Abstract
BACKGROUD AND PURPOSE: The choline (Cho)/creatine (Cr) ratio has been shown to be a reliable proton MR spectroscopy metabolic marker for differentiating squamous cell carcinoma (SCCA) from normal muscle in the upper aerodigestive tract. However, it is unclear whether the Cho/Cr ratio can be used to differentiate a malignant tumor from a benign neoplasm in the extracranial head and neck. Our purpose was to determine whether the Cho/Cr ratio can be used to differentiate benign from malignant tumors in this region.
METHODS: In vitro one-dimensional proton MR spectroscopy (2000/136,272 [TR/TE]) was performed at 11 T on tissue specimens obtained from glomus tumors (n = 3), inverting papilloma (n = 1), and schwannoma (n = 1). Cho/Cr area ratios were calculated and compared with similar, previously reported in vitro (11 T) findings and with samples of SCCA and normal muscle.
RESULTS: The Cho/Cr ratio was elevated in relation to muscle in all benign tumors at TE = 136 (glomus tumors = 4.52, inverting papilloma = 3.85, schwannoma = 2.2) and at TE = 272 (glomus tumors = 8.01, inverting papilloma = 2.1, schwannoma = 4.28). The average Cho/Cr ratio for benign lesions was 3.92 (TE = 136) and 6.11 (TE = 272). The Cho/Cr ratio was significantly higher in benign tumors than in both SCCA and muscle. The average Cho/Cr ratio for muscle at TEs of 136 and 272 was 1.16 and 1.31, respectively, whereas for SCCA the average Cho/Cr ratio at TEs of 136 and 272 was 1.67 and 2.45, respectively.
CONCLUSION: In our small group, the Cho/Cr ratio was significantly higher in benign tumors than in muscle and SCCA of the extracranial head and neck.
Proton MR spectroscopy provides a noninvasive method for evaluating some metabolic components of various diseases of the extracranial head and neck. Because this technique measures the presence of specific metabolites, it is independent of anatomic information and may be used to characterize lesions that are indeterminate on standard anatomic studies.
Recently, proton MR spectroscopy has been found capable of distinguishing malignant tumors of the extracranial head and neck from uninvolved muscle on the basis of the choline (Cho)/creatine (Cr) ratio (1, 2). Elevation of the Cho/Cr ratio is significantly higher in squamous cell carcinoma (SCCA) than in muscle (3); however, the Cho/Cr ratio of benign tumors of the extracranial neck have not been described. Information regarding the likelihood of malignancy may be helpful in diagnosing impalpable tumors with ambiguous imaging characteristics located in the parapharyngeal space or adjacent to the skull base; moreover, such information would directly affect the surgical approach and resection of these tumors. The intent of this study was to determine whether the Cho/Cr ratio can be used to differentiate benign from malignant tumors arising in the extracranial head and neck.
Methods
One-dimensional proton MR spectroscopy was performed at a high field strength (11 T) in this prospective in vitro analysis of five benign tumors arising in the upper aerodigestive tract. The lesions consisted of three glomus tumors, one inverting papilloma, and a vagal schwannoma. Cho/Cr area ratios were calculated and compared with similar, previously reported in vitro (11 T) findings (2) and with samples of SCCA and normal muscle.
Tissue Procurement
The institutional review board at our institution approved the tissue procurement protocol. One to two cubic centimeters of benign tumor, SCCA, and muscle samples were obtained at the time of surgical resection. Special care was taken by the surgeon to ensure that the samples consisted only of muscle, SCCA, or benign tumor. All specimens were placed in plastic vials and immediately frozen in liquid nitrogen to preserve the metabolites. Samples were then stored in a −80°C freezer until the time of imaging. In preparation for proton MR spectroscopy, the samples were thawed to room temperature, minced, and washed with D2O phosphate-buffered saline (PBS) (three times) to remove as much residual water as possible. Tissue samples were then placed on glass wool plugs (saturated with D2O) in 5-mm tubes so that they could be positioned between the coils of the proton probe. The temperature of the sample was maintained at 37°C in the probe during spectral acquisition.
