Abstract
BACKGROUND AND PURPOSE: Our hypothesis was that the carotid plaques associated with retinal and cerebrovascular symptomatology and asymptomatic presentation may be differ from each other. The aim of this study was to identify the sonographic and histopathologic characteristics of plaques that corresponded to these three clinical manifestations.
METHODS: The echo process involved duplex preoperative imaging of 71 plaques (67 patients, 21 plaques were associated with retinal, 25 with cerebrovascular symptoms, and 25 were asymptomatic), which was performed in a longitudinal fashion. Appropriate frames were captured and digitized via S-video signal in a computer and digitized sonograms were normalized by two echo-anatomic reference points: the gray scale median (GSM) of the blood and that of the adventitia. The GSM of the plaques was evaluated to distinguish dark (low-GSM) from bright (high-GSM) plaques. Subsequent to endarterectomy, the plaques were sectioned transversely, and a slice at the level of the largest plaque area was examined for the relative size of necrotic core and presence of calcification and hemorrhage.
RESULTS: Retinal symptomatology was associated with a hypoechoic plaque appearance (median GSM: 0), asymptomatic status with a hyperechoic plaque appearance (median GSM: 34), and cerebrovascular symptomatology with an intermediate plaque appearance (median GSM: 16) (P = .001). The histopathologic characteristics did not disclose differences between the three clinical groups. The hypoechoic plaque appearance was associated only with the presence of hemorrhage (median GSM for the hemorrhagic plaques, 6, and for the non-hemorrhagic ones, 20 [P = .04]). The relative necrotic core size and the presence of calcification did not show any echomorphologic predilection.
CONCLUSION: Our results showed that distinct echomorphologic characteristics of plaques were associated with retinal and cerebrovascular symptomatology and asymptomatic status. Histopathologically, only the presence of hemorrhage proved to have an echomorphologic predilection.
An overview analysis revealed that the annual rate of subsequent stroke was 1.3% in asymptomatic patients, 2.2% in patients with amaurosis fugax (AF), 3.7% in those with transient ischemic attack (TIA), 6.1% in patients with minor stroke, and 9% in patients with major stroke (1). These findings suggest that a hierarchical profile of worsening clinical characteristics is running in parallel with a hierarchical progression of increasing risk of subsequent stroke. In addition, this observation allows us to put forward a hypothesis that, in the presence of a carotid atheroma, different plaque characteristics might be associated with various clinical manifestations (ie, asymptomatic status, AF, TIA, and stroke).
The aim of this study was to determine in a cross-sectional investigation the attributes of carotid plaques in terms of sonographic and histopathologic characteristics that corresponded to the various neurovascular presentations (asymptomatic status, retinal symptoms [amaurosis fugax, AF], cerebrovascular symptoms [hemispheric TIA, stroke]).
Methods
Materials Used and Study Design
Seventy-one carotid bifurcation plaques (67 patients, 49 men and 18 women; mean age, 70.74 years; range, 50–86 years) were studied. Duplex imaging revealed 65% to 95% stenosis. Twenty-five (35.2%) plaques were associated with an ipsilateral asymptomatic presentation, 21 (29.6%) with AF, 19 (26.8%) with hemispheric TIA, and six (8.5%) with stroke. The inclusion criterion was the eligibility of the patient for carotid endarterectomy. Asymptomatic patients were considered candidates for carotid endarterectomy if the stenosis was greater than 60%, and a disorder that could seriously complicate surgery was absent (2).
Each plaque was treated as an independent case (unit of the study) and defined the side of interest on each patient. Sixty-three patients presented with unilateral and four patients with bilateral carotid atheromata that could be subjected to analysis.
The sonographic characteristics and stenosis of the carotid plaques under study were evaluated at presentation. The assignment of symptoms was performed by a neurologist who was unaware of the previous clinical reports and duplex findings and was in accordance with the recommendations of the Committee for the Classification of Cerebrovascular Disease III (3). Only neurovascular symptoms on the side of interest, which had occurred within the last 3 months prior to recruitment to the study, were noted. Patients with symptoms on the appropriate side prior to the 3rd month from recruitment were excluded. Asymptomatic patients were considered those who had never experienced a symptom on the side of interest.
Patients with cardioembolic conditions established by clinical and electrocardiographic evaluation (ie, atrial fibrillation, aortic or mitral valve disease, recent myocardial infarction occurring less than 6 weeks from the neurovascular event, prosthetic cardiac valves, and heart failure) were excluded by a cardiologist to implicate the carotid lesions under study as the most likely cause of their cerebrovascular symptoms. Electrocardiograms were part of the routine clinical investigation of patients in our hospital. Lacunar symptomatology, diagnosed by a neurologist based on clinical and brain CT examination, was an additional exclusion criterion, because this condition is not associated with carotid disease.
All patients underwent a brain CT scan upon recruitment, and all ischemic or hemorrhagic infarctions on the side of interest were noted by a neuroradiologist. Patients with cerebral hematomas were excluded, because this condition is not associated with carotid disease. CT scanning is part of the routine clinical investigation of patients with carotid atheromata at our hospital.
