Abstract
Summary: We describe a case of translocation of temporo-parietal language function (Wernicke’s area) to the contralateral hemisphere in a right-handed patient with a left temporo-parietal glioma. This translocation was identified by functional MR imaging (fMRI) and validated by direct cortical stimulation during gross-total resection. The current case exemplifies how preoperative fMRI can identify unexpected language organization as a result of tumor growth, affording surgery to patients who may otherwise be deemed inoperable.
Functional MR imaging (fMRI), for the purpose of presurgical mapping, is used to determine the laterality of language function and the proximity of a lesion to language centers. Because disease can alter the map of language function in an unexpected fashion, fMRI has the potential to reveal cortical reorganization that could affect the neurosurgeon’s decision to offer surgery. In the present case, we describe a right-handed patient with confirmed left-lateralized Broca’s area and the isolated translocation of Wernicke’s function to the right hemisphere. We conclude that this is most likely due to tumor growth in the left temporo-parietal region. We define translocation as the switch of most cortical function to the homologous or near-homologous regions in the contralateral hemisphere. Translocation was identified by fMRI and validated during awake craniotomy as well as neuropsychological testing after gross-total resection of the expected location of Wernicke’s area.
Case Report
A 62-year-old right-handed man presented with a 5-year history of headaches. He was 100% right-handed as measured by the Edinburgh Handedness Inventory (1). Routine MR imaging revealed a predominantly nonenhancing left temporo-parietal lesion measuring 3.1 cm × 2.8 cm involving the expected location of Wernicke’s area. Specifically, the lesion involved the superior-temporal gyrus, portions of the supramarginal and angular gyri, and subcortical white matter.
Picture naming (termed “confrontation naming”) is known to deteriorate in patients with Wernicke’s aphasia. Therefore, we tested the patient with the Boston Naming Test, a subset of the Boston Diagnostic Aphasia Examination (2). The age-standardized mean is defined as the correct identification of 56 of a total 60 pictures without hesitation. The range for normal performance is 49–59 pictures. The patient named 57 pictures without hesitation. Thus, despite a lesion in the expected location of Wernicke’s area, the patient performed within the normal range for confrontation naming.
The patient was scheduled for resection of the lesion. To localize the patient’s language function for presurgical planning, blood oxygen level dependent (BOLD) fMRI was performed during a preoperative imaging session. A 1.5-T system (General Electric Medical Systems, Milwaukee, WI) was used to acquire two trials of T2-weighted gradient-echo echo planar images (4000/60 [TR/TE]; section thickness, 4.5-mm skip 0; matrix size, 128 × 128; field of view, 240 × 240 mm) (Fig 1). Three trials of block-designed language tasks were performed.
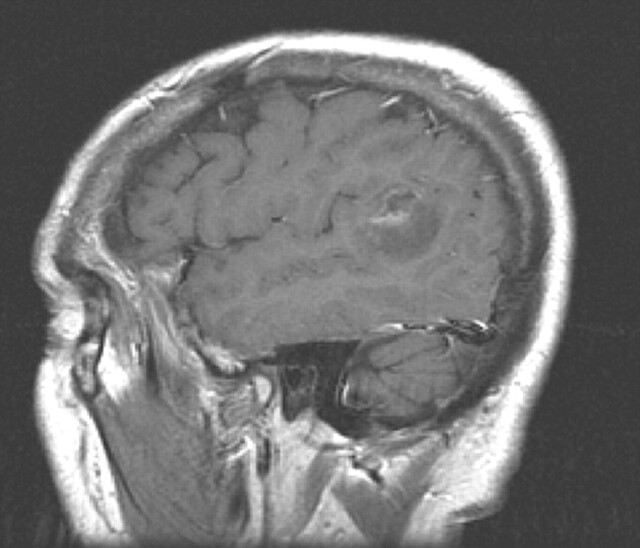
Fig 1.
Postcontrast left sagittal image from a 62-year-old right-handed man, showing a mostly nonenhancing temporo-parietal neoplasm involving superior temporal gyrus and portions of the supramarginal and angular gyri and underlying white matter, the expected location of Wernicke’s area.
Three fMRI language trials were acquired (Fig 2). Each trial was 93 images long and consisted of four active periods each flanked by a resting baseline of matched length. Stimuli were projected onto a screen at the base of the patient’s feet. Each fMRI language trial consisted of randomized periods of homonym judgment, synonym generation, and picture naming. When the patient was presented with two words, he was instructed to decide whether the two words were homonyms. When shown one word, he was told to generate a synonym for that word. Finally, when given a picture, the patient was asked to name the picture. During the baseline periods, a crosshair was projected onto the screen, and the patient was told to focus on it. Tasks were designed to elicit nonverbal forced choice response to minimize head movement.