Spectral Analysis
Proton MR spectroscopy of benign tumors (n = 5), SCCA (n = 19), and uninvolved muscle (n = 13) was performed on a 500-MHz pulsed spectroscopy system. The water signal was suppressed using a presaturation pulse centered over the water frequency. One-dimensional proton MR spectroscopy was performed with a Carr-Purcell-Meiboom-Gill sequence with data acquired at TEs of 136 and 272, over a width of 7042.25 Hz (14.0806 ppm), by use of 8192 data points, 128 averages, an acquisition time of 0.582 seconds, and a TR of 2000. Exponential line-broadening of 5.00 Hz was applied to the 1D time domain data before Fourier transformation. The spectra obtained were phase-corrected (zero-order phase correction) for analysis. TEs of 136 and 272 were chosen because they are the values typically used for localized clinical MR spectroscopy.
Statistical Analysis
Peak areas under the Cho (3.2 ppm) and Cr (3.02 ppm) resonances were estimated in all cases as the product of the peak height (PH) and the linewidth at half maximum height (FWHM). Peak heights were determined relative to a flat baseline drawn manually through the noise. The analysis was based on the assumption that the shape of each resonance approximated a gaussian density function. The properties of a gaussian function allow for the area under the curve (AUC) of a particular resonance to be expressed as AUC = PH × FWHM × √π/4(lne2). In turn, the Cho/Cr ratio (R) for the AUCs was calculated using the formula R = PHCho × FWHMCho/(PHCr × FWHMCr). Cho/Cr ratios were determined for TEs of 136 and 272 for all three groups of tissue samples. All measurements were made by a single observer who was aware of the origin of the spectra.
The Cho/Cr ratios (R) obtained from direct measurements of the specific Cho and Cr resonances were logarithmically transformed using Y = log10R. This ensured the validity of the assumption of gaussian errors with constant variance necessary for accurate analysis of variance. Positive Y values correspond to AUCCho > AUCCr, and negative values correspond to AUCCho < AUCCr. A Y value of 0 corresponds to AUCCho = AUCCr.
To summarize, the statistical analyses were performed following logarithmic transformation of the Cho/Cr ratio to assure the validity of the statistical analyses. The Cho/Cr ratios obtained represent the antilog of the Y values calculated after appropriate statistical analysis. We attempted to determine whether there was a difference in the Cho/Cr ratios between benign tumor and SCCA and between benign tumor and muscle measured in vitro. To accomplish this, the Y value was calculated for all three tissue sample groups at TEs of 136 and 272. Statistical comparison between the three groups was performed using Wilcoxon's rank-sum test with Van der Waerden scores.
Results
Our results showed a marked elevation of the Cho/Cr ratio in all five benign tumors (Table 1 and Figs 1–3). The Y values and standard deviations for benign tumors, SCCA, and muscle are summarized in Table 2 (and see Figs 4 and 5). The following discussion is based on the antilog of Y, which was derived from the Cho/Cr ratios that were measured directly from the spectral resonances (2) (Figs 4 and 5). No Cho resonance was detectable in one sample of normal tissue, and this sample was not included in the analysis, thereby reducing the number of analyzed normal tissue samples to 12.
TABLE 1:
In vitro proton MR spectroscopy (11 T) choline/creatine ratios for five benign head and neck tumors
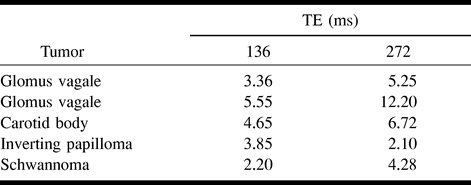
fig 1.
