Abstract
BACKGROUND AND PURPOSE: Peak CSF velocities detected in individual voxels in the subarachnoid space in patients with Chiari I malformations exceed those in similar locations in the subarachnoid space in healthy subjects. The purpose of this study was to test the hypothesis that the peak voxel velocities are decreased by craniocervical decompression.
METHODS: A consecutive series of patients with symptomatic Chiari I malformations was studied before and after craniocervical decompression with cardiac-gated, phase contrast MR imaging. Velocities were calculated for each voxel within the foramen magnum at 14 time points throughout the cardiac cycle. The greatest velocities measured in a voxel during the cephalad and caudad phases of CSF flow through the foramen magnum were tabulated for each patient before and after surgery. The differences in these velocities between the preoperative and postoperative studies were tested for statistical significance by using a single-tailed Student’s t test of paired samples.
RESULTS: Eight patients with a Chiari I malformation, including four with a syrinx, were studied. Peak caudad velocity diminished after craniocervical decompression in six of the eight patients, and the average diminished significantly from 3.4 cm/s preoperatively to 2.4 cm/s postoperatively (P = .01). Peak cephalad velocity diminished in six of the eight cases. The average diminished from 6.9 cm/s preoperatively to 3.9 cm/s postoperatively, a change that nearly reached the significance level of .05 (P = .055).
CONCLUSION: Craniocervical decompression in patients with Chiari I malformations decreases peak CSF velocities in the foramen magnum. The study supports the hypothesis that successful treatment of the Chiari I malformation is associated with improvement in CSF flow patterns.
Studies of CSF flow in the foramen magnum with phase contrast MR (PC MR) have suggested that CSF physiology is significantly abnormal in patients with Chiari I malformation (1). The PC MR results, together with measurements of CSF pressure, suggest that the Chiari I malformation produces an anatomic and physiologic block to the flow of CSF in the foramen magnum. In patients with a Chiari I malformation, the arterial pressure wave in the cranial vault produces an enlarged cervical subarachnoid pressure wave that may contribute to the development of a syrinx and to clinical signs and symptoms (1). Despite evidence of abnormal flow in a Chiari I malformation, PC MR measurements of flow have not provided a consistent and reliable distinction between normal and abnormal CSF flow. Criteria based on PC MR to select patients for decompression have not yet been established. The discrepancies between studies may reflect the complexity of CSF flow and the variations in the methods used to measure flow.
The complexity of flow is demonstrated by the presence of heterogeneities of flow in healthy subjects and greater heterogeneities in many patients with a Chiari I malformation (2). The velocity of flow recorded by any one technique depends on the size and location of the region of interest used to sample flow. The choice of plane to study flow, which is a midsagittal plane in some studies and an axial plane in others, also affects the flow velocities measured. When flow data are acquired in a sagittal plane, CSF flow is sampled only in a portion of the subarachnoid space in the foramen magnum and regional variations within the selected section are averaged. When flow is sampled in the axial plane, the size and location of the region of interest determine the degree to which velocities in the abnormal range are averaged, together with velocities in the normal range within the subarachnoid space.
In one study, velocities in the subarachnoid space were studied voxel by voxel to identify the peak velocity in the caudad and in the cephalad direction during the cardiac cycle (3). The measurement reflects both the temporal and spatial maximum of CSF velocity, which differs from the measurement of the temporal maximum for a region of interest, which has been referred to as “peak velocity.” The temporally and spatially resolved peak velocities reflect more precisely the greatest magnitudes of the CSF flow in jets than does a peak velocity measurement for a region of the subarachnoid space, in which regions of reduced or normal flow may be averaged with regions of increased flow. The peak voxel velocity measured during the cephalad and caudad flow though the foramen magnum in patients with a Chiari I malformation exceeds that in healthy subjects. These previously measured peak velocities probably underestimated the true velocities, because only the component of velocity in the superior-inferior direction is measured, and only in one section. Nonetheless, a significant difference was noted in peak velocities between patients and controls (3). Therefore, the temporally and spatially resolved peak velocity has some promise for furthering the study of CSF flow abnormalities in patients. The maximal cephalad CSF velocities in the Chiari I patients exceed those in the controls by the larger margin. Therefore, we hypothesized that the maximal cephalad velocities would be most affected by the surgical treatment with suboccipital craniectomy.