Sonographic Grading of Carotid Stenosis
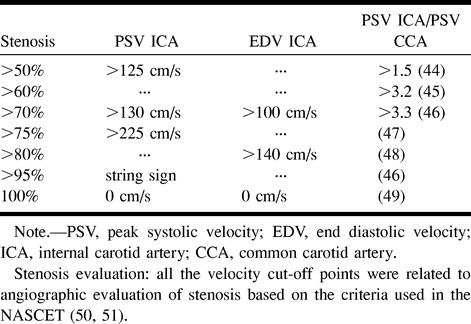
The severity of carotid stenosis was assessed by duplex sonography upon recruitment by use of the ATL HDI 3000 (Advanced Technology Laboratories, Bothell, WA) scanner. This entailed a hemodynamic evaluation of the index stenotic vessel based on standard criteria (Table). Imaging was performed by one well-trained operator (TJT) who was unaware of the clinical profile of the patients at the time of examination.
Grading of carotid stenosis (references 44–51)
Sonographic Characteristics of Carotid Plaques
In order to compare the sonographic characteristics of carotid plaques, certain standards were kept during all the phases of the echo analysis: 1) duplex scanning, 2) image capturing to a computer, 3) digitization, 4) normalization, and 5) analysis of echomorphologic characteristics. This echo process was performed at presentation by the same well-trained operator (TJT) who also evaluated the degree of stenosis and was unaware of the clinical profile of the patients. This ensured uniformity and an unbiased approach in the analysis.
Sonographic Scan Settings of Carotid Plaque Images
The plaques were scanned on an ATL HDI 3000 sonographic device in longitudinal, anterolateral projection at a minimal depth. All patients were scanned from the side, in the supine position, without head tilt. Transcutaneous real-time gray scale mode (real-time b mode) was used with a 7-MHz linear probe, at a medium frame rate, TGC (time gain compensation) vertical to the blood vessel, linear postprocessing curve, minimal persistence, and maximum dynamic range (60 dB).
The gain, the magnification, and the exact angle of the projection under study, ie, the three subjective parameters in the scan settings, were adjusted in such a way that in the selected static frames of the sonographic images of the carotid plaques, the blood, adventitia, and plaque were fulfilling certain criteria. These were: 1) the blood, in the vicinity of the plaques, was dark and echoically uniform, 2) the adventitia, again in the vicinity of the plaques, was thick, horizontal, bright, and echoically uniform (ie, homogeneous, with no variation of brightness from area to area), and 3) the atherosclerotic plaque was well-delineated, horizontal, and most informative in terms of echoic characteristics and with maximum thickness. Only frames fulfilling these criteria were considered appropriate for analysis. Plaque imaged failing to fulfil the aforementioned criteria (especially those related to the adventitia) were excluded from the analysis.
In the case of poor luminal-edge visualization, especially in hypoechoic (dark) plaques, color images in parallel to the b-mode (gray scale) images were studied to assist in the delineation of their luminal margin (the color images were frozen on the screen and an automatic switch-off of the color provided by the ATL HDI 3000 yielded the b-mode images on the same plane).
One inherent problem with the delineation of the luminal edge of the plaque by means of color centered on the fact that the laminar flow could be so slow along the arterial wall, such that filtration could remove the color from the image in many luminal areas, giving the impression of thrombus. This problem, however, has been eliminated to a great extent in the new digital systems, such as the ATL HDI 3000, by lowering the threshold for displaying the gray scale information, giving priority to the writing color information.
Capturing Settings of the Sonographic Images of Carotid Plaques to a Computer
The appropriate frames were captured via an S-video signal to a computer (Dell Dimension XPS P90), where they were digitized. The capturing device used was a SCREEN MACHINE II version 1.1 (Fast Multimedia AG). The settings of this device (source settings, display settings, effect settings, color settings, and capturing resolution [736 × 560 pixels]) were fixed during the capture of this material. All b-mode images were digitized in a computer in GIF format.
