Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most debilitating and invasive tumors. Although previous reports have demonstrated the critical role microRNA-181a (miR-181a) serves in the progression of ESCC, how miR-181a induces epithelial-mesenchymal transition (EMT) remains to be elucidated. In the present study, the expression profiles of TGF-β1 and Smad4 proteins in 88 patients with ESCC and 21 adjacent non-cancerous tissues were analyzed using immunohistochemistry. The expression of miR-181a in ESCC cells (ECA109 and TE-1) and HEEC was analyzed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The role of miR-181a in ESCC was analyzed using miR-181a mimics and inhibitor in the same system. Migration, proliferation and apoptosis of cells were assessed using wound-healing assays and cell proliferation assays and flow cytometry, respectively. The expression levels of TGF-β1 and Smad4 in ESCC cell lines transfected with miR-181a mimics and inhibitor were measured using RT-qPCR and western blotting. The expression of E-cadherin and vimentin was also assessed following transfection. The findings demonstrated that expression of TGF-β1 was upregulated, in contrast to Smad4 expression which was downregulated. Expression levels of Smad4 affected the prognosis of patients with ESCC. Higher expression of miR-181a promoted migration and proliferation but inhibited apoptosis of ESCC cells. miR-181a promoted EMT by modulating Smad4 expression in ESCC cells. Overall, these findings revealed that miR-181a induced EMT in ESCC via the TGF-β/Smad pathway in ESCC. Consequently, miR-181a is a potential novel target against ESCC.
Keywords: microRNA-181a, epithelial-mesenchymal transition, transforming growth factor-β, apoptosis, esophageal squamous cell carcinoma
Introduction
Esophageal cancer (EC) is one of the commonest malignant tumors of the digestive system worldwide (1). In China, the most dominant histological subtype is esophageal squamous cell carcinoma (ESCC). According to the 2015 Chinese Cancer Statistics, ESCC is the fourth most fatal disease in China (2). ESCC is characterized by a high malignancy, uncomplicated metastasis and poor survival rates (3). Given the difficulty in early stage diagnosis of ESCC, a number of patients diagnosed with ESCC often have advanced metastases. Moreover, the prognosis of patients with ESCC remains very low; even after surgery and chemotherapy and radiotherapy, the 5-year overall survival rate of patients with ESCC is <25% (4).
Epithelial-mesenchymal transition (EMT) is a critical physiological process that regulates invasion and metastasis of cancer cells (5). It enhances the invasion and metastasis of cancer cells by modulating the expression of E-cadherin and overexpression of vimentin (6). This underlines the integral role EMT serves in metastasis of cancers. However, the molecular mechanism underlying EMT in ESCC remains to be elucidated.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs of ~19–25 nucleotides. They participate in post-transcriptional regulation of gene expression for processes such as invasion, migration, metastasis and EMT (7). A previous study reported differential expression of 22 miRNA in three pairs of ESCC and paracancerous tissues. In particular, compared with adjacent tissues, the expression of 6 miRNAs was upregulated, whereas the expression of the remaining 16 miRNAs was significantly modulated. Notably, miR-181a was among the upregulated miRNAs in the ESCC tissues. The overexpression of miR-181a in ESCC tissues compared with adjacent tissues was further validated by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (8). EMT is regulated by the TGF-β/Smad signaling pathway, which also mediates a number of essential cancer processes (9–11). Therefore, the present study explored the role of miR-181a in EMT and the interaction between miR-181a, EMT and the TGF-β/Smad signaling pathway in ESCC.
Materials and methods
Patients and samples
The protocol for the present study was approved by the Ethics Review Committees of the Affiliated Hospital of North Sichuan Medical College. Between February 2016 and June 2017, EC and paired adjacent non-cancerous tissue samples were surgically removed from patients attending The Affiliated Hospital of North Sichuan Medical College (Sichuan, China). The average age of the patients was 65 years (range 40–77 years). Together, 88 histopathologically confirmed ESCC tissues (66 males; 22 females) and 21 adjacent non-cancerous tissues were collected and analyzed. The adjacent non-cancerous tissues were obtained 5 cm from boundaries of cancerous tissues. The following inclusion criteria were used: i) ESCC was confirmed by pathological diagnosis after surgery; ii) patients had no other tumors; iii) patients had not received neoadjuvant chemotherapy, radiotherapy or other adjuvant therapy; and iv) patients had complete clinicopathological data. No exclusion criteria were used. After extraction, all samples were immediately frozen and stored in liquid nitrogen at −80°C or fixed in 10% formalin for future paraffin embedding.
Cell culture
The TE-1 cells were purchased from the Chinese Academy of Sciences, HEEC and ECA109 were obtained from the Institute of Molecular Biology, North Sichuan Medical College. All cells were verified by using the short tandem repeat profiling technique. The cells were then cultured in Dulbecco's modified Eagle's medium (HyClone; Cytiva) supplemented with a mixture of 1% penicillin-streptomycin (Wuhan Boster Biological Technology, Ltd.) and 10% fetal bovine serum (Thermo Fisher Scientific, Inc.). Incubation was performed for 3–5 days, at 37°C under 5% CO2.
