Abstract
BACKGROUND AND PURPOSE: We applied functional MR imaging with a learning task in healthy elderly volunteers and in patients with Alzheimer's disease to study brain activation during memory performance. The purpose was to determine the feasibility of functional MR imaging during a learning task in healthy elderly volunteers and in patients with Alzheimer's disease and to test our hypothesis that brain activation is decreased in the medial temporal lobe (MTL) memory system in patients with Alzheimer's disease compared with control volunteers.
METHODS: In 12 patients with mild to moderate forms of Alzheimer's disease and 10 elderly control volunteers, activation of the MTL memory system was studied. We used two learning tasks that required the encoding of new information into memory. After the functional MR imaging experiment, participants were tested for recognition of the encoded objects.
RESULTS: In the elderly control volunteers, activation during memory encoding was observed in medial and lateral temporal lobe structures (fusiform, parietal and occipital parts, and hippocampal formation) and in the frontal cortex, as reported previously in studies of young control volunteers. Focusing on the MTL, we observed that activation was significantly decreased in patients with Alzheimer's disease compared with control volunteers in the left hippocampus and parahippocampal gyrus bilaterally during the first encoding task but not during the second (P < .05, uncorrected).
CONCLUSION: Functional MR imaging with a learning task seems feasible in elderly volunteers and in patients with Alzheimer's disease. The measured functional signal decrease in MTL areas warrants further exploration of the (early) diagnostic usefulness of functional MR imaging in cases of Alzheimer's disease and other dementias.
Patients with Alzheimer's disease characteristically show a profound anterograde amnesia that becomes evident during the early stages of the disease (1). This condition is thought to be related to the early occurring pathologic alterations in the medial temporal lobe (MTL) system (2). Volumetric measurements and visual assessment of MTL atrophy by use of MR imaging are sensitive markers of Alzheimer's disease, even for mild forms of the disease (3–8). However, a certain amount of tissue has to be lost before these techniques show the characteristic abnormalities, and it may be hypothesized that dysfunction of the MTL system occurs before atrophy is present to the extent that it can be assessed. We speculate that methods that measure activation of brain structures during memory tasks may be used to measure such changes in function, even before morphologic changes are apparent.
Positron emission tomography studies have revealed hippocampal activity during the encoding of objects and faces (9–11), incremental learning (12), and associative learning (13). Functional MR imaging offers the possibility to study individual participants noninvasively using the blood oxygen level–dependent technique (14). With functional MR imaging, encoding of complex color pictures or visual associations of line drawings was shown to lead to activation in caudal portions of the parahippocampal gyrus and hippocampal formation (15–17). Functional MR imaging may thus provide potential for studying functional alterations that occur early in Alzheimer's disease.
In the present study, we applied paradigms involving the visual encoding of complex color pictures and visual associations of line drawings to study the activation pattern in patients with mild to moderate forms of Alzheimer's disease and in healthy elderly control volunteers. The purpose of this study was twofold. First, we wanted to know whether functional MR imaging with a learning task was feasible in elderly healthy volunteers and in patients with Alzheimer's disease. Based on previous findings, we expected that these tasks would result in activation of bilateral temporal and inferior frontal cortices. Second, we wanted to compare brain activation of elderly volunteers and patients with Alzheimer's disease. Our hypothesis was that in the MTL, brain activation would be decreased in patients compared with control volunteers.