Methods
We retrospectively reviewed the medical records of adult patients with Chiari I malformation who underwent craniocervical decompression between July 1999 and December 2001 and identified eight patients who underwent CSF flow studies preoperatively and repeat studies 3 months postoperatively. The preoperative studies in these patients have been reported previously (3). Patients less than 18 years of age were excluded, because normal peak CSF flow velocities have not been recorded for this age group. Inclusion criteria for this study included a 3- and 6-month follow-up. Preoperative and postoperative neurologic symptoms and signs were recorded.
Each patient in the series underwent T1- and T2-weighted sagittal MR imaging of the cervical spine to evaluate for tonsillar descent into the foramen magnum and syringomyelia and with PC MR imaging study of CSF flow in the foramen magnum. Cardiac-gated PC MR images were acquired of the foramen magnum when the subject had achieved a steady heart rate. The pulse sequence used was a commercially available PC flow sequence with a flip angle of 20°; a TR/TE of 20/5 ms; a section thickness of 5 mm; a field of view of 180 mm; a matrix of 256 × 256; and an encoding velocity of 10 cm/s. For the acquisition, a section was chosen perpendicular to the spinal canal at the point below the tonsils in which sufficient CSF was present for visualization of CSF flow. Fourteen image frames were acquired at regular time intervals throughout the R-R interval, with the R wave from a chest electrocardiographic electrode used for the trigger impulse. The offset velocity, estimated from the velocity of stationary tissue, was used to correct for phase shifts introduced by eddy currents.
Flow analysis was performed with commercially available software resident on the image workstation. A cursor was placed to include the entire subarachnoid space (including the spinal cord) and to exclude the vertebral arteries. The program then calculated the flow velocity for each voxel at each time point from the phase shift in the region of interest. The time course of the velocity was inspected and compared with the expected shape of the curve. Aliasing was corrected by the method of Lee et al (4). The peak caudad (systolic) and craniad (diastolic) velocities for all voxels during the cephalad and during the caudad phase of CSF flow in the cardiac cycle were recorded.
In each patient, a postoperative MR study with PC flow analysis was performed 3 months following surgery, with the same MR technique. Temporally and spatially resolved peak systolic and diastolic velocities were recorded. The preoperative and postoperative averages for the maximal systolic and diastolic velocities were calculated, and differences in the means were tested with a one-tailed Student t test of paired samples.
The effect of the surgical decompression on the neurologic signs and symptoms was assessed by comparing the incidence of specific signs and symptoms in the preoperative and postoperative periods. For each patient, the presence or absence of occipital headaches, Valsalva-induced headaches, motor signs or symptoms, sensory signs or symptoms, vertigo, and cranial nerve dysfunction were evaluated. Patients with either an objective sign of motor weakness or a symptom of weakness were for this study considered as having a motor abnormality. Either a sign of hypesthesia or a symptom of diminished sensation was tabulated as a sensory abnormality.
Results
Eight consecutive patients with symptoms referable to Chiari I malformation underwent craniocervical decompression (see Table 1 at http://www.ajnr.org). Four of the eight had syringomyelia. Five had signs or symptoms such as difficulty swallowing or vertigo related to cranial nerve dysfunction. They all underwent a suboccipital craniectomy, C1 laminectomy, and duraplasty. In some cases, the arachnoid was opened for exploration. Following surgery, all patients had improvement of at least some signs or symptoms (see Table 2 at http://www.ajnr.org). In one case with a large syrinx, the cyst diminished substantially in size. In three cases, the syrinx, which was small preoperatively, did not change in size. In seven cases, suboccipital headaches were present preoperatively, and in six of the seven, the headaches were relieved following surgery. All five patients who presented with Valsalva-induced headaches had complete postoperative resolution of the headaches. Hand weakness, which was present in four cases, resolved after surgery in all four patients. Cranial nerve dysfunction resolved in three of the five patients. Some atypical signs and symptoms—for example, pain attributable to reflex sympathetic dystrophy in one patient—did not resolve.