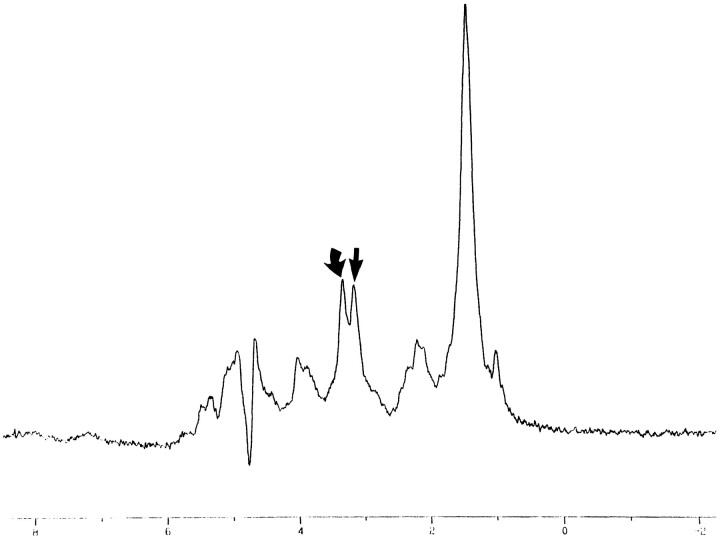
Inverting papilloma. In vitro 1D proton MR spectrum (2000/136/128) shows elevation of the Cho/Cr ratio. Curved arrow indicates Cho; straight arrow, Cr
TABLE 2:
Logarithmic transformation of the in vitro choline/creatine ratio area

fig 4.
Strap muscle. In vitro 1D proton MR spectrum (2000/136/128) shows similar areas of the Cho/Cr ratio. Curved arrow indicates Cho; straight arrow, Cr
The observed Cho/Cr ratios of the three tissue groups calculated at a TE of 136 (2000/136/128) ranged from 2.20 to 5.55 for benign tumors (Table 1), 0.82 to 3.84 for SCCA, and 0.53 to 2.14 for normal tissue (2). All statistical tests were based on means of the Y values, as in Table 2. The mean Y values were 0.57 for benign tumors, 0.22 for SCCA, and 0.07 for muscle (Table 2). The Cho/Cr ratios that correspond to these mean Y values are 3.72 for benign tumors (100.57), 1.67 for SCCA (100.223), and 1.17 for normal tissue (100.07). A previous investigation showed a significant difference between the elevation in the Y value in SCCA as compared with muscle (2). The results of our investigation show a substantial elevation in the Cho/Cr ratio in benign tumors (Figs 1–3). The observed difference in the elevated Y value of benign tumors that corresponds to the elevated Cho/Cr ratio is significantly higher than the corresponding Y values for SCCA (P = .0021) and muscle (P = .002).
The observed Cho/Cr ratios of the three tissue groups were also calculated at a TE of 272 (2000/272/128). The mean Y values were 0.72 (benign tumors), 0.39 (SCCA), and 0.12 (muscle) (Table 2). The Cho/Cr ratios that correspond to these mean Y values are 5.25 for benign tumors (100.72), 2.45 for SCCA (100.39), and 1.32 for normal tissue (100.12). A previous investigation showed a significant difference between the elevation in the Y value of SCCA as compared with muscle at a TE of 272 (2). The results of our studies obtained at a TE of 272 are similar to those obtained at a TE of 136 and show a substantial elevation in the Cho/Cr ratio of benign tumors. The observed difference in the elevated Y value of benign tumors that corresponded to the elevated Cho/Cr ratio was shown to be significantly higher than the corresponding Y values for SCCA (P = .015) and muscle (P = .003).
Discussion
Elevation of the Cho/Cr ratio has been identified in a variety of malignant tumors arising in the colon, prostate, breast, female reproductive tract, and brain. Similar findings have been identified in malignant tumors of the upper aerodigestive tract (1, 2). However, the characteristics of the Cho/Cr ratio have not been established for benign tumors of the extracranial head and neck (3–6).
Choline, a nutrient present in most foods, is absorbed from the diet and is ubiquitous throughout the body (7). The molecule is transported intracellularly by a low-affinity system with its mechanism coupled to the synthesis of phosphatidylcholine (8). Once intracellular, Cho is initially phosphorylated by choline phosphotransferase to form phosphocholine. This latter molecule is the precursor of phosphatidylcholine, which is formed by the Kennedy pathway (8). Choline and its derivatives are thought to represent important constituents in the phospholipid metabolism of cell membranes (9). The Cho peak (resonance at 3.2 ppm) is composed of choline, phosphocholine, phosphatidylcholine, and glycerophosphocholine. Elevation of this peak has been found in several tumors and is thought to represent increased membrane phospholipid biosynthesis and also to be an active marker for cellular proliferation (1, 2, 10–12).