Normalization of the Digital Sonographic Images of Carotid Plaques
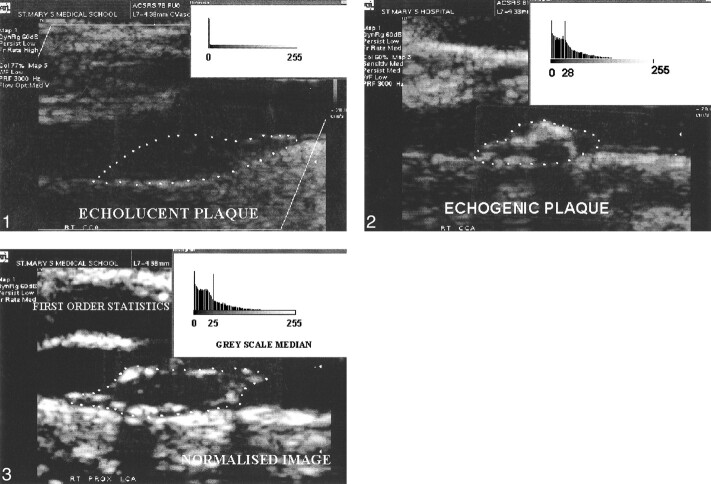
All digitized sonographic plaque images were normalized in a computer by using two echo-anatomic reference points: the GSM (gray scale median) of the blood and the GSM of the adventitia. The software used was the commercially available and user-friendly Adobe Photoshop (Adobe Systems Inc., version 3). GSM was a feature of the First Order Statistics (4) and represented the median of the frequency distribution of gray tones of the pixels included in the region of interest (median echo level, GSM of the region) in a scale of 256 gray tones (0 = darkest tone; 255 = brightest tone). This feature has been used as an index to describe the overall brightness of the region of interest. Dark (hypoechoic) regions were associated with a GSM that tended to approach 0, whereas bright (hyperechoic) regions were associated with a GSM that tended to approach 255 (Figs 1 and 2).
fig 1.
Hypoechoic (echolucent) plaque (GSM = 0).fig 2. Hyperechoic (echogenic) plaque (GSM = 28).fig 3. Normalized image of a carotid plaque (GSM = 25)
Normalization was a gray scale transformation achieved using linear scaling in a range of gray tones from 0 to 255. This process modified all images in a way that, in the resultant normalized images, the GSM of the blood was in the range of 0–5 tones, and the GSM of the adventitia was in the range of 180–200 tones. Previous studies performed in our laboratory (5, 6) demonstrated an excellent interobserver variability of this method and of the evaluation of the plaque echomorphologic characteristics by means of GSM.
The aim of normalization was to make feasible the comparison of the echomorphologic characteristics of all digital carotid plaque images. This was achieved by eliminating the operator-dependent, sonographic gain-induced variability of the echomorphologic characteristics of carotid plaques that has been produced during the operator's attempt to acquire the best images for echo analysis.
Sonographic Characteristics of the Normalized Images of Carotid Plaques
Sonographic characteristics of the normalized images of carotid plaques were determined by the GSM (Fig 3).
Measurement of the Sonographic Plaque Area
These were performed by means of the Image-Pro Plus software for Windows (version 1.2.01, Media Cybernetics). For the calibration, predetermined segments of 1 cm in the horizontal and vertical axes within the image were used. These were produced during the scanning process.
Histopathologic Characteristics of Carotid Plaques
Seventy-one carotid endarterectomy specimens were entered into the study. The time range between the onset of cerebrovascular symptoms and the operation was 3 months. Inclusion criteria demanded the ability of the vascular surgeon to remove the plaque en bloc, without fragmentation or significant distortion. No attempts were made to evaluate the presence and the degree of surface ulceration or thrombus. After endarterectomy was done, the fresh specimens were rinsed briefly in normal saline solution to remove the surface blood and were immersed in 10% formalin fixative and subsequently in a decalcifying solution (formic acid). The plaques were partly decalcified in order to be sectioned subsequently. The specimens were sectioned transversely with a disposable microtome knife at 4-mm intervals by one blinded histopathologist (MS), and the blocks were numbered in sequence, starting at the proximal end. For most of the specimens, five to six blocks were available.
Each block was processed through paraffin, sectioned at 5 μm, and then three slices per block at its proximal end were stained in sequence with Hematoxylin and Eosin (H&E), Martius scarlet blue, and Perls stain. All the sections were examined for the presence of atheroma, necrotic core, hemorrhage, fibrosis, calcification, and thrombosis. Images of H&E sections were captured from a microscope (Olympus BX50) to a computer (Dell Dimension XPS P90) via an S-video signal where they were digitized. For the evaluation of the relative necrotic core, the minimum magnification was used (×2 objective), whereas higher ones were used for hemorrhage and calcification (×10–×20 objective). The capturing device that has been used was an Image-Pro Plus, version 1.2.01 for Windows (Media Cybernetics). The settings of this device were fixed during the capture of this material. All images were digitized in a computer in TIF format. For the relative area evaluation, a graticule of 1 mm in horizontal and perpendicular direction was used (Fisher Scientific, UK).
Digital sections were examined to assess the following histologic features at each level: relative size of necrotic core (size of the necrotic core over the size of the plaque), presence of hemorrhage, and calcification. These features were evaluated at each level, but they were more marked at the level of the largest plaque area, which frequently corresponded to the level of the maximal stenosis. This study focused on this particular level. Morphometric analysis of the other levels will be addressed in future studies.
The necrotic core was usually located at the deeper regions of the plaque and consisted of cholesterol clefts and amorphous material without any viable cells or admixed collagen (Fig 4). Calcifications were manifested as dark blue, sharply demarcated regions devoid of cells in the H&E stains (Fig 4).
fig 4.