Methods
Participants
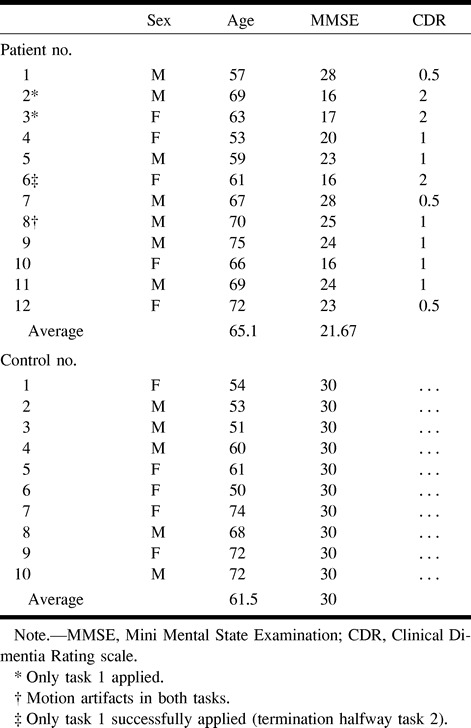
Twelve consecutive patients with Alzheimer's disease (seven male and five female patients) from the memory outpatient clinic of the Academisch Ziekenhuis Vrije Universiteit were asked to participate. Written informed consent for the functional MR imaging procedure was obtained, and the protocol was approved by the Institutional Review Board of the hospital. The clinical diagnosis of probable Alzheimer's disease was made by a senior neurologist according the criteria set forth by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Associations (18). Disease severity was assessed according to the Clinical Dementia Rating scale (19), and cognitive status was expressed, independently from the Clinical Dementia Rating, by the Mini-Mental State Examination scores (20). The mean age of the patients was 65.1 years, and the mean Mini-Mental State Examination score was 21.67. Ten elderly volunteers, comparable in age and level of education, served as control participants. The mean age was 61.5 years (five male and five female participants). Eight of these control volunteers were spouses of patients, and two were employees of the University. None of the control volunteers had ongoing medical problems or complained of memory disturbances. Details regarding the patients and control volunteers are provided in the Table.
Subject characteristics
Data Acquisition
Imaging was performed on a 1.5-T MR unit with a standard circularly polarized head coil. Imaging started with scout images being obtained in the three orthogonal directions. The scout images were used to position the structural images perpendicular to the long axis of the hippocampus. High-resolution structural imaging of the whole brain was performed with a 3D gradient-echo, T1-weighted sequence, with the following parameters: 15/7/1 (TR/TE/excitations); magnetization-prepared gradient-echo, inversion recovery–prepared; inversion time, 300 ms; flip angle, 8 degrees; matrix size, 256 × 256; field of view, 220 × 220 mm; section thickness, 2 mm; number of sections, typically 82. Next, functional imaging was performed using a gradient-echo, echo-planar sequence with the following parameters: 4000/64/1; flip angle, 90 degrees; matrix size, 64 × 128 interpolated to 128 × 128; field of view, 220 × 220 mm; section thickness, 6 mm; intersection gap, 1.02 mm. Echo-planar images were also positioned perpendicular to the long axis of the hippocampus and covered the entire brain; in most cases, 23 sections were needed.
Paradigm
Participants were positioned with a specially molded foam pad to restrict motion of the head. Two functional MR imaging paradigms during which pictures had to be encoded were administered. Pictures were projected on a screen at the end of the imaging table with a data projector, located outside the imaging room. A laptop computer was connected to the data projector, and the screen was seen via a mirror that was positioned above the head coil. If necessary, participants wore functional MR imaging–compatible glasses, to ensure optimal visual acuity.
Task 1: Encoding Complex Color Pictures
Encoding of complex color pictures with functional MR imaging was first described by Stern et al (17) and has been shown to activate the lingual and fusiform gyri and hippocampal formation bilaterally. We applied an encoding task with color pictures, showing landscapes and daily scenes (emotionally neutral: traffic, unrecognizable persons viewed from a distance), in 12 patients with Alzheimer's disease and 10 control volunteers. While participants were in the imager but before functional imaging was begun, two different pictures were presented to the participants, each for approximately 1 min (the so-called familiar pictures). During this preimaging period, participants were asked to describe what was shown in these two pictures to make sure they analyzed them with enough detail and to make sure that participants became familiar with the pictures. Then, functional imaging was begun. In total, 82 images were acquired per participant (total imaging time, 5 min 28 s). For the first two images, a familiar picture was presented (data not used in the analysis). For the next 80 images, either eight epochs of 10 images each (three control volunteers and five patients) or four epochs of 20 images each (seven control volunteers and seven patients) were alternated; the first epoch consisted of the presentation of a new picture (never seen before) every 4 s (ie, one picture per traverse, 10 or 20 in total). This epoch was called the N condition. The next epoch consisted of the presentation of the familiar pictures every 4 s in random order (ie, one picture per traverse, 10 or 20 in total). This epoch was called the F condition. These two conditions were repeated until 80 images were obtained. Thus, during the whole paradigm, all participants were presented with two familiar pictures and 40 novel pictures. Participants were instructed to view the pictures carefully to be able to recognize them later in a memory test in the imager. The presentation of novel and familiar color pictures contrasted presumed encoding of novel versus familiar scenes into memory.