Creatine plays a role in the maintenance of energy metabolism. It may be obtained from the diet or synthesized de novo within the liver, kidneys, and pancreas by precursor molecules, which include arginine, glycine, and S-adenosylmethionine (9, 13). Creatine is converted to creatine phosphate by the enzyme creatine kinase. Creatine phosphate acts as a store for high-energy phosphates in the cytosol of muscles (9). Additionally, because phosphocreatine is a high-energy phosphate compound, it has been postulated that it may help to sustain levels of adenosine triphosphate in energy-demanding tissues, such as actively proliferating tumors. The Cr resonance observed at 3.02 ppm is believed to comprise both phosphocreatine and creatine (13, 14). Currently, the exact levels and concentrations of Cr in normal and diseased tissue (ie, tumor or inflammation) are unknown (9).
The results of our in vitro (11 T) investigation show elevation of the Cho/Cr ratio in inverting papillomas, glomus tumors, and schwannomas. These findings suggest that the Cho/Cr ratio may be used to differentiate benign tumors from surrounding nonneoplastic tissues. However, the elevation of the Cho/Cr ratio cannot be used to differentiate benign from malignant tumors of the extracranial head and neck.
The elevation of the Cho/Cr ratio in inverting papillomas, glomus tumors, and schwannomas may reflect neoplastic cellular proliferation present within these tumors. Inverting papillomas are characterized histologically by neoplastic epithelial proliferation that results in extension of the epithelium into the underlying stroma (15). Schwannomas and glomus tumors are of neural crest origin. Schwannomas are tumors whose proliferative capacity is demonstrated by their capacity to produce abundant amounts of Schwann cells and collagen (16). Glomus tumors are neoplasms of the paraganglia of the head and neck. These tumors can be infiltrative and may be quite advanced at the time of presentation (17).
In our samples, the Cho/Cr ratio was not only elevated but was significantly higher in benign tumors than in malignant ones. This finding in head and neck tumors is most likely analogous to the presence of elevated Cho/Cr ratio in intracranial meningiomas, which are thought to be slow-growing benign neoplasms. Previous reports have demonstrated higher Cho/Cr ratios in meningiomas than in high-grade gliomas (18). Elevation of Cho may be due to cellular proliferation and/or cell density. That is, benign tumors that are hypercellular, such as meningiomas, may show elevated Cho without harboring true malignancy (19, 20). In brain tumors, a diagnosis of malignancy can only be made when lipids and lactate are present on MR spectra and cannot rely solely on the presence of high Cho levels (19, 20). In addition, inflammatory processes that are histologically benign may also show a high Cho peak (21). This is believed to be the result of a large number of inflammation-related cells and does not indicate malignancy. On the basis of these observations, we are not surprised by the finding of significantly elevated Cho levels in histologically benign head and tumors. It is unclear whether this is a consistent finding present in a variety of solid benign head and neck tumors, and further studies are necessary to evaluate this interesting observation.
Previous investigations have found elevation of Cho levels in benign thyroid and breast tumors (22–24). However, the degree of Cho elevation in these benign lesions does not appear to be as high as that seen in the benign tumors evaluated in our study (22–24). In fact, MacKinnon et al (23) suggested that a threshold Cho/Cr ratio of 1.7 could be used to differentiate benign breast tumors from invasive carcinoma. There was no evidence of such a threshold value in our results. Thus, on the basis of this initial investigation, elevation of the Cho/Cr ratio relative to that of muscle cannot be used to distinguish benign from malignant tumors of the extracranial head and neck. Future studies are needed to determine whether the significant elevation that we observed in the Cho/Cr ratio between benign tumors and SCCA is consistent and can potentially be used to differentiate benign from malignant tumors in this region.
Our spectroscopic analysis was performed using a ratio analysis based on the measurements of peak heights. Both peak height and peak area ratios have been used to evaluate differences in the Cho/Cr ratio between tumors and normal tissue (1, 2, 4–6). Because our tissues were studied at high field strengths, the spectra we obtained showed no significant line broadening that could potentially result in misinterpretation of the peak intensity analysis.