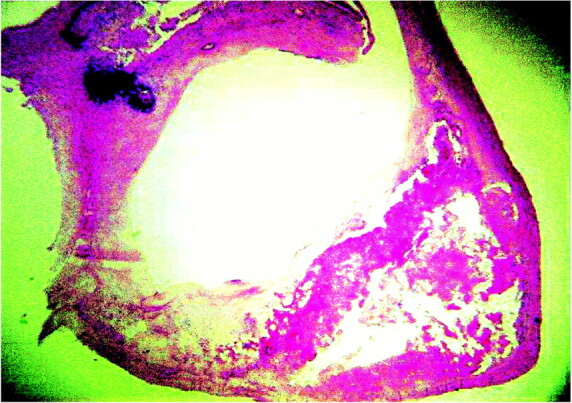
Microscopic appearance of a carotid plaque with necrotic core and calcification (H&E stain)
Intraplaque hemorrhage appeared as debris containing degenerated red blood cells as well as macrophage engulfment of hemosiderin and giant cell development. These areas are different from the hemorrhage produced by surgical manipulation, in which intact red cells are seen, and certainly is not responsible for the present cerebrovascular symptom. For the purposes of this study, only areas of hemorrhage or calcification occupying at least 30% of the plaque section, as calculated in the Image-Pro Plus software, were taken into account.
Results
Three groups were considered for analysis: plaques associated with retinal symptoms (n = 21, 29.5%), plaques associated with cerebrovascular symptoms (n = 25, 35.2%), and plaques of asymptomatic status (25, 35.2%).
The one-sample Kolmogorov-Smirnov test was performed on the distribution of the systolic blood pressure of the patients and demonstrated abnormality (P = .001). This result dictated the use of a non-parametric test in the analysis of the systolic blood pressure.
The median systolic blood pressure in our material was 140 mm Hg (maximum, 200 mm Hg; minimum, 115 mm Hg). The distributions of the systolic blood pressure corresponding to the three clinical groups (AF, TIA/stroke, asymptomatic) were similar (median value for AF, 140 mm Hg; TIA/ stroke, 140 mm Hg; asymptomatic status, 140 mm Hg; Kruskal-Wallis test, P = .9).
For the delineation of the sonographic luminal margin of the plaques, color Doppler was needed in 51/71 (71.8%) plaques. In terms of the plaque area offered for echomorphologic analysis, 64 (90.1%) plaques could be fully analyzed (100%), three (4.2%) could be analyzed by 80%, one (1.4%) could be analyzed by 70%, and three (4.2%) could be analyzed by 50%. The relative area offered for the sonographic analysis was calculated in the Image-Pro Plus program (version 1.2.01).
The one-sample Kolmogorov-Smirnov test was performed on the distribution of the sonographic plaque area (longitudinal view, mm2) and demonstrated normality (P = .47). This result dictated the use of a parametric test in the analysis of the sonographic plaque area.
The mean sonographic plaque area in our population was 82.84 mm2 (maximum, 369 mm2; minimum, 10 mm2). The distributions of the sonographic plaque area corresponding to the three clinical groups (AF, TIA/stroke, asymptomatic) were similar (mean values for AF, 94.95 mm2; TIA/stroke, 70.9 mm2; asymptomatic, 84.6 mm2) (one-way analysis of variance, P = .27)
The one-sample Kolmogorov-Smirnov test was performed on the distribution of GSM and demonstrated abnormality (P < .05). This result dictated the use of a non-parametric test in the analysis of GSM.
The median GSM in our population was 11 (maximum, 98; minimum, 0). Comparison of the distributions of GSM corresponding to retinal, cerebrovascular, and asymptomatic groups demonstrated that these groups were distinct in terms of the GSM (Kruskal-Wallis test, P = .001). The corresponding median GSMs were: 0 for the retinal group, 16 for the cerebrovascular group, and 34 for the asymptomatic group.
The one-sample Kolmogorov-Smirnov test was performed on the distribution of stenosis and demonstrated abnormality (P = .0001). This result dictated the use of a non-parametric test in the analysis of stenosis.
The median stenosis in our material was 90% (maximum, 95%; minimum, 65%). The distributions of the stenosis corresponding to the three clinical groups (AF, TIA/stroke, asymptomatic) were similar (median value for AF, 90%; TIA/ stroke, 90%; asymptomatic status, 83%; Kruskal-Wallis test, P = .34).
The one-sample Kolmogorov-Smirnov test was performed on the distribution of the histologic plaque area at the level of its largest transverse area (mm2) and demonstrated normality (P = .106). This result dictated the use of a parametric test in the analysis of the histologic plaque area.
The mean histologic plaque area in our population was 42.8 mm2 (maximum, 122 mm2; minimum, 14 mm2). The distributions of the histologic plaque area corresponding to the three clinical groups (AF, TIA/stroke, asymptomatic) were similar (mean values for AF, 49.8 mm2; TIA/stroke, 37.8 mm2; asymptomatic, 41.92 mm2) (one-way analysis of variance, P = .115).
The one-sample Kolmogorov-Smirnov test was performed on the distribution of the relative necrotic core size and demonstrated normality (P > .05). This result dictated the use of a parametric test for the analysis of the relative necrotic core size.