Immunohistochemistry
ESCC and non-cancerous tissues were fixed with 10% paraformaldehyde for 12 h at room temperature, dehydrated using an Excelsior ES automatic dehydrator (Thermo Shandon, Inc.), embedded in paraffin using an automatic Biological Tissue Embedding machine (Leica Microsystems, Inc.) and sliced into thin 4-µm thick sections. The sections were then incubated with 3% H2O2 for 30 min at 37°C to block endogenous peroxidase activity, and incubated with primary anti-TGF-β1 mouse 3C11 (1:50; cat. no. sc-130348; Santa Cruz Biotechnology, Inc.) and anti-Smad4 rabbit EP618Y (1:100; cat. no. ab40759; Abcam) monoclonal antibodies according to the manufacturers' protocols for 12 h at 4°C. Following the primary antibody incubation, the sections were incubated with anti-mouse or anti-rabbit IgG molecules from the Maxvision™ 2 HRP-Polymer Anti-Mouse/Rabbit IHC kit (MXB Biotechnologies) for 30 min at 37°C. Sections were then stained with DAB from the kit for 2 min at room temperature to reveal the expression of TGF-β1 in the cytoplasm and/or cytomembrane and Smad4 in the cytoplasm and/or nucleus. The tissues were then assessed by qualified pathologists. The expression profile of 500 tumor cells (100 cells/field) in five fields was observed under high power (magnification, ×200) using a light microscope. The score for intensity of staining was based on the degree of staining of the cancer cells: 0, the degree of stained cancer cell <5%; 1, 5–25% cell staining; 2, 26–50% cell staining; 3, 51–75% cell staining; and 4, >75% cell staining. The average labeling index of TGF-β1 and Smad4 was assessed according to the total scores in each field. Expression of TGF-β1 and Smad4 was graded as negative (−) for a score <2, weakly positive (+) for a score 2–3, moderately positive (++) for a score between 4–5 and strongly positive (+++) for a score between 6–7. Overall, a score ≥2 was regarded as positive expression. The scoring was performed by two independent, double blinded pathologists.
Cell transfection
The miR-181a mimics, mimics negative control (mimics NC), miR-181a inhibitor and inhibitor negative control (inhibitor NC) were purchased from Sangon Biotech Co., Ltd. Details of the miRNAs, their inhibitors and controls are shown in Table I. Briefly, ECA109 and TE-1 cells were first seeded into 6-well plates (5×105 cells/well). After reaching 40–50% confluence, Lipofectamine 6000® (Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect with miR-181a mimics (20 µM), mimics NC (20 µM), miR-181a inhibitor (20 µM) and inhibitor NC (20 µM) in DMEM supplemented with 10% fetal bovine serum without 1% penicillin/streptomycin for 6 h at 37°C. Negative control (NC) served as the control group. The medium was replaced after 6 h to remove the remaining liposomes. After 48 h transfection, cells were collected for RT-qPCR analysis and after 72 h for western blot analysis.
Table I.
Sequence of the miR-181a mimics and inhibitor and their negative controls.
| miRNA | Sequence (5–3) |
|---|---|
| miR-181a | Sense: AACAUUCAACGCUGUCGGUGAGU |
| mimics | Antisense: ACUCACCGACAGCGUUGAAUGUU |
| mimics | Sense: UUGUACUACACAAAAGUACUG |
| NC | Antisense: GUACUUUUGUGUAGUACAAUU |
| miR-181a inhibitor | ACUCACCGACAGCGUUGAAUGUU |
| Inhibitor | CAGUACUUUUGUGUAGUACAA |
| NC |
miR, microRNA; NC, negative control.
Wound healing assays
The ECA109 and TE-1 cells were seeded into 6-well plates (5×105 cells/well) and transfected as above; after 48 h, when the cell density reached 90% confluence, the cells were scratched using a 100-µl pipette tip. The cells were cultured in 10% fetal bovine serum-free DMEM at 37°C and thereafter washed twice using PBS. Images were captured at 0 and 24 h after scratching using a light microscope at ×100 magnification (Nikon Corporation). The wound zone distances were measured using ImageJ 1.51 software (National Institutes of Health).
Cell proliferation assays
Briefly, transfected Cells were cultured for 48 h in 6-well plates. Thereafter, 8×103 cells (100 µl/well) were seeded into 96-well plates and cultured overnight. Cells in each group were divided into four sub-groups (0, 12, 24 and 48 h), with each subgroup containing six duplicates. Culture medium without cells served as the negative control. Cell viability was assessed based on the Enhanced Cell Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology). Briefly, 10 µl of CCK-8 solution was added in each well before a 2 h incubation at 37°C. The optical density of the cells was measured using a microplate reader (Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm.
Flow cytometry
After 48 h transfection, the inter-cellular collagen in ECA109 and TE-1 cells was digested using 0.25% pancreatic enzymes (without EDTA; Beijing Solarbio Science & Technology Co., Ltd.), centrifuged (39.5925 × g) for 5 min at room temperature and washed twice with cold PBS. The PBS was discarded after centrifugation. The cells were stained for 15 min in the dark and at room temperature based on Annexin V-FITC/PI Apoptosis Detection kit (Nanjing KeyGen Biotech Co., Ltd.). was used to stain cells at room temperature for 15 min in the dark. Early (Annexin V-positive, PI-negative) and late (Annexin V-positive and PI-positive) apoptosis of the cells were assessed using a NovoCyte 3130 flow cytometer (ACEA Biosciences. Inc.; Agilent Technologies, Inc.). Analysis was performed using 10,000 cells per sample using FlowJo 10.5 software (FlowJo LLC).