Quantitative analysis showed that the Cho/Cr ratios calculated for tumor and normal tissue were dependent on TE. The difference in the Cho/Cr ratio between tumor and normal tissues increased with longer TEs. These alterations in Cho/Cr ratios with respect to TE are most likely due to relaxation effects caused by differences in T1 and T2 relaxation times, as the proton MR spectra in our study were obtained under partial relaxation conditions. We were able to determine in a controlled setting the difference in Cho/Cr ratios produced by different TEs. However, the difference in TE did not affect the significance of the difference identified between benign tumor and muscle and between benign tumor and SCCA. Therefore, it is probably not necessary to perform experiments using both TEs in a clinical setting. On the basis of our findings, we would recommend using a TE of 136 as opposed to one of 272. This would reduce the acquisition time and help provide information concerning the presence of lactate based on the inversion of this resonance that occurs at this TE.
We performed fully relaxed proton MR spectroscopy on three tumor tissue samples to determine whether the differences in the in vitro Cho intensities between normal tissue and tumor are due to changes in relaxation time or to concentration. We assumed a T1 of 1500 for Cho, since this is what has been found for the T1 of Cho in other cell types. To be on the safe side, we used a TR of 1000 and performed the pulse and acquisition study. No difference was observed in the relative ratios of the peak intensities as compared with a TR of 2000. Thus, the changes we observed are, in all probability, due to increases in the concentration of Cho and Cr and not to changes in the relaxation time (1, 2).
The tissue preparation used in our analysis was similar to that used in other investigations (1, 2). A potential pitfall of this tissue preparation technique is membrane degradation, which results in the release of high concentrations of Cho-containing compounds that are normally located within the cell membrane. The end result could be artifactual elevation of Cho that leads to overestimation of its actual intracellular free concentration. The method used for sample preparation for in vitro spectroscopy is a standard technique that has been shown to minimize possible artifactual changes in in vitro spectra, including Cho elevation (1, 2, 8, 24–30). In order to release this membrane/lipid-associated form of Cho, phospholipases need to be activated. These phospholipases are calcium-ion dependent. The PBS solution used in our experiment was a calcium- and magnesium-ion free deuteration, which minimizes the action of the phospholipases. The act of dicing tissue has not been shown to activate plasma membrane degradative processes (1, 2, 8, 24–30).
An alternative method of tissue preparation is perchloric acid (PCA) extraction. A drawback of PCA extraction is that this technique can induce acid hydrolysis of membrane Cho components and, thus, increase its visibility on proton MR spectra. PCA extraction of cells is typically used if one is interested in the differences in the type of metabolites being produced and in determining their “absolute” concentrations within a cell under different conditions. We chose not to use PCA extraction because of the potential for acid hydrolysis and the fact that this method cannot be used in vivo to detect differences in metabolite levels in normal versus tumor tissues. The ultimate purpose of the 1D in vitro proton MR spectroscopy studies of histologically confirmed tumors was to obtain a spectrum that would provide us with data similar to that which may be observed in an intact tumor on a whole-body clinical MR system.
Despite the small number of benign tumors, we were able to show a marked elevation of the Cho/Cr ratio in all three benign histologic samples. Our study only evaluated spectral peaks assigned to Cho and Cr for several reasons. Elevation of Cho/Cr has been identified in a variety of malignant tumors and these spectral peaks can be evaluated on clinical 1.5-T units. Previous studies focusing on spectroscopic evaluation of the upper aerodigestive tract have also shown elevation of the Cho/Cr ratio to be a reliable indicator for differentiating malignant from normal tissue (1, 2). It is possible that the application of statistical classifiers, such as neural networks, may improve the ability of proton MR spectroscopy to identify specific histologic types of tumors (31).