The mean relative necrotic core size in our population was 0.26 (maximum, 0.71; minimum, 0). Comparison of the distributions of relative necrotic core size corresponding to retinal, cerebrovascular, and asymptomatic groups demonstrated a trend of separation among them regarding relative necrotic core size, but this did not attain statistical significance (one-way analysis of variance, P = .38). The corresponding mean relative necrotic core sizes were: 0.32 for the retinal group, 0.26 for the cerebrovascular group, and 0.22 for the asymptomatic group.
The prevalence of calcification in our material was 76.1%. In a more detailed analysis, this prevalence was 13/21 (61.9%) for the retinal group, 19/25 (76%) for the cerebrovascular group, and 22/25 (88%) for the asymptomatic group (χ2 test, P = .13). The prevalence of hemorrhage in the examined material was 47.9%. In a more detailed analysis, this prevalence was 12/21 (57.1%) for the retinal group, 19/25 (44%) for the cerebrovascular group, and 19/25 (44%) for the asymptomatic group (χ2 test, P = .6).
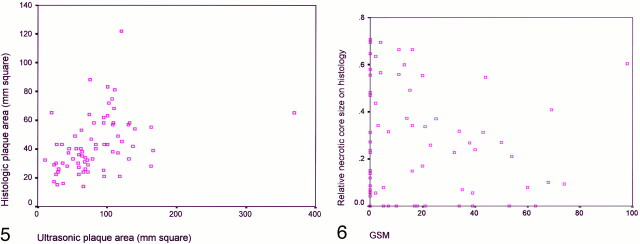
The sonographic and histologic area of the plaques were related (Pearson's correlation, P = .001, r = 0.386) (Fig 5)
fig 5.
Correlation of sonographic (longitudinal) and histologic (transverse) plaque area (Pearson's correlation, P = .001, r = 0.386).fig 6. Correlation of GSM and relative necrotic core size (Pearson's correlation, P = 0.37, r = −0.1)
Further analysis demonstrated that the GSM and relative necrotic core size were unrelated (Pearson's correlation, P = .37, r = 0.1) (Fig 6). In addition, the median GSMs were almost equal in the calcified and non-calcified plaques (nine for the calcified plaques and 11 for the non-calcified plaques, Mann-Whitney U test, P = .65). Interestingly, the median GSM was lower in the hemorrhagic than in the non-hemorrhagic plaques (six for the hemorrhagic plaques and 20 for the non-hemorrhagic ones, Mann-Whitney U test, P = .04).
Discussion
It has previously been shown that stroke, hemispheric TIA, and AF are predictors of a subsequent stroke in a descending order of likelihood (stroke was a strong predictor, AF was a weak predictor) (7, 8). In addition, a neurovascular symptom (AF or hemispheric TIA or non-disabling stroke) and asymptomatic status are predictors of a subsequent stroke in a descending order of likelihood, as suggested by the medical arms of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (9) and the Asymptomatic Carotid Atherosclerosis Study (ACAS) (2) (26% cumulative risk of ipsilateral stroke at 2 years in NASCET versus 6.3% of stroke risk at 2.7 years in ACAS).
Based on the distinct rates of the incident stroke conferred by the various clinical types of the previous symptomatology (asymptomatic status, retinal symptoms, cerebrovascular symptoms), a hypothesis that different carotid plaque characteristics were associated with each of these clinical types was put forward. The present study was conducted with the goal of recognizing these characteristics from the echomorphologic and histopathologic point of view.
The sonographic characteristics of carotid plaques have been the subject of much research interest in recent years. These characteristics have been classified into those that describe the overall distribution of gray tones (overall brightness), and those that describe the spatial variation of gray tones of the plaque in the image (10). Based on the former classification, plaques can be designated as either “echolucent,” (anechoic, hypoechoic), corresponding to dark plaques on sonograms or as “echogenic” (hyperechoic), corresponding to bright plaques on sonograms. Based on the latter classification, plaques can also be designated as either “homogeneous,” corresponding to a uniform echo pattern or “heterogeneous,” corresponding to a non-uniform echo pattern (11).
Our results have shown that retinal symptomatology was associated with hypoechoic plaque appearance, asymptomatic status with hyperechoic plaque appearance, and cerebrovascular symptomatology with intermediate echoic results. The systolic blood pressure, degree of stenosis, sonographic and histologic plaque area, relative necrotic core size, presence of calcification, and hemorrhage failed to reveal a difference between the three clinical groups (AF, TIA/stroke, asymptomatic).
One study has established an inverse correlation between the carotid plaque GSM and the number of emboli detected in the ipsilateral middle cerebral artery on transcranial Doppler sonograms, suggesting that the hypoechoic plaques are embologenic and the hyperechoic ones non-embologenic (12). This might explain the association of AF with the hypoechoic plaques and the relationship between asymptomatic status and hyperechoic plaques, as demonstrated in our study.