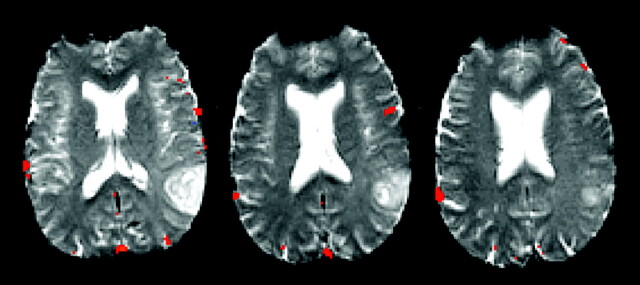
Fig 2.
Axial fMRI results showing Broca’s area in the left hemisphere and activity most consistent with Wernicke’s area in the right hemisphere. The figure shows voxels at a correlation coefficient of 0.46 (P = 4.0 × 10−6) or higher. The language map is shown at this correlation coefficient, because it was the most robust representation of language activity in both hemispheres while minimizing the noise that would be seen at even lower thresholds.
The output language map was a concatenated analysis of the function associated with all three tasks rather than each task separately. The tasks were analyzed together for maximum predictive power of essential language areas. Presumably, this type of analysis emphasizes global language function and can be a more consistent predictor of laterality than the task specific activity.
Images were aligned and analyzed by using AFNI software. Alignment plots revealed the patient’s head motion to be minimal, spanning a maximum of 1 mm of linear trend in the x direction. A 2D, followed by a 3D, motion correction was performed to correct for both in-plane and out-of-plane head motion. A standard spatial Gaussian filter of 2.0 full width–half maximum was applied to the images before statistical calculation. No cluster thresholding or erosion processes were used.
Language activation was determined by statistical correlation and was calculated at a range of correlation coefficients. Right-sided temporo-parietal activity was first seen at a correlation coefficient of 0.59 (P = 5.0 × 10−10). Left-sided frontal activity (Broca’s area) was also present at this correlation coefficient; however, left temporal activity (Wernicke’s area) was absent. Left-sided temporal activity first appeared at a correlation coefficient of 0.53 (P = 5.5 × 10−8).
Two region of interest calculations were evaluated: 1) the inferior frontal lobe (the expected location of Broca’s area) and 2) the posterior aspect of the superior temporal gyrus and the angular and supramarginal gyri (the expected location of Wernicke’s area). Laterality indices were calculated as follows: laterality index = 100(left region of interest − right region of interest)/(left region of interest + right region of interest).
Indices revealed a left hemispheric dominance for Broca’s area and a right hemispheric dominance for Wernicke’s area for all correlation coefficients tested. We tested a variety of thresholds to demonstrate that the split dominance was present independent of thresholding.
A voxel-wise signal intensity profile of both the superior temporal and inferior frontal activity showed a time-course that matched the stimulus presentation, lessening the possibility that the signal intensity was noise. Artifact due to edge effect was ruled out because these voxels were not in close enough proximity to the axial cortical edge. Further, the voxel-wise profiles of these areas did not suggest head movement–induced voxel variability, because the percent change from baseline was approximately 2%.
The patient underwent awake craniotomy for language mapping and resection. Language areas were identified by direct cortical stimulation of the left hemisphere. The current threshold for cortical stimulation was determined by first establishing the minimum current required to elicit a motor response to stimulation of the precentral gyrus by an Ojemann bipolar stimulator (Radionics, Inc., Burlington, MA). Intraoperative mapping involved epicortical recordings of somatosensory evoked potentials and direct cortical bipolar stimulation at 60 Hz, 1-ms width, 1-second trains, beginning at 2 mA and terminating, in this case, at 10 mA. The precentral gyrus was localized at 6 mA by noting facial muscle fasciculations and tongue deviation.
With this threshold current, Broca’s area was then unambiguously identified in the left hemisphere. Stimulation of the inferior frontal gyrus during a vocal counting exercise elicited halting arrest. This arrest was repeatable over multiple sites of stimulation in the inferior frontal gyrus.