The importance of identifying a consistent and reproducible metabolic marker for tumors of the upper aerodigestive tract rests in the potential for proton MR spectroscopy to identify early recurrent tumors and for prospective treatment monitoring. The ability to detect recurrent tumors by physical examination and imaging (CT and MR) is difficult after treatment, as surgery and radiation therapy result in scarring and fibrosis of underlying tissues. The granulation tissue and scarring that often follow treatment may be indistinguishable from tumor by CT and MR imaging (32, 33). Early results suggest that proton MR spectroscopy may be used to differentiate recurrent tumor from posttreatment changes and may be used to assess early treatment response in patients who are treated nonsurgically (34). Elevation of the Cho/Cr ratio detected in an indeterminate mass after treatment is suggestive of recurrent tumor, whereas a low ratio is suggestive of posttreatment changes (34). Early results from our institution suggest that persistent elevation of the Cho/Cr ratio during treatment compared with pretreatment proton MR spectroscopy is suggestive of unresponsive tumor, whereas reduction of the Cho/Cr ratio is suggestive of tumor that is responding to therapy (34). Earlier detection of recurrent tumor after surgery has the potential to increase the likelihood of cure in those patients in whom initial treatment fails. Further studies are necessary to confirm these initial observations.
Conclusion
This study represents our initial experience in attempting to identify the spectral characteristics of benign tumors of the upper aerodigestive tract. In our small group, the Cho/Cr ratio was significantly higher in these benign tumors than in muscle and SCCA of the extracranial head and neck. These findings should serve as a basis for further experiments that investigate the spectral profile of these types of tumors.
fig 2.
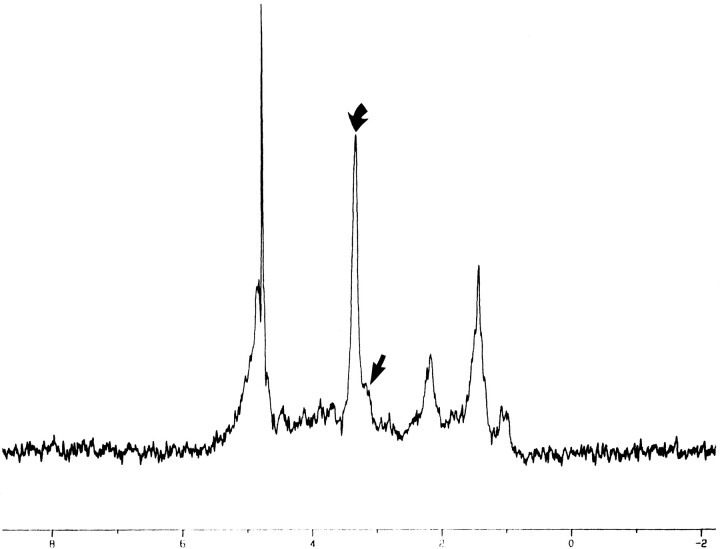
Glomus tumor. In vitro 1D proton MR spectrum (2000/136/128) shows elevation of the Cho/Cr ratio. Curved arrow indicates Cho; straight arrow, Cr
fig 3.
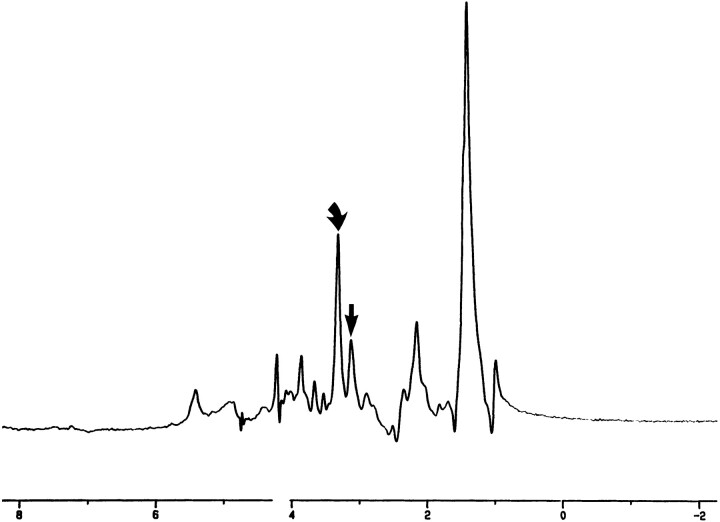
Vagal schwannoma. In vitro 1D proton MR spectrum (2000/136/128) shows elevation of the Cho/Cr ratio. Curved arrow indicates Cho; straight arrow, shoulder of Cr resonance
fig 5.
