick red colored fibers, whereas other patients did not have an identifiable AC (Fig 2).
Fig 2.
T2-weighted and diffusion tensor-based color images at the level of the AC. Right-to-left running red fibers are identified in control subject and one patient with callosal agenesis, whereas other patients did not show these transverse fascicles on diffusion tensor maps (white arrows).
Corticospinal tracts and major thick bundles in brain stem of the patients of ACC were not different from those of normal controls, except for the third patient, in whom there was not any identifiable internal capsule in left hemisphere.
Discussion
Previously published studies reported the findings of callosal agenesis at conventional MR imaging as separation and parallel orientation of lateral ventricles, radial orientation of interhemispheric gyri, colpocephaly, and longitudinal white matter tracts at the superomedial aspect of lateral ventricle. To our knowledge, diffusion tensor MR imaging findings of callosal agenesis have not been reported, although measurement of anisotropy in normal corpus callosum was proposed by Chepuri et al (5).
Between 8 and 20 weeks of gestation, the corpus callosum is formed by development of callosal precursors and midline crossing fibers from the hemispheric cortex (2). As shown in the FT of controls, the corpus callosum connects contralateral hemispheric fibers as lobe-to-lobe correspondence (eg, frontal-to-frontal lobes through the genu or anterior body of corpus callosum and parietal-to-parietal lobes through the posterior body and splenium). In the case of partial ACC, we demonstrated fiber connections from various regions other than frontal lobe through the partially developed genu portion of the corpus callosum. With these findings, we can postulate that developing axonal fibers may grow in another direction if their original pathway is blocked, such as in failure of midline crossing.
In the case of ACC, aberrant migration of the fibers occurs and anteroposterior connection develops between callosal fibers—eg, LCFs, or Probst bundle (1). Neuroanatomic study by using horseradish peroxidase technique revealed that fibers leaving from the anterior Probst bundle take a U-turn ipsilaterally without crossing midline and return to the bundle (6). Until now, various staining techniques including peroxidase method have been the only way to describe fiber orientation of specific fascicles. In vivo depiction of fiber architecture in humans was not available before the introduction of diffusion tensor MR imaging. Diffusion tensor MR imaging clearly demonstrates the connectivity of the Probst bundle, and the findings support the embryogenesis that failure of decussation of neurons and axons from cerebral vesicles at the midline leads to anteroposterior connection between aberrant axons.
The anterior commissure is usually hypoplastic in ACC, but it can be enlarged or normal and its function and role are still controversial (7, 8). In our cases, slightly thickened AC was seen in one of three patients, but more investigation with larger number of patients is needed for clarifying the fact. As stated by Meyer et al (8), abnormalities in septum pellucidum, fornices, and gyral architecture can be seen in ACC; however, these structures are too small or have low anisotropy to be described by FT. We showed only a two-dimensional color map at the section of AC because it was very difficult to define regions of interest in AC with the resolution of our protocol; therefore, three-dimensional architecture of AC was demonstrated in only one case (Fig 1). High-field-strength and high-spatial-resolution diffusion tensor MR imaging techniques will show more detailed architecture of these fascicles in the future.
We still do not have a proper quantification method for white matter fiber bundles that allows conversion of multiple data into standardized form. Standardized techniques for objective results of FT and adequate quantification method are the future work of diffusion tensor imaging in clinical imaging.
Conclusion
Diffusion tensor MR imaging was useful in the evaluation of white matter configuration in callosal dysgenesis and contributed to the understanding of axonal guidance and fiber connections in the developing brain.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D Project (02-PJI-PG3-21301-0013), Ministry of Health and Welfare, Korea.
References
- 1.Probst M. Über den Bau des vollständing balkenlosen Groβhirns. Arch Psychiatr 1901;34:709–786 [Google Scholar]
- 2.Barkovich AJ, Norman D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. AJNR Am J Neuroradiol 1988;9:493–501 [DOI] [PubMed] [Google Scholar]
- 3.Barkovich AJ. Apparent atypical callosal dysgenesis: analysis of MR findings in six cases and their relationship to holoprosencephaly. AJNR Am J Neuroradiol 1990;11:333–339 [PMC free article] [PubMed] [Google Scholar]
- 4.Mori S, Crain BJ, Chacko VP, van Zijl PCM. Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–269 [DOI] [PubMed] [Google Scholar]
- 5.Chepuri NB, Yen Y, Burdette JH, et al. Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol 2002;23:803–808 [PMC free article] [PubMed] [Google Scholar]
- 6.Ozaki HS, Murakami TH, Toyoshima T, Shimada M. The fibers which leave the Probst’s longitudinal bundle seen in the brain of an acallosal mouse: a study with the horseradish peroxidase technique. Brain Res 1987;400:239–246 [DOI] [PubMed] [Google Scholar]
- 7.Barr MS, Corballis MC. The role of the anterior commissure in callosal agenesis. Neuropsychology 2002;16:459–471 [PubMed] [Google Scholar]
- 8.Meyer BU, Roricht S. In vivo visualization of the longitudinal callosal fascicle (Probst’s bundle) and other abnormalities in an acallosal brain. J Neurol Neurosurg Psychiatry 1998;64:138–139 [DOI] [PMC free article] [PubMed] [Google Scholar]