RNA extraction and mRNA expression analysis
Total RNA was extracted from cultured cells based on the manufacturer's protocols with an EASYspin Plus tissue/cell RNA extraction kit (Aidlab Biotechnologies Co., Ltd.). Reverse transcription and amplification of the RNA (1 µg) were performed based on the all-in-One™ miRNA qRT-PCR Detection kit (GeneCopoeia, Inc.); cDNA synthesis was performed at 37°C for 60 min and at 85°C for 5 min. The resultant cDNA was diluted 5 times before amplification with qPCR (20 µl of cDNA), which was performed at 95°C for 10 min for the initial denaturation, followed by 40 cycles of denaturation at 95°C for 10 sec, elongation at 60°C for 20 sec and final extension at 72°C for 10 sec. For mRNA, reverse transcription was performed based on the Thermo Scientific RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). cDNA was synthesized from 1 µg of total RNA through reaction conditions of 65°C for 5 min, 25°C for 5 min, 42°C for 60 min and 70°C for 5 min. The resultant cDNA was diluted 20 times before qPCR; qPCR (25 µl total volume) was performed using the Bestar Sybr Green qPCR Master mix (DBI Bioscience) under the following temperature conditions: Initial denaturation for 2 min at 95°C, followed by 40 cycles of denaturation at 95°C for 10 sec, primer annealing for 30 sec at 55°C and extension for 30 sec at 72°C. U6 and GAPDH were used as controls for endogenous miRNA and mRNA. All experiments were performed in triplicate. The relative gene expression levels between experimental set ups and controls were analyzed using an automated computer based on the 2−ΔΔCq equation (12). The primers (Sangon Biotech Co., Ltd.) used in the present study are listed in Table II.
Table II.
Primer sequences of reverse transcription-quantitative PCR products.
| Gene | Primer sequence (5–3) |
|---|---|
| miR-181a | F: CGGTAACATTCAACGCTGTCG |
| R: GTGCAGGGTCCGAGGT | |
| TGF-β1 | F: ATGGTGGAAACCCACAACGAA |
| R TGCTGAGGTATCGCCAGGAAT | |
| Smad4 | F: CCATTTCCAATCATCCTGCTC |
| R: GAAGGGTCCACGTATCCATCA | |
| E-cadherin | F: TTGTGGCAGAGTGTAATGCTG |
| R: GTCCCTGGTCTTCTTGGTCA | |
| Vimentin | F: AGAGAACTTTGCCGTTGAAGC |
| R: ACGAAGGTGACGAGCCATT | |
| U6 | F: CGCTTCGGCAGCACATATA |
| R: TTCACGAATTTGCGTGTCAT | |
| GAPDH | F: CATGAGAAGTATGACAACAGCCT |
| R: AGTCCTTCCACGATACCAAAGT |
miR, microRNA; F, forward; R, reverse.
Western blot analysis
Total proteins of transfected cells were extracted, separated and solubilized in lysis buffer (Beyotime Institute of Biotechnology). The concentration of proteins of interest (E-cadherin, Vimentin, TGF-β1, Smad4 and GAPDH) was measured based on the Bradford Protein Assay kit (Tiangen Biotech Co., Ltd.). Briefly, equivalent amounts of protein (55 µg/lane) per sample were separated using 10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore; 0.22 µm). The membranes were blocked by 3 h incubation at room temperature in 5% skimmed milk dissolved in TBS-0.1% Tween. The membranes were then incubated with primary antibodies (Wuhan Boster Biological Technology, Ltd.) against E-cadherin (Rabbit; cat. no. PB9561; dilution, 1:500-1:2,000), vimentin (Rabbit; cat. no. PB9359; dilution, 1:500-1:2,000), TGF-β1 (Rabbit; cat. no. BM4876; dilution, 1:100-1:400), Smad4 (Rabbit; cat. no. BA1397; dilution, 1:100-1:400) and GAPDH (Rabbit; cat. no. BA2913; dilution, 1:500-1:2,000) at 4°C overnight. Second incubation with secondary antibodies (Wuhan Boster Biological Technology, Ltd.) anti-rabbit IgG (H+L)-HRP (Rabbit; cat. no. BA1054; dilution, 1:5,000-1:10,000) goat antibodies was performed for 1 h at room temperature. Chemiluminescence of the protein bands was performed based on the BeyoECL Star kit (Beyotime Institute of Biotechnology). The images for the protein bands were captured using the gel imaging equipment (Bio-Rad Laboratories, Inc.). The expression level of the proteins was assessed using ImageJ (v1.51; National Institutes of Health). GAPDH was used as an endogenous control. Antibody dilution were as follows: E-cadherin (1:1,000), vimentin (1:1,000), TGF-β1 (1:300), Smad4 (1:300), GAPDH (1:500) and goat anti-rabbit IgG (H+L)-HRP (1:5,000).