We then tested Wernicke’s function. While stimulating superior temporal, supramarginal, and angular gyri, the patient named the same Boston Naming pictures that he had identified successfully preoperatively. The patient’s grammatic structure and comprehension remained intact during stimulation. A single site that elicited phonemic paraphasic errors during picture naming was identified. This site was located on the immediate inferior border of the tumor in the superior temporal lobe. The phonemic paraphasias were as follows: after correctly identifying “unicorn” and “funnel” without stimulation, stimulation of this single site elicited “municourse” and “shunnel,” respectively. No other temporo-parietal areas revealed any dysfunction on stimulation. This finding is in direct opposition to what is expected in a right-handed patient shown to be left-side dominant for Broca’s area. Increasing stimulus current to10 mA failed to elicit evidence of any other language error in the temporo-parietal areas. This essential inability to identify Wernicke’s area by direct cortical stimulation is in concert with the fMRI data, which demonstrated that the patient’s Wernicke’s area had translocated to the contralateral hemisphere.
The patient underwent gross-total resection that included the expected location of Wernicke’s area, namely, superior temporal supramarginal and angular gyri as well as portions of underlying white matter. The resection extended immediately adjacent to the single functional site. The patient suffered no postoperative language dysfunction. Postoperative neuropsychological testing showed an improvement in scores compared with preoperative testing. He correctly identified 59 of 60 pictures during confrontation naming. Pathology returned a diagnosis of anaplastic astrocytoma.
Discussion
The translocation of Wernicke’s area in an adult due to the presence of an anaplastic astrocytoma is documented here by fMRI, direct cortical stimulation, and pre- and postoperative neuropsychological testing. The translocation of Wernicke’s area to a position contralateral to Broca’s area has not, to our knowledge, previously been described.
Language function has been shown to demonstrate plasticity in children; however, in pediatric populations, studies on language are mostly restricted to patients with epilepsy (3) and congenital lesions (4) as assayed by positron emission tomography (PET) and WADA. It is not possible to know whether the translocation we report here was due to an early lesion present during the period of language development or is the result of later plasticity. Transfer of language dominance has been noted in pediatric cases, but these reports describe either the shift or development of both Broca’s and Wernicke’s function together in the expected nondominant right hemisphere (5). To our knowledge, the isolated translocation of Wernicke’s area has not been demonstrated in the pediatric population, regardless of pathologic condition.
The literature in adults also supports cortical reorganization. Most of these cases describe intrahemispheric (6) cortical reorganization. Similar to the present case, however, there is evidence of an increase in the volume of fMRI and PET activity in the right-sided language cortex in patients with stroke (7, 8). It is likely that cerebrovascular events effect functional reorganization differently than tumors. In contrast to the sudden onset of aphasia associated with middle communicating artery infarction, slow-growing tumors offer the brain more time to recover and may result in a different mode of reorganization and should be considered separately.
There is sparse discussion of adult low-grade brain tumors and language reorganization in the literature. Holodny et al (9) used fMRI to describe the translocation of Broca’s area in isolation from temporal language function following the development of a low-grade frontal glioma. In Holodny et al (9), however, the fMRI results were not confirmed by direct cortical stimulation.
Thiel et al (10) used PET to study adult patients with low-grade neoplasms in language areas. Patients with both low- and high-grade tumors in the dominant hemisphere demonstrated a mostly intrahemispheric reorganization with some additional right-hemispheric recruitment in the frontal lobe. The study by Thiel et al (10) did not support interhemispheric reorganization of temporal language areas, and the imaging results were not confirmed intraoperatively as with the present case. In addition, it is unclear whether any of the patient’s in the study had lesions specifically affecting Wernicke’s area proper and subcortical white matter.
Primary brain tumors, especially low-grade tumors, have been shown to have functional tissue preserved within the lesion itself (11). This has consequences for the determination of safe surgical resection. To this end, fMRI is becoming increasingly common to assess the likelihood that functioning brain is located both within lesions as well as in perilesional cortex. The use of BOLD fMRI in and around tumors, however, must be interpreted with caution. Negative findings may represent working language cortex that functions below the sensitivity of the technique and thus is not detected. This is especially true with high-grade lesions where the vascular autoregulation may be compromised and where, as a result, it is hypothesized that the BOLD sensitivity in and around the lesion is also compromised (12). Further, temporo-parietal language areas are more difficult to detect by using fMRI than frontal areas or motor cortex where the signal intensity is stronger, more discrete, and less affected by paradigm choice.
With this in mind, the likelihood that the current patient’s fMRI failed to detect left hemispheric activity is small. There are several lines of evidence in our study to suggest that the absence of function in the left temporo-parietal portion of our patient’s fMRI is a true negative. Broca’s area was identified unambiguously in the left hemisphere by both fMRI and direct cortical stimulation. No studies in normal brain have revealed the condition of true split dominance where most frontal language function is contralateral to most temporal function. Consequently, it is reasonable to expect that the anatomy encompassed by the patient’s left hemispheric tumor involved most, if not all, of the original temporo-parietal language function.
