Abstract
BACKGROUND AND PURPOSE: Non-Hodgkin lymphoma (NHL) of the larynx is a rare tumor. The aim of this study was to report the CT and MR features of laryngeal NHL in four patients to determine if there are any features that might be helpful to distinguish NHL from other laryngeal tumors.
METHODS: The CT and MR images of four patients with laryngeal NHL were retrospectively reviewed for tumor volume and distribution, appearance, local invasion, and lymphadenopathy.
RESULTS: Tumor volume ranged from 4 to 45 mL3. Tumor was based in the submucosal (2/4 [50%]), mucosal (1/4 [25%]), or both regions (1/4 [25%]) and was centered in the supraglottis (4/4 [100%]) but also involved the glottis (4/4 [100%]) and subglottis (2/4 [50%]). Laryngeal tumor involved the aryepiglottic folds (4/4 [100%)]), ventricles and false cords (4/4 [100%]), epiglottis (3/4 [75%]), paraglottis (3/4 [75%]), true cords (4/4 [100%]), anterior commissure (4/4 [100%]), and laryngeal cartilage (1/4 [25%]). The tumor extended into the hypopharynx (4/4 [100%]), strap muscles (1/4 [25%]), prevertebral muscles (1/4 [25%]), tongue base (1/4 [25%]), and walls of the oropharynx (1/4 [25%]) and nasopharynx (1/4 [25%]). Bilateral cervical lymphadenopathy with extracapsular tumor spread was present in one patient.
CONCLUSION: Laryngeal NHL is a tumor that usually has a large submucosal component centered in the surpaglottis. The tumor extends into the glottis, with less frequent spread to the subglottis, laryngeal cartilage, and strap muscles. Laryngeal NHL also involves the hypopharynx, with large tumors extending superiorly into the tongue base, oropharynx, and nasopharynx. A laryngeal tumor with a large supraglottic submucosal component should alert the ragiologist to the possibility of NHL.
Primary hematologic laryngeal tumors are rare, accounting for less than 1% of laryngeal tumors (1, 2). Non-Hodgkin lymphoma (NHL) is the second most common primary hemopoeitic tumor after plasmacytoma (3), but fewer than 100 reported cases of primary NHL exist in the general literature (4). To the best of our knowledge, only two reported cases of cross-sectional imaging of laryngeal NHL have been reported in the radiologic literature, one of which was a primary tumor (5) and the other was disseminated at diagnosis (6). The aim of this study was to describe the CT and MR features of laryngeal lymphoma in four additional patients.
Methods
The radiographic images obtained from four patients with NHL of the larynx (one male, three female; age range, 36–50 years [mean age, 43 years]) were retrospectively reviewed. All patients presented with symptoms of laryngeal tumor. Three of the patients had primary NHL, whereas one had NHL of the breast 4 years before the current relapse, which was localized to the larynx. All CT imaging (four patients) was performed on a spiral scanner to produce postcontrast axial images with 3–5-mm collimation. Images of the hyoid and laryngeal cartilages were also displayed with a bone window setting. All MR imaging (four patients) was performed on a 1.5-T unit to produce pre- and postcontrast T1-weighted spin-echo images and T2-weighted turbo spin-echo images with or without fat saturation. Images were obtained in at least two planes.
Tumor in the larynx was assessed for volume and distribution, appearance, local extension, and cervical lymphadenopathy. Images were reviewed by consensus by two radiologists (A.D.K., E.H.Y.). In addition, the clinical records were reviewed for clinical presentation, histologic characteristics, and patient outcome.
Results
CT and MR Features
Tumor volume.
The volume of tumor ranged from 4 to 45 mL3 (mean, 19 mL3).
Tumor distribution.
The tumor was based in the submucosa (two cases), mucosa (one case), or both regions (one case). The tumor was centered in the supraglottis (four cases) but also involved the glottis (four cases) and subglottis (two cases). In 100% of patients, the laryngeal tumor involved the aryepiglottic folds (four cases), ventricles and false cords (four cases), true cords (four cases), and anterior commissure (four cases); in 75% of patients, the tumor involved the epiglottis (three cases) and paraglottis (three cases); and in 25% of patients the tumor involved the thyroid lamina and arytenoid cartilage (one case).