Statistical analysis
Analyses were performed using GraphPad Prism 5.0 and SPSS v23.0 (IBM Corp.). Continuous data were expressed as mean ± standard deviation. Comparison between groups was performed using χ2 and t-test. One-way ANOVA followed by Tukey's post hoc test was performed for multiple group comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of TGF-β1 and Smad4 in ESCC tissues
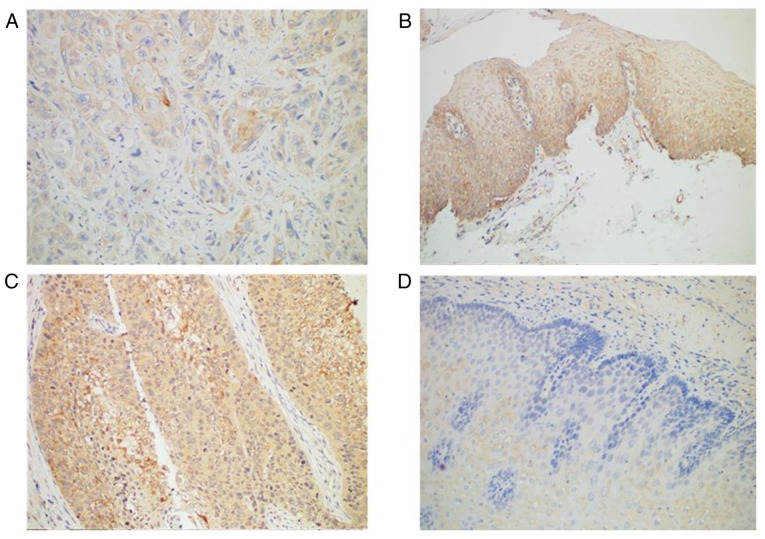
Immunohistochemistry revealed Smad4 protein was expressed in all normal tissues but only in 89.77% of ESCC tissues (Table III; Fig. 1A and B). TGF-β1 protein was expressed in most (72.73%) patients with ESCC (64 of 88 patients), compared with normal adjacent tissues in which TGF-β1 was negative (Table III; Fig. 1C and D). The expression profile of TGF-β1 and Smad4 was not associated with any of the clinicopathological factors (Table IV).
Table III.
TGF-β1 and Smad4 expression in ESCC tissues and non-cancerous tissues.
| TGF-β1 | Smad4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue type | Cases | Positive (%) | Negative (%) | χ2 | P-value | Positive (%) | Negative (%) | χ2 | P-value |
| Tissue | 20.8188 | 0.05 | 13.3134 | 0.05 | |||||
| Cancer | 88 | 64 (72.73) | 24 (27.27) | 79 (89.77) | 9 (10.23) | ||||
| Non-cancerous | 21 | 4 (19.05) | 17 (80.95) | 21 (100) | 0 (0) | ||||
ESCC, esophageal squamous cell carcinoma.
Figure 1.
The expression of TGF-β1 and Smad4 in ESCC and non-cancerous tissues by immunohistochemistry (magnification, ×200). (A) Weakly positive expression of Smad4 in ESCC. (B) Positive expression of Smad4 in non-cancerous tissues. (C) Positive expression of TGF-β1 in ESCC. (D) Negative expression of TGF-β1 in the basal layer of non-cancerous tissues. ESCC, esophageal squamous cell carcinoma.
Table IV.
TGF-β1 and Smad4 expression in relation to clinicopathological findings in ESCC.
| Characteristics | Number (n=88) | TGF-β1 expression | P-value | Smad4 expression | P-value |
|---|---|---|---|---|---|
| Age (years) | 0.75848 | 0.69893 | |||
| >65 | 49 | 35 | 45 | ||
| ≤65 | 39 | 29 | 34 | ||
| Sex | 0.60701 | 0.75139 | |||
| Male | 66 | 49 | 60 | ||
| Female | 22 | 15 | 19 | ||
| Histological grade | 0.61817 | 0.56364 | |||
| Well diff. | 37 | 29 | 33 | ||
| Moderately diff. | 45 | 32 | 40 | ||
| Poorly diff. | 6 | 3 | 6 | ||
| Depth of invasion | 0.72166 | 0.7216 | |||
| Upper | 8 | 5 | 7 | ||
| Middle | 30 | 22 | 28 | ||
| Lower | 50 | 37 | 44 | ||
| Lymphatic invasion | 0.28511 | 0.41298 | |||
| Yes | 37 | 27 | 34 | ||
| No | 51 | 37 | 45 |
ESCC, esophageal squamous cell carcinoma; diff., differentiated.