The TIA/stroke group was associated with an intermediate echoic plaque appearance. This group might also be of embolic origin, but probably it constitutes a heterogeneous group with other factors influencing its development besides the plaque echomorphology, ie, intracranial stenosis and impaired cerebral collateral supply. It seems that the emboli are more deleterious to the brain in the presence of a low cerebral flow state as compared to a normal state.
The NASCET collaborators (13) and others (14–16) demonstrated that the prevalence of intracranial stenosis in patients with stroke, hemispheric TIA, and AF followed a descending order (higher in stroke, lower in AF). In addition, patients with neurovascular symptoms were associated with a lower cerebral vasomotor response (an index of cerebrovascular collaterality) on transcranial Doppler sonography, xenon CT, and positron emission tomography scanning as compared to the asymptomatic ones (17–26). Interestingly, cerebrovascular reactivity was impaired to a greater extent in patients with stroke than in those with hemispheric TIA or AF (27, 28). Therefore, the higher prevalence of intracranial lesions or impaired cerebral vasomotor reactivity in patients with cerebrovascular symptoms (stroke, hemispheric TIA) than in the ones with retinal symptoms (AF) or asymptomatic status seems to be a crucial pathogenetic factor in the former (stroke and TIA patients), making the dependence of their symptoms on carotid plaques relatively less strong.
As to the plaque echomorphology, studies similar to ours conducted by other investigators reached the same results. Holdsworth et al (29) demonstrated that the majority of plaques associated with AF were hypoechoic, and the majority of plaques associated with hemispheric TIA, stroke, and an asymptomatic status were hyperechoic (29). The same author demonstrated that an almost equal proportion of echogenic plaques were associated with hemispheric TIA, stroke, and asymptomatic status (29). The criticism attached to the previous study, as contrasted with ours, rested on the inherent lack of objectivity of the visual evaluation of the plaque echo pattern. Our study was computer-based and therefore more reliable.
Systolic blood pressure failed to show differences between the three clinical groups (AF, TIA/stroke, asymptomatic). The reason might be the exclusion of the lacunar symptomatology from our material, which is associated with high blood pressure (30).
In addition, the sonographic and histologic plaque area and the degree of stenosis failed to separate the three clinical groups, emphasizing the importance of the plaque composition over the plaque size for the development of a symptom.
In our study, there was a tendency for the AF group to be associated with a larger relative necrotic core, absence of calcification, and presence of hemorrhage as compared to the TIA/stroke and asymptomatic groups, but this did not attain statistical significance. Future studies with larger samples of carotid plaques are needed to verify these tendencies fully.
In a similar study, Fisher and Ojemann (31) demonstrated that intraplaque hemorrhage was more prevalent in plaques associated with AF as compared to those with TIA. Plaques with stroke and asymptomatic status were associated with the lowest prevalence of hemorrhage.
Our results also demonstrated that the GSM was unrelated to the relative necrotic core size. In addition, the GSM was unable to distinguish the calcified from the non-calcified plaques but was adequate to separate the hemorrhagic from the non-hemorrhagic ones. This suggests that only the hemorrhage and not the lipid core or the calcification might be an echomorphologic determinant.
Other studies have shown that the prevalence or the size of the lipid core was higher in hypoechoic or heterogeneous plaques (32–36), hemorrhage was more prevalent in the hypoechoic or heterogeneous plaques (37–41), and calcification was more prevalent in hyperechoic plaques (42, 43).
Conclusion
In summary, the results of the current study demonstrated that retinal symptomatology was associated with hypoechoic plaque appearance, asymptomatic status with hyperechoic plaque appearance, and cerebrovascular symptomatology with intermediate echoic results. In addition, it has been shown that only hemorrhage was associated with hypoechoic appearance. The large lipid core or the presence of calcification did not show any echomorphologic predilection.