The tumor was diffuse, involving both sides of the larynx in all four patients but with predominant involvement of one side in two patients. The airway was narrowed by the tumor in all cases, producing a reduction in the airway of 25–50% (two cases), 50–75% (one case), and 75–100% (one case).
Tumor appearance.
Contrast-enhanced CT revealed that all tumors were homogeneous with contrast enhancement. MR imaging revealed that all tumors were heterogeneous and of mixed high and intermediate signal intensity on T2-weighted images, homogeneous and of intermediate signal intensity on T1-weighted images with homogeneous (two cases) or heterogeneous (two cases) contrast enhancement characteristics.
Local tumor invasion.
NHL extended outside the confines of the larynx to involve the hypopharynx in four patients (pyriform fossa [three cases] and posterior wall [three cases]), strap muscles (one case), prevertebral muscles (one case), tongue base (one case), and walls of the oropharynx (one case) and nasopharynx (one case).
Cervical lymphadenopathy.
Lymphadenopathy was present in one patient (25%). The lymphadenopathy was bilateral and involved the internal jugular chain and posterior triangle. The maximum size of the nodes was 25 mm, and there was extracapsular tumor spread but no necrosis.
Clinical details of the patients included in this study are provided in the Table.
Clinical details
| Case (No.) | Age (y)/Sex | Primary Lymphoma | Presenting Symptoms | Histology | Cervical Lymphadenopathy | Outcome |
|---|---|---|---|---|---|---|
| 1 | 45 /F | Yes | Sore throat. | NK/T cell | No | Disease-free at 36 months. |
| 2 | 50 /F | No; relapse localized to larynx 4 years after diagnosis of primary breast lymphoma. | Hoarse voice. | B cell | No | Disease-free at 11 months. |
| 3 | 36 /F | Yes | Hoarse voice, sore throat, weight loss, and fever. | NK/T cell | No | Colonic lymphoma diagnosed 1 month later. Died at 2 months. |
| 4 | 40 /M | Yes | Hoarse voice. | NK/T cell | Yes | Lost to follow-up. |
Discussion
NHL of the head and neck usually arises in the extranodal lymphatic system of Waldeyer’s ring. Involvement of nodal and extranodal extralymphatic sites is less common, the latter tending to involve the sinonasal tract, salivary glands, thyroid, and orbit more frequently than the larynx. Laryngeal NHL arises in patients of varying age (4–81 years) (7), although the mean age is 58 years and occurrence in children is extremely rare (3). This study showed a preponderance of laryngeal NHL in female patients (female-male ratio, 3:1), whereas other reports have shown a male preponderance. Although NHL of the larynx is rare, the incidence is increasing as a result of the prevalence of acquired immunodeficiency syndrome (8).
Patients with laryngeal NHL commonly present with the progressive hoarseness, cough, dysphagia, or a “lump in the throat.” In this study, two patients also had systemic symptoms of weight loss and fever; one of these patients had a delay between initial diagnosis and subsequent treatment, whereas the other patient had disseminated disease involving the colon shortly after the initial diagnosis of laryngeal NHL. The rapid dissemination to the gastrointestinal tract is a common feature of NK/T-cell lymphoma.