Expression of TGF-β1 and Smad4 is associated with the survival of patients
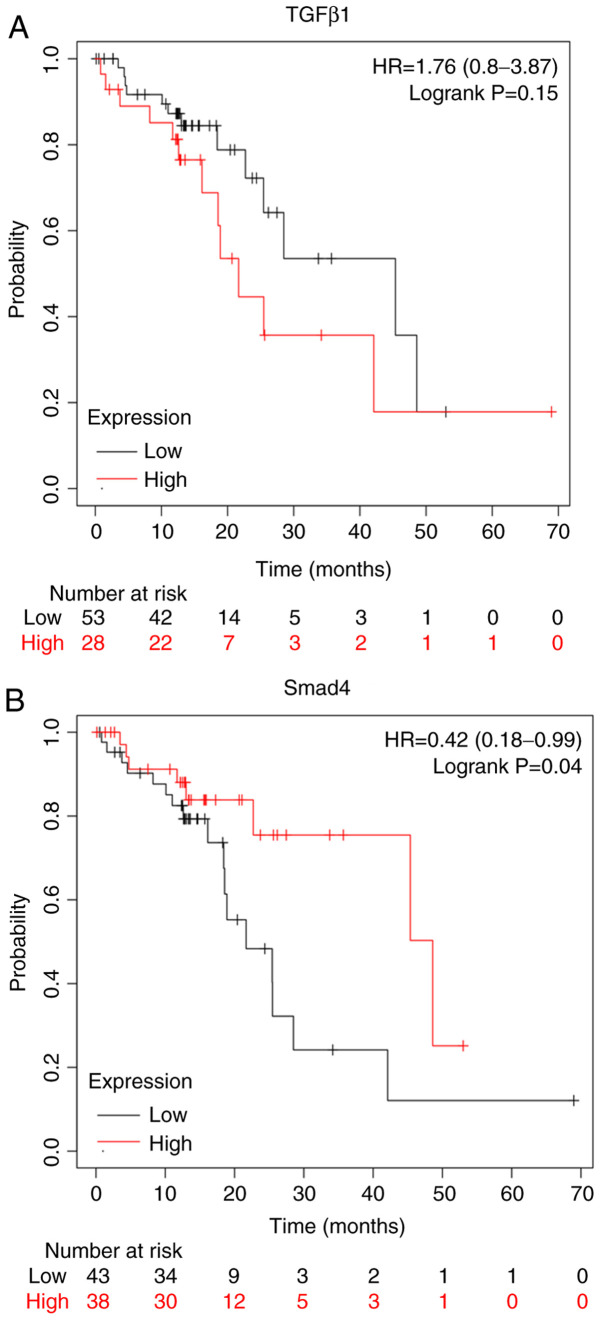
The Kaplan-Meier plot (www.kmplot.com/analysis/) for the expression of TGF-β1 and Smad4 proteins assessed the role of these proteins in the prognosis of patients with ESCC. Overexpression of TGF-β1 proteins conferred worse survival rates for patients with ESCC (Fig. 2A). On the other hand, the overexpression of Smad4 conferred better survival rates for patients with ESCC (Fig. 2B).
Figure 2.
Survival analysis of patients with ESCC according to TGF-β1 and Smad4 expression were determined by Kaplan-Meier analysis followed by the log-rank test. Data are presented as the means ± standard error of three experimental results. The t-test was applied to compare differences between groups (kmplot.com/analysis/). (A) Survival analysis of patients with ESCC was not related to TGF-β1 expression according to a Kaplan-Meier plot (P=0.15). (B) Survival analysis of patients with ESCC was related to Smad4 expression according to a Kaplan-Meier plot (P<0.05). ESCC, esophageal squamous cell carcinoma, HR, hazard ratio.
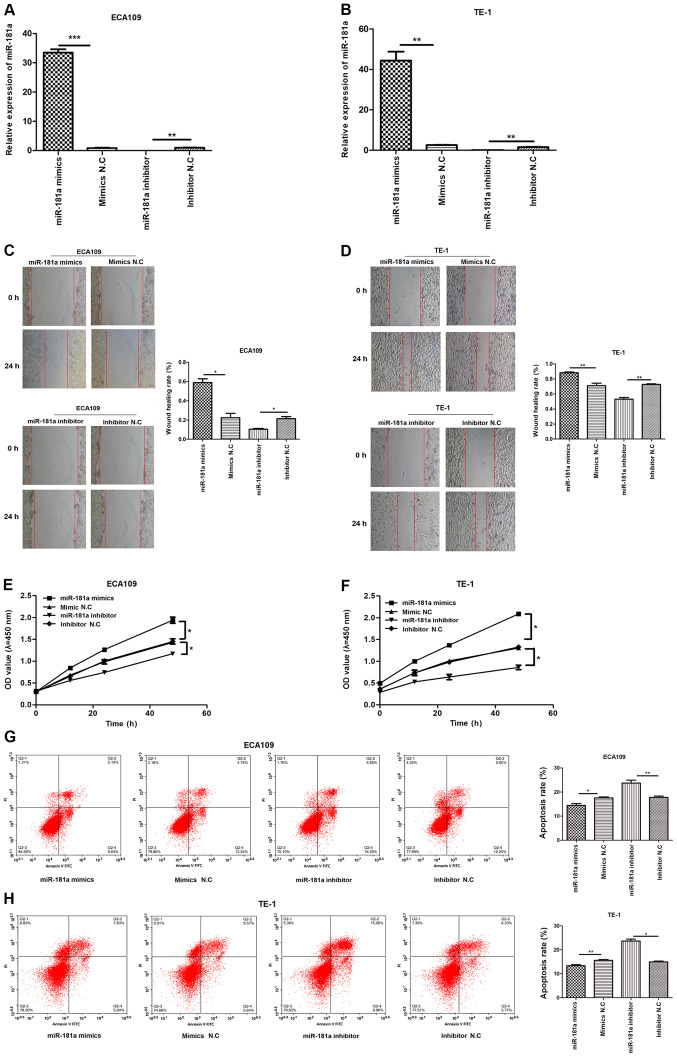
Overexpression of miR-181a promotes migration, proliferation but inhibits apoptosis of ESCC cells
RT-qPCR revealed miR-181a was overexpressed in ESCC cells (Fig. 3). To explore the potential role of miR-181a in ESCC cells, miR-181a expression was modulated by miR-181a mimics and inhibitor (Fig. 4A and B). Wound healing assay revealed that miR-181a promoted migration of ECA109 and TE-1 cells. Consequently, inhibition of miR-181a expression (Fig. 4C and D) markedly disrupted migration of ECA109 and TE-1 cells. CCK-8 assay further revealed that compared with controls, overexpression of miR-181a promoted proliferation of ECA109 and TE-1 cells (Fig. 4E and F). Flow cytometry part revealed inhibition of miR-181a enhanced apoptosis of ECA109 and TE-1 cells (Fig. 4G and H). Therefore, miR-181a potentially promoted tumorigenesis.