Further confirmation that fMRI correctly identified right hemispheric Wernicke’s dominance is found in the results of direct cortical stimulation of posterior language areas. Our experience with more than 220 awake language mappings routinely implicates multiple locations of temporal and parietal language dysfunction on stimulation of the dominant hemisphere. This was not seen in the current case. Despite a thorough survey at suprathreshold currents, stimulation of the patient’s temporo-parietal lobes caused only one disruption. Whether this corresponds to the few voxels of left hemispheric activity seen at fMRI is uncertain. The discovery of this single intraoperative site does not run contrary to the notion of translocation, because we expect that the patient was left-side dominant for Wernicke’s function before the development of the tumor. It is reasonable to assume that he may have some residual supportive function.
The single most important piece of supportive evidence is the result of the gross-total resection of approximately 3.1 cm × 2.8 cm of superior temporal, supramarginal, and angular cortex as well as portions of the underlying white matter in this patient’s dominant hemisphere. The patient awoke with language fully intact. In addition, although the surgical resection did not encompass the single site of temporal function revealed by direct cortical stimulation, it was visualized within approximately 5 mm of the resection site. Therefore, it is reasonable to assume that that functional area’s connectivity was probably disrupted at least in part by resecting adjacent cortex. This possibility reduces the likelihood of function in the left superior temporal lobe even further. We can say that fMRI predicted right hemispheric dominance correctly, because the patient’s left hemispheric Wernicke’s area and small portions of surrounding cortex were resected without language deficit. This was confirmed by postoperative neuropsychological testing.
Pathology returned a diagnosis of anaplastic astrocytoma. In light of the patient’s long history of symptoms and the radiographic evidence of only spotty enhancement, it is likely that this tumor developed as a low-grade lesion for a period of years before becoming anaplastic. The long period of slow growth after development of language takes place may be permissive for unique cortical reorganizations, such as translocations, not seen previously in stroke or pediatric cases. The phenomenon of translocation may be recognized more commonly as functional imaging becomes increasingly used to map language before awake craniotomy.
Conclusion
This case shows that fMRI should routinely be done preoperatively in patients with lesions in language cortex, particularly when brain tumors are deemed inoperable because of their proximity to essential language centers. Many surgeons would not offer surgery for a lesion in dominant temporo-parietal cortex in a right-handed patient in the absence of techniques such as fMRI. Even if an exploratory awake craniotomy were performed, the finding of Broca’s function in the left hemisphere would have suggested left language dominance and therefore a high risk to surgery. Further, direct cortical stimulation would not have provided any information about the functional capacity of the right hemisphere. The current case exemplifies the need for functional imaging in presurgical planning, because it demonstrates a translocation of Wernicke’s area to the contralateral hemisphere and correctly suggested that the lesion was safe to resect.
References
- 1.Oldfield RC. The assessment of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea & Febiger,1983
- 3.Muller RA, Rothermel RD, Behen ME, et al. Brain organization of language after early unilateral lesion: A PET study. Brain Language 1998;62:422–451 [DOI] [PubMed] [Google Scholar]
- 4.Staudt M, Lidzba K, Grodd W, et al. Right-hemispheric organization of language following early left-sided brain-lesions: functional MRI topography. Neuroimage 2002;16:954–967 [DOI] [PubMed] [Google Scholar]
- 5.Rausch R, Walsh GO. Right-hemisphere dominance in right-handed epileptic patients. Arch Neurol 1984;41:1077–1080 [DOI] [PubMed] [Google Scholar]
- 6.Warburton E, Price CJ, Swinburn K, Wise RJS. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry 1999;66:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thulborn, KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke 1999;30:749–754 [DOI] [PubMed] [Google Scholar]
- 8.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol 1995;37:723–732 [DOI] [PubMed] [Google Scholar]
- 9.Holodny AI, Schulder, Ybasco A, Liu WC. Translocation of Boca’s area to the contralateral hemisphere due to a growth of a left inferior frontal glioma. JCAT 2002;26:941–943 [DOI] [PubMed] [Google Scholar]
- 10.Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation Study. Ann Neurol 2001;50:620–629 [DOI] [PubMed] [Google Scholar]
- 11.Ojemann JG, Miller JW, Silbergeld DL. Preserved function in brain invaded by tumor. Neurosurgery 1996;39:253–265 [DOI] [PubMed] [Google Scholar]
- 12.Holodny AI, Schulder M, Liu WC, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 1999;20:609–612 [PMC free article] [PubMed] [Google Scholar]