TABLE 1:
Preoperative characteristics of eight patients who underwent craniocervical decompression
| Patient (No.) | Occipital Headache | Valsalva-Induced Headache | Motor | Sensory | Cranial Nerve Dysfunction | Syrinx | Caudad Pre-op | Cephalad Pre-op |
|---|---|---|---|---|---|---|---|---|
| 1 J B | + | + | 0 | 0 | + | 0 | 1.5 | 3 |
| 2 T C | + | + | 0 | + | 0 | 0 | 1.8 | 3.8 |
| 3 A Z | + | 0 | + | + | + | 0 | 2 | 2.5 |
| 4 J S | + | + | + | 0 | 0 | yes | 3.3 | 3.3 |
| 5 N A | + | + | 0 | 0 | + | yes | 3.4 | 14.2 |
| 6 J P | + | + | + | + | 0 | yes | 3.5 | 4.5 |
| 7 C T | 0 | 0 | 0 | + | + | yes | 4.5 | 8.9 |
| 8 M S | + | 0 | + | + | + | 0 | 7.3 | 14.6 |
TABLE 2:
Outcomes of eight patients who underwent craniocervical decompression
| Patient (No.) | Occipital Headache | Valsalva-Induced Headache | Motor | Sensory | Cranial Nerve Dysfunction | Syrinx | Percent Change, Postop |
|
|---|---|---|---|---|---|---|---|---|
| Caudad | Cephalad | |||||||
| 1 J B | Resolved | Resolved | 0 | 0 | Resolved | 0 | 7% | 43% |
| 2 T C | Resolved | Resolved | 0 | + | 0 | 0 | 28% | −11% |
| 3 A Z | Resolved | 0 | Resolved | + | Resolved | 0 | −35% | −16% |
| 4 J S | Resolved | Resolved | Resolved | 0 | 0 | No change | −30% | 61% |
| 5 N A | Resolved | Resolved | 0 | 0 | + | No change | −32% | −71% |
| 6 J P | Resolved | Resolved | Resolved | + | 0 | No change | −34% | −44% |
| 7 C T | 0 | 0 | 0 | + | + | Smaller | −38% | −58% |
| 8 M S | Resolved | 0 | Resolved | + | Resolved | 0 | −37% | −63% |
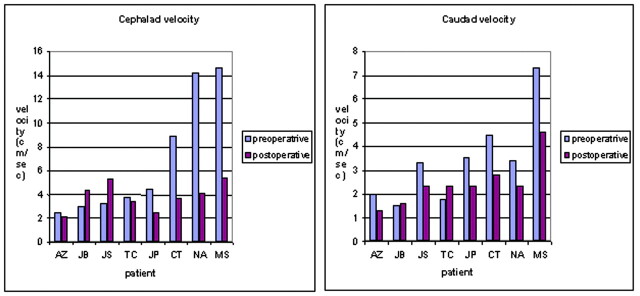
The effect of decompression on maximal CSF velocities is summarized in Figures 1 and 2. Peak caudad velocity ranged from 1.5 to 7.3 cm/s preoperatively and from 1.3 to 4.6 cm/s postoperatively. The mean peak caudad velocity decreased significantly, from 3.4 to 2.4 (P = .01). Peak cephalad velocities ranged from 2.5 to 14.6 cm/s preoperatively and from 2.1 to 5.4 cm/s postoperatively. The mean maximal cephalad velocity decreased from 6.9 to 3.9 cm/s, a larger difference than for the change in peak caudad velocity. The difference was near significance at the .05 level (P = .055). In three patients, either the caudad or cephalad peak velocity or both increased after surgery. No difference in symptom improvement was evident in the patients in whom velocities increased versus those in whom it decreased.