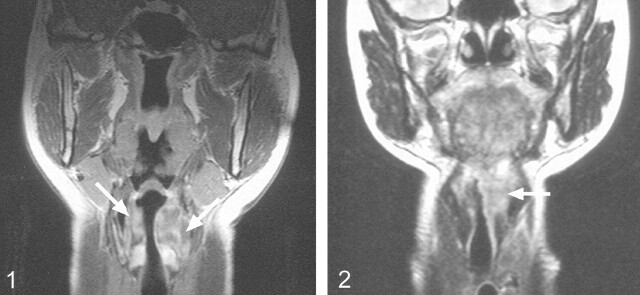
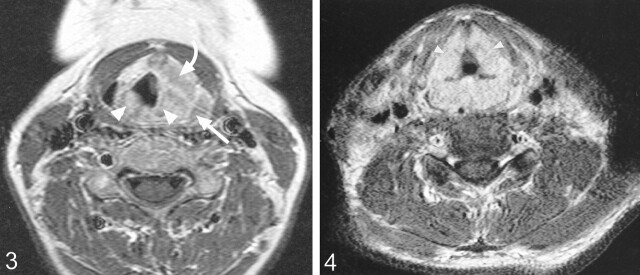
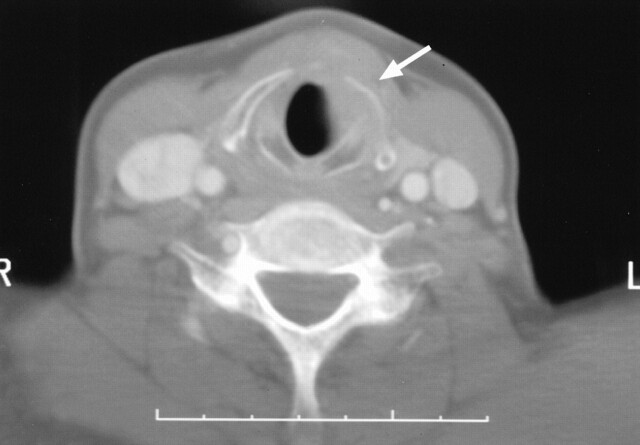
Imaging revealed that laryngeal NHL involved both the supraglottis and glottis in all patients. In all cases, the main bulk of the tumor was in the supraglottic region (Figs 1 and 2), involving the aryepiglottic folds and ventricles and false cords, with most cases involving also the epiglottis and paraglottic spaces (Figs 3 and 4). Inferior extension into the glottis occurred in all patients but was of smaller volume compared with that of the supraglottic component. Two patients had further minimal tumor extension into the subglottis. Two of the four NHL cases in this study were large submucosal tumors (Figs 1 and 2), whereas the third, in addition to a more diffuse involvement of the mucosa, had a large preepiglottic submucosal tumor. This finding of a submucosal tumor centered in the supraglottic region is in keeping with the MR appearance of a primary malignant lymphoma of the larynx reported by Takayama et al (5). Laryngeal NHL confined to the glottis was not encountered, which is not surprising considering the paucity of lymphatics within the true vocal cords. As illustrated in these cases, tumor in the glottis is usually secondary to spread from the adjacent regions. The distribution of NHL in this study is in accordance with the reported findings at endoscopic examination (1, 3, 7). At endoscopy, the supraglottis was the most frequent site of NHL, with involvement of the vestibule and aryepiglottic folds in 80% of patients and frequent involvement of the epiglottis. The glottis and subglottis were less frequently recorded as the sites of involvement (3); however, diffuse involvement of the hemilarynx or whole of the larynx is reported only rarely at endoscopy. Most tumors appear as a more localized polypoidal nonulcerated swelling. Contrast-enhanced imaging revealed that all tumors involved both sides of the larynx: in two, NHL was diffuse and symmetrical (Fig 4), whereas in two, there was asymmetrical involvement. This discrepancy is probably because laryngeal NHL is frequently a submucosal disease and the full extent therefore cannot be appreciated at endoscopy. Deep invasion occurred into the thyroid lamina and arytenoid cartilage and strap muscles (Fig 5). Tumor invasion of these structures, therefore, is not a useful sign in distinguishing lymphoma from the principle differential diagnosis of squamous cell carcinoma. Laryngeal NHL causes narrowing of the airway—one case in this study required a tracheotomy—and obstruction from the tumor can lead to death (3).
Fig 1.
Coronal T1-weighted (494/15) contrast-enhanced MR image obtained in a 50-year-old female patient shows a submucosal laryngeal NHL centered in the supraglottis (arrows)
Fig 3.
Axial T1-weighted (425/15) contrast-enhanced MR image obtained in a 50-year-old female patient shows laryngeal NHL in the supraglottis involving both aryepiglottic folds (arrowheads), left paraglottic space (curved arrow), and extension into the left pyriform fossa (straight arrow)
Fig 2.