SCCA. In vitro 1D proton MR spectrum (2000/136/128) of SCCA obtained from the same patient as in figure 3 shows elevation of the Cho/Cr ratio. Curved arrow indicates Cho; straight arrow, Cr
References
- 1.Mukherji SK, Schiro S, Castillo M, et al. Proton MR spectroscopy of squamous cell carcinoma of the upper aerodigestive tract: in vitro characteristics. AJNR Am J Neuroradiol 1996;17:1485-1490 [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherji SK, Schiro S, Castillo M, et al. Proton MR spectroscopy of squamous cell carcinoma of the extracranial head and neck: in vitro and in vivo studies:. AJNR Am J Neuroradiol 1997;18:1057-1072 [PMC free article] [PubMed] [Google Scholar]
- 3.Mafee MF, Barany M, Gotsis ED, et al. Potential use of in vivo proton spectroscopy for head and neck lesions. Radiol Clin N Am 1989;27:243-254 [PubMed] [Google Scholar]
- 4.Gill SG, Thomas DGT, Van Bruggen NV, et al. Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr 1990;14:497-504 [DOI] [PubMed] [Google Scholar]
- 5.Delikatny EJ, Russell P, Hunter JC, et al. Proton MR and human cervical neoplasia: ex vivo spectroscopy allows distinction of invasive carcinoma of the cervix from carcinoma in situ and other preinvasive lesions. Radiology 1993;188:791-796 [DOI] [PubMed] [Google Scholar]
- 6.Negendak WG, Brown TR, Evelhoch JL, et al. Proceedings of a national cancer institute workshop: MR spectroscopy and tumor cell biology. Radiology 1992;185:875-883 [DOI] [PubMed] [Google Scholar]
- 7.Thompson GA. Phospholipid metabolism in animal tissues. In: Ansell GB, Jawthorne JN, Dawson RMC, eds. Form and Function of Phospholipids. Amsterdam: Elsevier 1973;67-96
- 8.Lean CL, Newland RC, Ende DA, Bokey EL, Smith ICP, Mountford CE. Assessment of human colorectal biopsies by H-1 MRS: correlation with histopathology. Magn Reson Med 1993;30:525-533 [DOI] [PubMed] [Google Scholar]
- 9.Miller BL. A review of chemical issues in 1-H NMR spectroscopy: n-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991;4:47-52 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed 1992;5:226-233 [DOI] [PubMed] [Google Scholar]
- 11.Negendank W. Studies of human tumors by MRS: a review. NMR Biomed 1992;5:303-324 [DOI] [PubMed] [Google Scholar]
- 12.Van Zijl PC, Moonen CT, Gillen J, et al. Proton magnetic resonance spectroscopy of small regions (1 ml) localized inside superficial human tumors: a clinical feasibility study. NMR Biomed 1990;3:227-232 [DOI] [PubMed] [Google Scholar]
- 13.Rodwell VW. Conversion of amino acids to specialized products. In: Martin DW, Mayes PA, Rodwell VW, eds. Harper's Review of Biochemistry. 18th ed. Los Altos, CA: Lange Medical Publications 1981;299-305
- 14.De Cestaines JD, Larsen VA, Podo F, Carpinella G, Briot O, Hensiksen O. In vivo P-31 MRS of experimental tumors. NMR Biomed 1993;6:345-365 [DOI] [PubMed] [Google Scholar]
- 15.Batsakis JG. Squamous cell “papilloma” oral cavity, sinonasal tract, and larynx. In: Batsakis JG, ed. Tumors of the Head and Neck. Baltimore: William & Wilkins 1979;130-143
- 16.Batsakis JG. Tumors of the peripheral nerves. In: Tumors of the Head and Neck. Baltimore: William & Wilkins 1979;313-333
- 17.Batsakis JG. Paragangliomas of the head and neck. In: Tumors of the Head and Neck. Baltimore: William & Wilkins 1979;369-380