References
- 1.Wilterdink JL, Easton D. Vascular event rates in patients with atherosclerotic cerebrovascular disease. Arch Neurol 1992;49:857-863 [DOI] [PubMed] [Google Scholar]
- 2. Endarterectomy for asymptomatic carotid artery stenosis:. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study JAMA 1995;273:1421-1428 [PubMed] [Google Scholar]
- 3. Special report from the National Institute of the Neurological Disorders and Stroke: Classification of Cerebrovascular Disease III. Stroke 1990;21:637-676 [DOI] [PubMed] [Google Scholar]
- 4.Qin W. Automatic tumor detection for MRI liver images:. PhD thesis, Imperial College, London, UK 1996, p 48
- 5.Elatrozy T, Nicolaides A, Tegos T, Zarka AZ, Griffin M, Sabetai M. The effect of image standardisation on the echodensity of symptomatic and asymptomatic carotid bifurcation plaques. Int Angiol 1998;17:179-186 [PubMed] [Google Scholar]
- 6.Tegos TJ, Sabetai MM, Nicolaides AN, Pare G, Elatrozy TS, Dhanjil S, Griffin M. Comparability of the ultrasonic tissue characteristics of carotid plaques. J Ultrasound Med 2000; (in press) [DOI] [PubMed]
- 7. The Dutch TIA trial study group. Predictors of major vascular events in patients with a transient ischemic attack or nondisabling stroke. Stroke 1993;24:527-531 [DOI] [PubMed] [Google Scholar]
- 8.Dennis MS, Bamford JM, Sandercock PAG, Warlow CP. A comparison of risk factors and prognosis for transient ischemic attacks and minor ischemic strokes. Stroke 1989;20:1494-1499 [DOI] [PubMed] [Google Scholar]
- 9. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. N Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 10.Raeth U, Schlaps D, Limberg B, et al. Diagnostic accuracy of computerised B-scan texture analysis and conventional ultrasonography in diffuse parenchymal and malignant liver disease. J Clin Ultrasound 1985;13:87-99 [DOI] [PubMed] [Google Scholar]
- 11.De Bray JM, Baud JM, Dauzat M. Consensus Concerning the Morphology and the Risk of Carotid Plaques. Cerebrovasc Dis 1997;7:289-296 [Google Scholar]
- 12.Tegos TJ, Sabetai MM, Nicolaides AN, Robless P, Kalodiki E, Elatrozy TS, Ramaswami G, Dhanjil S. Correlates of embolic events detected on transcranial Doppler in patients with carotid atheroma. J Vasc Surg 2000; (in press) [DOI] [PubMed]
- 13.Kappelle LJ, Eliasziw M, Fox AJ, Sharpe BL, Barnett HJM. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. Stroke 1999;30:282-286 [DOI] [PubMed] [Google Scholar]
- 14.Thiele BL, Young JV, Chikos PM, Hirsch JH, Strandness DE. Correlation of arteriographic findings and symptoms in cerebrovascular disease. Neurology 1980;30:1041-1046 [DOI] [PubMed] [Google Scholar]
- 15.Harrison MJG, Marshall J. Arteriographic comparison of amaurosis fugax and hemispheric transient ischemic attacks. Stroke 1985;16:795-797 [DOI] [PubMed] [Google Scholar]
- 16.Pessin MS, Dunkan GW, Mohr JP, Poskanzer DC. Clinical and angiographic features of carotid transient ischemic attacks. N Engl J Med 1977;296:358-362 [DOI] [PubMed] [Google Scholar]
- 17.Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Non-invasive assessment of CO2 vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 1988;19:963-969 [DOI] [PubMed] [Google Scholar]
- 18.Silvestrini M, Troisi E, Matteis M, Cupini LM, Caltagirone C. Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke 1996;27:1970-1973 [DOI] [PubMed] [Google Scholar]
- 19.Derdeyn CP, Yundt KD, Videen TO, Carpenter DA, Crubb RL, Powers WJ. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 1998;29:754-758 [DOI] [PubMed] [Google Scholar]
- 20.Brown MM, Wade JPH, Bishop CCR, Ross Russell RW. Reactivity of cerebral circulation in patients with carotid occlusion. J Neurol Neurosurg Psychiatry 1986;49:899-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke 1992;23:171-174 [DOI] [PubMed] [Google Scholar]
- 22.Gur AL, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients. Stroke 1996;27:2188-2190 [DOI] [PubMed] [Google Scholar]
- 23.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 1999;30:593-598 [DOI] [PubMed] [Google Scholar]
- 24.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483-489 [DOI] [PubMed] [Google Scholar]
- 25.Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid occlusive disease. J Vasc Surg 1995;21:338-345 [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi H, Fukuyama H, Nagahama H, et al. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive disease from PET. J Neurol Neurosurg Psychiatry 1996;61:18-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor AR, Merrick MV, Gillespie I, et al. Prevalence of impaired cerebrovascular reserve in patients with symptomatic carotid artery disease. Br J Surg 1994;81:45-48 [DOI] [PubMed] [Google Scholar]
- 28.Bullock R, Mendelow AD, Bone I, Patterson J, Macleod WN, Allardice G. Cerebral blood flow and CO2 responsiveness as an indicator of collateral reserve capacity in patients with carotid arterial disease. Br J Surg 1985;72:348-351 [DOI] [PubMed] [Google Scholar]