Coronal T2-weighted (2500/100) image obtained in a 36-year-old female patient shows a laryngeal NHL centered in the supraglottis (arrow)
Fig 5.
Axial CT scan obtained in a 50-year-old female patient shows laryngeal NHL invading the left thyroid lamina (arrow)
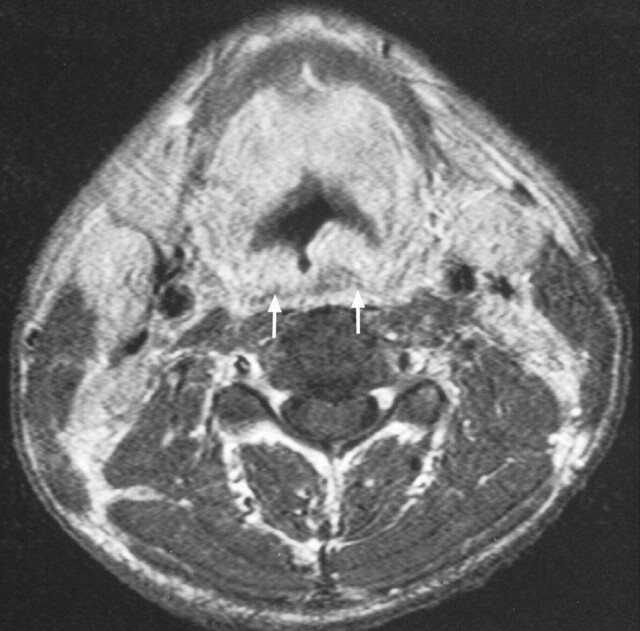
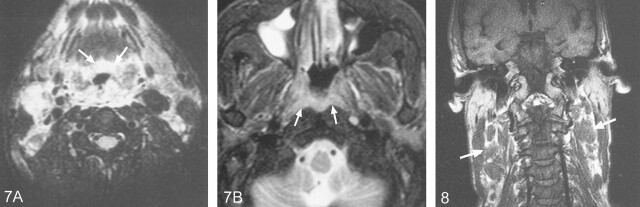
NHL extended into the hypopharynx in all cases involving the posterior wall or pyriform fossae (Figs 3 and 6) and extension along the pharynx to the tongue base (Fig 7A) was also seen. Once again, these findings were not of value in distinguishing carcinoma from lymphoma. In one patient, in whom there had been a delay in diagnosis, there was further circumferential tumor extension along the walls of the oropharynx and nasopharynx (Fig 7B), and this extensive superior involvement of the pharynx should raise the suspicion of a lymphoma. In keeping with the literature (3), a quarter of the cases had cervical lymphadenopathy. The one patient with lymphadenopathy in this series had a delay before treatment was instituted for NK-T cell lymphoma. The nodes showed extracapsular tumor spread (Fig 8). There was no necrosis, a feature that has been described in other cases of NHL of the head and neck (9).
Fig 6.
Axial T1-weighted (543/12) contrast-enhanced MR image obtained in a 40-year-old male patient shows lymphomatous involvement of the posterior hypopharyngeal wall (arrows)
Fig 7.
Axial T2-weighted (2200/100) fat-suppression MR images obtained in a 40-year-old male patient shows extension of the laryngeal lymphoma into the tongue base (A, arrows) and the nasopharynx (B, arrows)
B-cell lymphomas are reported to be more common than T-cell lymphomas (ratio, of 6:1 [3]), although in this small series most tumors were NK-T cell lymphomas. Laryngeal NHL is more frequently a high-grade rather than a low-grade tumor (3), with some low-grade tumors arising from mucosa-associated lymphoid tissue (10). There is also a case report of a rare B-cell lymphoma with Hodgkin-like transformation (11). Most primary laryngeal NHLs are radiosensitive, with a good prognosis, and chemotherapy is reserved for disseminated cases (12). Laryngeal NHL remains localized for long periods (7). Primary NHL of the larynx may eventually disseminate to other sites in the upper respiratory tract, stomach, lung, orbit, or skin (7, 13) after a long disease-free interval (7). However, as demonstrated by one case in this series with colonic lymphoma, laryngeal NHL may be the first manifestation of a more aggressive disseminated disease, and in these cases prognosis is usually poor.