- 18.Pruel MC, Caramanos Z, Collins DL, et al. Accurate noninvasive diagnosis of human brain tumors by using magnetic resonance spectroscopy. Nat Med 1996;2:323-325 [DOI] [PubMed] [Google Scholar]
- 19.Rand SD, Prost R, Haughton V, et al. Accuracy of single voxel proton MR spectroscopy in distinguishing neoplastic from nonneoplastic brain lesions. AJNR Am J Neuroradiol 1997;18:1695-1704 [PMC free article] [PubMed] [Google Scholar]
- 20.Krouwer HGJ, Kim TA, Rand SD, et al. Single voxel proton MR spectroscopy of nonneoplastic brain lesions suggestive of a neoplasm. AJNR Am J Neuroradiol 1998;19:1695-1704 [PMC free article] [PubMed] [Google Scholar]
- 21.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton spectroscopy. AJNR Am J Neuroradiol 1999;20:1619-1627 [PMC free article] [PubMed] [Google Scholar]
- 22.Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia: discrimination between benign and malignant neoplasms. Am J Med 1994;96:383-388 [DOI] [PubMed] [Google Scholar]
- 23.MacKinnon WB, Barry PA, Malycha PL, et al. Fine-needle biopsy specimens of benign lesions distinguished from invasive cancer ex vivo with proton MR spectroscopy. Radiology 1997;204:661-666 [DOI] [PubMed] [Google Scholar]
- 24.Johnson M, Selinsky B, Cavis et al. In vitro NMR evaluation of human thyroid lesions. Invest Radiol 1989;24:666-671 [DOI] [PubMed] [Google Scholar]
- 25.Mountford CE, Lean CL, Hancock R, et al. Magnetic resonance spectroscopy detects cancer in draining lymph nodes. Invasion Metastasis 1993;13:57-51 [PubMed] [Google Scholar]
- 26.Moreno A, Rey M, Montane JM, et al. 1H NMR spectroscopy of colon tumors and normal mucosal biopsies: elevated taurine levels and reduced polyethyleneglycol absorption in tumors may have diagnostic significance. NMR Biomed 1993;6:111-118 [DOI] [PubMed] [Google Scholar]
- 27.Halliday KR, Fenoglio-Preiser C, Sillerud IO. Differentiation of human tumors from non-malignant tissue by natural abundance C-13 NMR spectroscopy. Magn Reson Med 1988;7:394-411 [DOI] [PubMed] [Google Scholar]
- 28.Mountford CE, Lean C, Mackinnon WB, Russell P. The use of proton MR in cancer pathology. In: Webb GA, ed. Annual Reports on NMR Spectroscopy 1993;27:172-215 [Google Scholar]
- 29.Sivaraja M, Turner C, Souza K, Singer S. Ex vivo two-dimensional proton nuclear magnetic resonance spectroscopy of smooth muscle tumors: advantages of total correlated spectroscopy over homonuclear J-correlated spectroscopy. Cancer Res 1994;54:6037-6040 [PubMed] [Google Scholar]
- 30.Russell P, Lean CL, Delbridge L, et al. Ex vivo diagnosis of potentially metastatic follicular tumours by magnetic resonance spectroscopy. Am J Med 1994;96:383-388 [DOI] [PubMed] [Google Scholar]
- 31.Arle JE, Morriss C, Wang ZJ, Zimmerman RA, Phillips PG, Sutton LN. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg 1997;86:755-761 [DOI] [PubMed] [Google Scholar]
- 32.Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiographic appearance of the irradiated larynx, I: expected changes. Radiology 1994;193:141-148 [DOI] [PubMed] [Google Scholar]
- 33.Mukherji SK, Mancuso AA, Kotzur IM, et al. Radiographic appearance of the irradiated larynx, II: primary site response. Radiology 1994;193:149-154 [DOI] [PubMed] [Google Scholar]
- 34.Mukherji SK, Kwock L, Schiro S. Magnetic resonance spectroscopy of the extracranial head & neck. In: Mukherji SK, ed. Clinical Applications of MR Spectroscopy. New York: Wiley-Liss 1998;163-202