- 29.Holdsworth RJ, McCollum PT, Bryce JS, Harrison DK. Symptoms, stenosis and carotid plaque morphology. Is plaque morphology relevant. Eur J Vasc Endovasc Surg 1995;9:80-85 [DOI] [PubMed] [Google Scholar]
- 30.Fisher CM. Lacunar infarcts-a review. Cerebrovasc Dis 1991;1:311-320 [Google Scholar]
- 31.Fisher CM, Ojemann RG. Clinico-pathologic study of carotid endarterectomy plaques. Rev Neurol 1986;142:573-589 [PubMed] [Google Scholar]
- 32.Gronholdt MLM, Wiebe BM, Laursen H, Nielsen TG, Schroeder TV, Sillesen H. Lipid- rich carotid artery plaques appear echolucent on ultrasound B- mode images and maybe associated with intraplaque hemorrhage. Eur J Vasc Endovasc Surg 1997;14:439-445 [DOI] [PubMed] [Google Scholar]
- 33.Kagawa R, Moritake K, Shima T, Onada Y. Validity of B- mode ultrasonographic findings in patients undergoing carotid endarterectomy in comparison with angiographic and clincopathologic features. Stroke 1996;27:700-705 [DOI] [PubMed] [Google Scholar]
- 34.Hatsukami TS, Thackray BD, Primozich JF, et al. Echolucent regions in carotid plaque: preliminary analysis comparing three- dimensional histologic reconstructions to sonographic findings. Ultrasound Med Biol 1994;20:8:743-749 [DOI] [PubMed] [Google Scholar]
- 35.El-Barghouty , Levine T, Ladva S, Flanagan A, Nicolaides AN. Histological verification of computerised carotid plaque characterisation. Eur J Vasc Endovasc Surg 1996;11:414-416 [DOI] [PubMed] [Google Scholar]
- 36.Gronholdt MLM, Nordrstgaard BG, Wiebe BM, Wilhjem JE, Sillesen H. Echo-lucency of computerised ultrsound images of carotid atherosclerotic plaques are associated with increased levels of triglucerides-rich lipoproteins as well a increased plaque lipid content. Circulation 1998;97:34-40 [DOI] [PubMed] [Google Scholar]
- 37.Van Dame H, Vivario M. Pathological aspects of carotid plaques. surgical and clinical significance. Int Angiol 1993;12:4:299-311 [PubMed] [Google Scholar]
- 38.Van Dame H, Trotteur G, Vivario M, Limet R. Echographic characterization of carotid plaques. Acta Chir Belg 1993;93:233-238 [PubMed] [Google Scholar]
- 39.Kardoulas DG, Kathamouris AN, Gallis PT, et al. Ultrasonographic and histologic characteristics of symptom- free and symptomatic carotid plaque. Cardiovasc Surg 1996;4:580-590 [DOI] [PubMed] [Google Scholar]
- 40.Weinberger J, Marks SJ, Gaul JJ, et al. Atherosclerotic plaque at the carotid artery bifurcation. J Ultrasound Med 1987;6:363-366 [DOI] [PubMed] [Google Scholar]
- 41.Bluth EI, Kay D, Merritt CRB, Sullivan M, et al. Sonographic characterization of carotid plaque: Detection of hemorrhage. AJNR Am J Neuroradiol 1986;146:1061-1065 [DOI] [PubMed] [Google Scholar]
- 42. European Carotid Plaque Study Group. Carotid artery plaque composition- relationship to clinical presentation and ultrasound B- mode imaging. Eur J Vasc Endovasc Surg 1995;10:23-30 [DOI] [PubMed] [Google Scholar]
- 43.Ratliff DA, Gallagher PJ, Hames TK, et al. Characterisation of carotid artery disease: comparison of duplex scanning with histology. Ultrasound Med Biol 1985;11:6:835-840 [DOI] [PubMed] [Google Scholar]
- 44.Polak JF, Dopkin GR, O'Leary DH, Wang AM, Cutler SS. Internal carotid artery stenosis. accuracy and reproducibility of color-Doppler-assisted imaging. Radiology 1989;173:793-798 [DOI] [PubMed] [Google Scholar]
- 45.Moneta GL, Edwards JM, Papanicolaou G, et al. Screening for asymptomatic carotid artery stenosis: Duplex criteria for discriminating 60% to 99% stenosis. J Vasc Surg 1995;21:989-994 [DOI] [PubMed] [Google Scholar]
- 46.Hood DB, Mattos MA, Mansour A, et al. Prospective evaluation of new duplex criteria to identify 70% internal carotid artery stenosis. J Vasc Surg 1996;23:254-262 [DOI] [PubMed] [Google Scholar]
- 47.Withers CE, Gosink BB, Keighhtley AM, et al. Duplex carotid sonography. Peak systolic velocity in quantifying internal carotid artery stenosis. J Ultrasound Med 1990;9:345-349 [DOI] [PubMed] [Google Scholar]
- 48.Moneta GL, Taylor DC, Zierler RE, Kazmers A, Beach K, Strandness DE. Asymptomatic high grade internal carotid artery stenosis: is stratification according to risk factors or duplex spectral analysis possible. J Vasc Surg 1989;10:475-483 [DOI] [PubMed] [Google Scholar]
- 49.Robinson ML, Sacks D, Perlmutter GS, Marinelli DL. Diagnostic criteria for carotid Duplex sonography. AJR Am J Roentgenol 1988;151:1045-1048 [DOI] [PubMed] [Google Scholar]
- 50.Fox JF:, How to measure carotid stenosis. Radiology 1993;186:316-318 [DOI] [PubMed] [Google Scholar]
- 51.Eliasziw M, Fox AJ, Sharpe BL, Barnett HJM. Carotid artery stenosis: external validity of the North American Carotid Endarterectomy Trial Measurement Method. Radiology 1997;204:229-233 [DOI] [PubMed] [Google Scholar]