Conclusion
Imaging demonstrates that laryngeal NHL is usually centered in the supraglottis and spreads to involve the glottis and less frequently the subglottis. Diffuse, often asymmetric tumor involvement is found. Laryngeal NHL is usually submucosal or has a submucosal component, which may be heterogeneous at MR imaging. Deep tumor invasion into cartilage or muscle may occur, as may cervical lymphadenopathy. In all cases, there is involvement of the hypopharynx, but superior extension along the pharynx to the oropharynx and nasopharynx may occur. Although no specific imaging features exist to help in the diagnosis of lymphoma, the finding of submucosal tumor centered in the supraglottis should raise the possibility of NHL, especially if there is superior extension to the oropharynx and nasopharynx.
Fig 4.
Axial T1-weighted (543/12) contrast-enhanced MR image obtained in a 40-year-old male patient shows laryngeal NHL with symmetrical involvement of the false vocal cords.
Fig 8.
Coronal T1-weighted (425/13) image obtained in a 40-year-old male patient shows cervical lymphadenopathy with extracapsular tumor spread (arrows)
References
- 1.Morgan K, MacLennan KA, Narula A, et al. Non-Hodgkin’s lymphoma of the larnyx (stage IE). Cancer 1989;64:1123–1127 [DOI] [PubMed] [Google Scholar]
- 2.Anderson HA, Maisel RH, Cantrell RW. Isolated laryngeal lymphoma. Laryngoscope 1976;86:1251–1257 [DOI] [PubMed] [Google Scholar]
- 3.Horny HP, Kaiserling E. Involvement of the larynx by hemopoietic neoplasms an investigation of autopsy cases and review of the literature. Pathol Res Pract 1995;191:130–138 [DOI] [PubMed] [Google Scholar]
- 4.Marianowski R, Wassef M, Amanou L, et al. Primary T-cell non-Hodgkin lymphoma of the larynx with subsequent cutaneous involvement. Arch Otolaryngol Head Neck Surg 1998;124:1037–1040 [DOI] [PubMed] [Google Scholar]
- 5.Takayama F, Takashima S, Momose M, et al. MR imaging of primary malignant lymphoma in the larynx. Eur Radiol 2001;11:1079–1082 [DOI] [PubMed] [Google Scholar]
- 6.Saleh EM, Mancuso AA, Stringer SP. CT of submucosal and occult laryngeal masses. J Comput Assist Tomogr 1992;16:87–93 [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Sakura M, Takooda S, et al. Primary non-Hodgkin’s lymphoma of the larynx. J Laryngol Otol 1997;111:571–574 [DOI] [PubMed] [Google Scholar]
- 8.Simo R, Hartley C, Malik T, et al. Primary non-Hodgkin’s lymphoma of the larynx in an AIDS patient. J Laryngol Otol 1998;112:77–80 [DOI] [PubMed] [Google Scholar]
- 9.King AD, Lei KIK, Ahuja AT. MRI of primary non-Hodgkin’s lymphoma of the palatine tonsil. Br J Radiol 2001;74:226–229 [DOI] [PubMed] [Google Scholar]
- 10.Horny HP, Ferlito A, Carbone A. Laryngeal lymphoma derived from mucosa-associated lymphoid tissue. Ann Otol Rhinol Laryngol 1996;105:577–583 [DOI] [PubMed] [Google Scholar]
- 11.Fung EK, Neuhauser TS, Thompson LD . Hodgkin-like transformation of a marginal zone B-cell lymphoma of the larynx. Ann Diagn Pathol 2002;6:61–66 [DOI] [PubMed] [Google Scholar]
- 12.Pisani P, Dosdegani R, Policarpo M, et al. Non-Hodgkin’s lymphoma of the larynx: clinical cases. Acta Otorhinolaryngol Ital 1999;19:97–101 [PubMed] [Google Scholar]
- 13.Ansell SM, Habermann TM, Hoyer JD, et al. Primary laryngeal lymphoma. Laryngoscope 1997;107:1502–1506 [DOI] [PubMed] [Google Scholar]