Fig 1.
Graph displaying the peak caudad and cephalad velocities (in cm/s) in the subarachnoid space of the foramen magnum in patients with a Chiari I malformation before and after suboccipital decompression. In most patients, the caudad (systolic) and cephalad (diastolic) velocities are diminished following surgery.
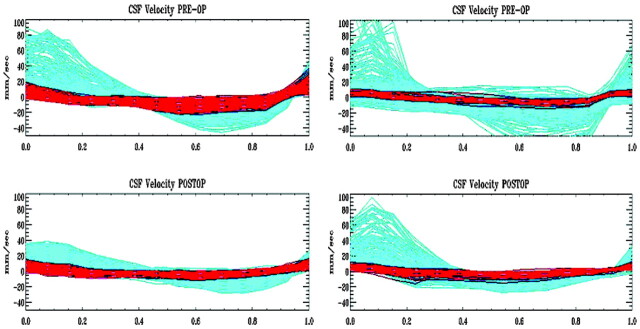
Fig 2.
Graph of the velocities (in mm/s) at each of 14 time points in the cardiac cycle in each voxel in the subarachnoid space for TC (left) and MS (right) before (top row) and after (bottom row) decompression of the craniocervical junction. Time courses of voxels in the anterior subarachnoid space are colored red, and those in the posterior subarachnoid space colored green. Note that the velocities in a cephalad direction (positive values) are reduced following decompression. Velocities in the anterior subarachnoid space are more affected than those in the posterior subarachnoid space.
Examples of the PC cine of CSF flow in the foramen magnum in a patient before and after craniooccipital decompression can be viewed at http://www.ajnr.org.
Discussion
In the present study, we found diminished maximal velocities after craniocervical decompression in adult patients with Chiari I malformation. This study adds support to the theory that the Chiari I malformation is associated with abnormal CSF flow in the foramen magnum. The study did not show correlation between changes in maximal CSF velocities and degree of clinical improvement. The physiologic significance of increased velocity and inhomogeneous flow of CSF in Chiari I patients requires more study.
Other measurements of CSF flow in Chiari I patients before and after suboccipital decompression are not readily comparable to our data because of differences in methodology. In one study, peak velocities during systole and diastole were measured in the foramen magnum and in the cervical spine before and after surgery. In that study, flow measurements were averaged for the entire subarachnoid space, whereas we measured flow on a voxel-by-voxel basis (5). In another study of eight patients who underwent suboccipital decompression, peak velocities were measured in a sagittal rather than an axial section and were averaged for a region of interest (6). This is again unlike our study, in which the peak velocity recorded consisted of the foramen magnum voxel with the highest velocity. In another study of 10 Chiari I patients who had surgical treatment, the mean systolic and diastolic velocities were measured (7). Some investigators have employed axial sections as we did. In one such study, postoperative measurements were not reported (8). In another study, CSF flow was measured at C5–C6 and in the foramen magnum of 20 patients who underwent suboccipital decompression (1). Flow, reported as peak flow rate (in mL/s), increased as a result of surgery in a caudal direction but not in a cephalad direction. To the best of our knowledge, our study is the first in which a temporally and spatially resolved peak velocity was recorded.
CSF flow within the foramen magnum, especially in patients with a Chiari I malformation, is complex; a single flow measurement such as the extreme velocity or the average velocity may not serve well as an index of abnormality. We tested the hypothesis that peak velocities are affected by suboccipital decompression, because we expected that these measurements would be more sensitive to the presence of the regional high velocity jets; however, the methods we used are not optimized. We used voxels with dimensions of 1.4 × 1.4 × 5 mm, which may not be sufficiently small to eliminate partial volume averaging.