Figure 3.
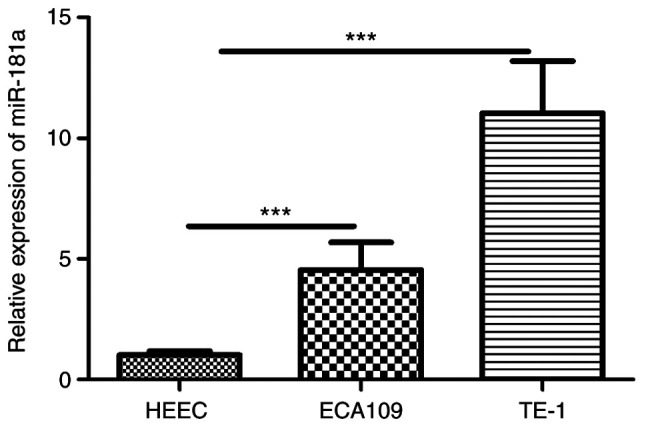
The expression of miR-181a is upregulated in ESCC cell lines. miR-181a-5p expression was detected in two ESCC cell lines (ECA109, TE-1) and the normal esophageal epithelial cell (HEEC) by reverse transcription-quantitative PCR. Data are expressed at the mean ± standard deviation (n=3) of one representative experiment. ***P<0.001, vs HEEC cell line. miR, microRNA; ESCC, esophageal squamous cell carcinoma.
Figure 4.
Overexpression of miR-181a promotes the migration, proliferation and inhibitors apoptosis of ESCC cells although downregulation of miR-181a shows the opposite effects. ECA109 and TE-1 cells were transfected with mimics, inhibitor and negative control. Cells were divided into four groups: miR-181a mimics, mimics NC, miR-181a inhibitor and inhibitor NC. (A) The relative expression of miR-181a of ECA109 was assessed using RT-qPCR 48 h after transfection. (B) The relative expression of miR-181a of TE-1 was assessed using RT-qPCR 48 h after transfection. (C) The migration abilities of ECA109 cells were detected by wound healing assays (magnification, ×100). (D) The migration abilities of TE-1 cells were detected by wound healing assays (magnification, ×100). (E) The proliferation of ECA109 cells was determined by the CCK-8 assay. (F) The proliferation of TE-1 cells was determined by the CCK-8 assay. (G) The apoptosis rate of ECA109 cells was measured by flow cytometry. (H) The apoptosis rate of TE-1 cells was measured by flow cytometry. The date is expressed at the means ± standard deviation (n=3) of one representative experiment. *P<0.05, **P<0.01, ***P<0.001. miR, microRNA; ESCC, esophageal squamous cell carcinoma; NC., negative control; RT-qPCR, reverse transcription-quantitative PCR; OD, optical density; PI, propidium iodide.
miR-181a may promote EMT in ECA109 and TE-1 cells via the TGF-β/Smad pathway
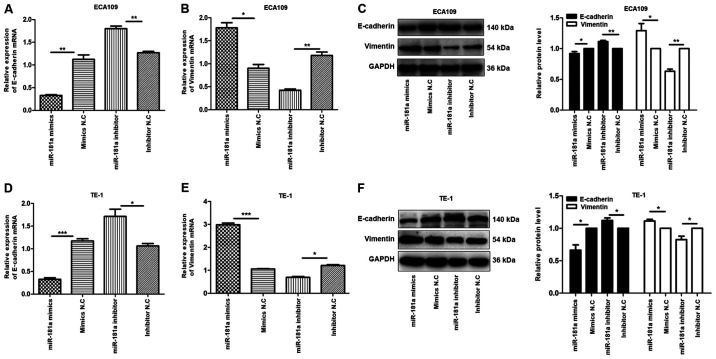
To explore the mechanism underlying EMT by miR-181a, the expression of E-cadherin and vimentin proteins in ECA109 and TE-1 cells transfected with miR-181a mimics or miR-181a inhibitor was evaluated. E-cadherin and vimentin proteins are markers for EMT (13). Compared with controls, E-cadherin mRNA was underexpressed in miR-181a mimics group, compared with the upregulated expression of vimentin mRNA in the same group of cells, while the miR-181a inhibitor group exhibited the opposite effect (Fig. 5A, B, D and E). The miR-181a mimics group showed a reduction of E-cadherin protein and enhancement of vimentin protein, while miR-181a inhibitor group exhibited the opposite effect in ECA109 and TE-1 cells (Fig. 5C and F). These findings demonstrated that miR-181a regulates EMT in ECA109 and TE-1 cells.
Figure 5.
Overexpression of miR-181a promotes the EMT of ESCC cells. Instead, the deregulation of miR-181a suppressed EMT of ESCC cells. The relative mRNA and protein expression of E-cadherin, Vimentin was detected in ECA109 and TE-1 cells following transfection with mimics, inhibitor and negative control by RT-qPCR and western blot. GAPDH was used as an internal control. Values are standardized to an average of 1.0 in the negative control samples. Cells were divided into four groups: miR-181a mimics, mimics NC, miR-181a inhibitor, inhibitor NC. (A-C) The relative mRNA and protein expression of E-cadherin, Vimentin in ECA109 were assessed using RT-qPCR and western blotting. (D-F) The relative mRNA and protein expression of E-cadherin, Vimentin in TE-1 were assessed using RT-qPCR and western blotting. Data were presented as the means ± standard error of three experimental results. The t-test was applied to compare differences between groups where appropriate. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; EMT, Epithelial-mesenchymal transition; ESCC, esophageal squamous cell carcinoma; NC, negative control; RT-qPCR, reverse transcription-quantitative PCR.
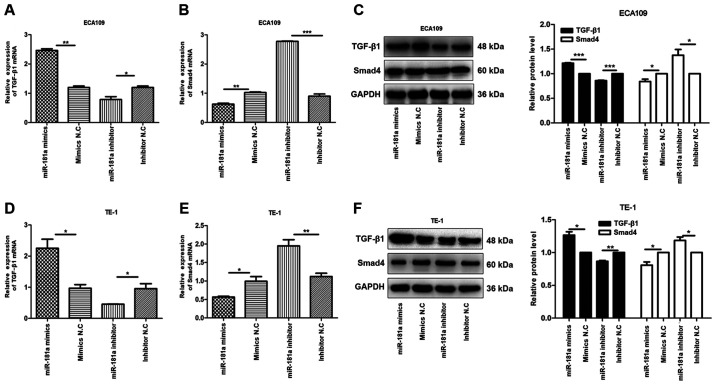
Research shows that TGF-β/Smad pathway serves a critical role in EMT (14)
To explore this hypothesis, the expression of TGF-β1 and Smad4 mRNAs and proteins in transfected ECA109 and TE-1 cells were evaluated. It was found that, compared with controls, the expression of TGF-β1 mRNA and protein was high in the miR-181a mimics group, compared with Smad4 mRNA and protein which were underexpressed. Conversely, miR-181a inhibition modulated the expression of TGF-β1 mRNA and protein but upregulated that of Smad4 mRNA and protein in ECA109 and TE-1 cells (Fig. 6). Overall, miR-181a promoted EMT in ESCC via the TGF-β/Smad4 pathway.
Figure 6.
miR-181a alters the expression of TGF-β1 and Smad4 of ESCC cells. The relative mRNA and protein expression of TGF-β1, Smad4 were detected in ECA109 and TE-1 cells following transfection with mimics, inhibitor and negative control by RT-qPCR and western blotting. GAPDH was used as an internal control. Values are standardized to an average of 1.0 in the negative control samples. Cells were divided into four groups: miR-181a mimics, mimics NC, miR-181a inhibitor, inhibitor NC. (A-C) The relative mRNA and protein expression of TGF-β1 and Smad4 in ECA109 were assessed using RT-qPCR and western blotting. (D-F) The relative mRNA and protein expression of TGF-β1 and Smad4 in TE-1 were assessed using RT-qPCR and western blotting. Data were presented as the means ± standard error of three experimental results. The t-test was applied to compare differences between groups where appropriate. *P<0.05, **P<0.01, ***P<0.001. miR, microRNA; ESCC, esophageal squamous cell carcinoma; RT-qPCR, reverse transcription-quantitative PCR; NC, negative control.
Discussion
The present study explored the role of miR-181a in ESCC. Our previous study (8) found that, compared with adjacent non-cancerous tissues, miR-181a was upregulated in ESCC. The present study further validated the overexpression of miR-181a in ESCC cells. Further analyses revealed that miR-181a promoted migration and proliferation but inhibited apoptosis of the ESCC cells, consistent with a previous study (15).
The TGF-β signaling pathway serves a significant role in cancer progression, mediated by three ligands: TGF-β1, TGF-β2 and TGF-β3. Binding of TGF-β on TβRII initiates activation and transphosphorylation of TβRI, which activates downstream mediators (16). Overexpression of TGF-β promotes EMT by enhancing migration and invasion of cancer cells (17). The present study found the expression of TGF-β1 was upregulated in cancer tissues, compared with the modulated Smad4. Furthermore, in contrast to TGF-β1, Smad4 was associated with the survival of patients with ESCC. However, the expression of both TGF-β1 and Smad4 had no bearing on any of the clinicopathological factors.
Upregulated expression of miR-181a in pancreatic cancer tissues promotes EMT by downregulating RKIP (18). miR-181a also accelerates proliferation, invasion and EMT in gastric cancer by inhibiting RASSF6 via the MAPK signaling pathway (19). Overexpression of miR-181a also promotes proliferation, migration and metastasis of prostate cancer cells by suppressing TGIF2 (20). In ovarian cancer, upregulated miR-181a expression promotes EMT and modulates cell apoptosis by inducing paclitaxel resistance (21). miR-181a also induces invasion and migration and promotes EMT of lung cancer cells by disrupting PTEN expression (22). The present study found miR-181a promotes EMT via TGF-β/Smad pathway. In particular, miR-181a modulates expression of E-cadherin in ESCC, compared with vimentin, a mesenchymal marker, which was upregulated. Thus, in ESCC, upregulation of miR-181a transforms epithelial cells to mesenchymal phenotype. Overexpression of miR-181a disrupted expression of Smad4. Even though overwhelming evidence strongly underlines the oncogenic property of miR-181a, one study (23) reported that upregulation of miR-181a expression inhibited proliferation and induced apoptosis of leukemia cells. miRNAs are both tumor suppressor genes and oncogenic genes, so miR-181a may serve a different role in different types of cancer. Future studies will further investigate the role of miRNA-181a in different types of cancer to resolve the above conflicting findings.