Furthermore, CSF flow that is oblique to the superior-inferior phase encoding gradient used in this study is underestimated. Sampling velocities in only one axial section may have missed some of the larger velocities. Therefore, although a temporally and spatially resolved velocity measurement has potential importance in assessing the severity of the Chiari I malformation, much refinement in the analysis of the technique may be possible. Furthermore, the number of subjects studied is small, with the possible result that smaller differences such as the preoperative to postoperative change in systolic pressure did not achieve statistical significance. Furthermore, the precision and accuracy of the PC MR methodology has limitations that have been noted previously (8). Finally, an independent observer did not assess the patients before and after surgery.
Studies of CSF flow in the foramen magnum with PC MR have suggested that the Chiari I malformation produces an anatomic and physiologic block to the flow of CSF in the foramen magnum (1, 9). In patients with Chiari I malformation, the arterial pressure wave in the cranial vault produces and an enlarged cervical subarachnoid pressure wave that may contribute to the development of a syrinx and to clinical signs and symptoms. Our results are consistent with the theory of a physiologic block of CSF flow in the Chiari I malformation. A surgically produced decrease in physiological obstruction would likely reduce the maximal velocities. Additional studies and more sophisticated methods of flow analysis are probably required to determine which flow patterns are not physiologic, which criteria predict the development of a syrinx or symptoms, and what changes in flow characterize successful surgical treatment.
Conclusion
Despite limitations and the small number of cases, this study shows that craniocervical decompression decreases peak caudad and possibly cephalad velocities in Chiari I patients. These data support the theory that successful surgical therapy of a Chiari I malformation is associated with a reduction in CSF velocities in the foramen magnum.
Footnotes
Present affiliation: Department of Radiology, Indiana University, Indianapolis, IN.
References
- 1.Heiss SD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg 1999;91:553–562 [DOI] [PubMed] [Google Scholar]
- 2.Quigley MF, Quigley MA, Haughton V, Nicosia M, Iskanddar BJ. Temporal and spatial patterns of CSF flow in the foramen magnum in normal subjects and Chiari I patients Radiology (in press) [DOI] [PubMed]
- 3.Haughton V, Korosec F, Medow J, et al. Peak systolic and diastolic CSF velocity in the foramen magnum in adult Chiari I patients and normal subjects. AJNR Am J Neuroradiol 2003;24:169–176 [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AT, Pike GB, Pelc NJ. Three-point phase-contrast velocity measurements with increased velocity-to-noise ratio. Magn Reson Med 1995;33:122–126 [DOI] [PubMed] [Google Scholar]
- 5.Brugieres P, Idy-Peretti I, Iffenecker C, et al. CSF flow measurement in syringomyelia. AJNR Am J Neuroradiol 2000;21:1785–1792 [PMC free article] [PubMed] [Google Scholar]
- 6.Armonda RA, Citrin CM, Foley KT, Ellenbogen RG. Quantitative cine-mode magnetic resonance imaging of Chiari I malformations: an analysis of cerebrospinal fluid dynamics. Neurosurgery 1994;35:214–224 [DOI] [PubMed] [Google Scholar]
- 7.Bhadelia RA, Bogdan AR, Wolpert SM, et al. Cerebrospinal fluid flow waveform: analysis in patients with Chiari I malformation by means of gated phase-contrast MR imaging velocity measurements. Radiology 1995;196:195–202 [DOI] [PubMed] [Google Scholar]
- 8.Hofmann E, Warmuth-Metz M, Bendszus M, Solymosi L. Phase-contrast MR imaging of the cervical CSF and spinal cord: volumetric motion analysis in patients with Chiari I malformation. AJNR Am J Neuroradiol 2000;21:151–158 [PMC free article] [PubMed] [Google Scholar]
- 9.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. J Neurosurg 1994;80:3–15 [DOI] [PubMed] [Google Scholar]