Overall, the present study revealed that miR-181a was overexpressed in ESCC cancer cells, where it promoted EMT by inhibiting Smad4 via the TGF-β/Smad pathway. Consequently, miR-181a is a potential target for the treatment of ESCC cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RX, XMZ and YSL performed the experiments. RX, XMZ, YSL and LR performed the data analysis and data validation. RX and XRH prepared and wrote the original draft and reviewed and edited the manuscript. RX and XRH designed and conceived the study. XRH contributed experimental materials. All authors read and approved the final manuscript. RX and XRH confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the Ethics Review Committee of The Affiliated Hospital of North Sichuan Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Zuo T, Zeng H, Zhang S, He J. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.1007/978-3-319-42740-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Cui Y, Zhang L, Wang W, Ma S, Liu H, Zang X, Zhang Y, Guan F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/β-catenin pathway. Mol Cell Biochem. 2019;460:93–103. doi: 10.1007/s11010-019-03573-0. [DOI] [PubMed] [Google Scholar]
- 4.Zeng H, Zheng R, Zhang S, Zuo T, Xia C, Zou X, Chen W. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer. 2016;7:232–237. doi: 10.1111/1759-7714.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, Zhang J, Wu X, Shan B. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol Res. 2013;21:165–171. doi: 10.3727/096504014X13887748696662. [DOI] [PubMed] [Google Scholar]
- 7.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 8.Li YH, Li YS, Zhang RJ, Zhou XM, He XR. Expression of miR-181a-5p, miR-320a and TGF-β1 in esophageal squamous cell carcinoma. J Cancer Control Treat. 2019;32:395–401. [Google Scholar]
- 9.Shukla SK, Khatoon J, Prasad KN, Rai RP, Singh AK, Kumar S, Ghoshal UC, Krishnani N. Transforming growth factor beta 1 (TGF-β1) modulates Epstein-Barr virus reactivation in absence of Helicobacter pylori infection in patients with gastric cancer. Cytokine. 2016;77:176–179. doi: 10.1016/j.cyto.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Sun NF, Xue Y, Dai T, Li X. D. and Zheng NX: Tripartite motif containing 25 promotes proliferation and invasion of colorectal cancer cells through TGF-β signaling. Biosci Rep. 2017 Jul 12; doi: 10.1042/BSR20170805. (Epub ahead of print). doi: 10.1042/BSR20170805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H, Kim N, Park JH, Nam RH, Choi YJ, Park SM, Choi YJ, Yoon H, Shin CM, Lee DH. Helicobacter pylori might induce TGF-β1-mediated EMT by means of cagE. Helicobacter. 2015;20:438–448. doi: 10.1111/hel.12220. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu D, Yu Y, Qi Y, Wu K, Liu D, Yang Y, Zhang C, Zhao S. Long non-coding RNA CASC2 enhances the antitumor activity of cisplatin through suppressing the Akt pathway by inhibition of miR-181a in esophageal squamous cell carcinoma cells. Front Oncol. 2019;9:350. doi: 10.3389/fonc.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8:8. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang H, Ma D, Zhang J, Zhao J, Yang M. MicroRNA-18a induces epithelial-mesenchymal transition like cancer stem cell phenotype via regulating RKIP pathway in pancreatic cancer. Ann Transl Med. 2020;8:433. doi: 10.21037/atm.2020.03.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mi Y, Zhang D, Jiang W, Weng J, Zhou C, Huang K, Tang H, Yu Y, Liu X, Cui W, et al. miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett. 2017;389:11–22. doi: 10.1016/j.canlet.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Zhiping C, Shijun T, Linhui W, Yapei W, Lianxi Q, Qiang D. MiR-181a promotes epithelial to mesenchymal transition of prostate cancer cells by targeting TGIF2. Eur Rev Med Pharmacol Sci. 2017;21:4835–4843. [PubMed] [Google Scholar]
- 21.Li L, Xu QH, Dong YH, Li GX, Yang L, Wang LW, Li HY. MiR-181a upregulation is associated with epithelial-to-mesenchymal transition (EMT) and multidrug resistance (MDR) of ovarian cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:2004–2010. [PubMed] [Google Scholar]
- 22.Li H, Zhang P, Sun X, Sun Y, Shi C, Liu H, Liu X. MicroRNA-181a regulates epithelial-mesenchymal transition by targeting PTEN in drug-resistant lung adenocarcinoma cells. Int J Oncol. 2015;47:1379–1392. doi: 10.3892/ijo.2015.3144. [DOI] [PubMed] [Google Scholar]
- 23.Wang JJ, Yu JP. miR-181a down-regulates MAP2K1 to enhance adriamycin sensitivity in leukemia HL-60 cells. Eur Rev Med Pharmacol Sci. 2019;23:2497–2504. doi: 10.26355/eurrev_201903_17397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.