Immediately after functional MR imaging, participants, while still lying in the imager, were tested for recognition, during which no imaging data were obtained. Fifty-five pictures were presented, half of which had not been seen before. Participants were asked to reply “yes” or “no” when presented with a picture, to indicate whether they had seen the picture before. A recognition score was determined per participant by linearly scaling the percentage of correct answers exceeding 50% (which can be expected by chance) from 0.0 (50% correct) to 1.0 (100% correct).
Task 2: Encoding Visual Associations of Line Drawings
During a second paradigm, participants had to encode line drawings into memory. Our group has shown that during this task, the fusiform and lingual gyrus, hippocampal formation, and frontal cortex show blood oxygen level–dependent signal increase (16). Participants were presented with line drawings depicting pairs of unrelated objects, with their corresponding nouns written underneath. The instruction was to memorize every pair of associated objects. This task was administered to 10 patients with Alzheimer's disease and 10 control volunteers and had the same epoch design and timing as did task 1. Thus, presentation of familiar line drawings (F condition) was alternated with presentation of novel drawings (N condition). Each F or N condition lasted for 10 images (three control volunteers and three patients) or 20 images (seven control volunteers and seven patients). Again, during the whole paradigm, participants were presented with two familiar pictures and 40 novel pictures. Immediately after functional MR imaging, while still in the imager, participants were tested for recognition: they saw all 40 novel line drawings with one of the two objects in each drawing left out. At the bottom of the drawing, two possibilities were presented for the missing object. Participants were asked to name the missing object by choosing one of the two possibilities. A recognition score was determined as for the first task.
Data Analysis
MTL atrophy was rated using the visual rating scale described earlier (8). The rating scale is based on a visual estimation of both the volume of the MTL, including the hippocampus proper, dentate gyrus, subiculum, and parahippocampal gyrus, and the volume of the surrounding CSF spaces, in particular the temporal horn of the lateral ventricle and the choroid fissure on both sides and on the left and right sides separately. Rating is performed on coronal sections according to example images published earlier (21), on a scale from 0 (no atrophy) to 4 (severe atrophy).
The first two functional MR images acquired were not used in the data analysis. Analysis was conducted with SPM99 software (22–24). First, echo-planar imaging data were realigned and coregistered to the structural images. After that, the echo-planar images were reoriented and resized into the standard anatomic space presented by Talairach and Tournoux (25). This was achieved by registration of the structural images of each participant to the SPM99 T1 template in standard space. Echo-planar imaging data were spatially smoothed using a gaussian kernel of 10 mm. Next, the data regarding each participant were modeled using a box car design, convolved with the hemodynamic response function (26). The box car had the same on/off frequency as the alternation frequency of the N and F conditions. We then calculated for each participant a “contrast image,” depicting for every voxel in the brain the estimated signal increase in the N condition compared with the F condition. Each participant's contrast-enhanced image was then fed into a statistical test. This is called the functional MR imaging equivalent of a random effects analysis (27).
First, we applied a one-sample t test to assess the average brain activation (ie, N versus F condition) for control volunteers and for patients with Alzheimer's disease separately (P < .001; minimal cluster size, 108 mm3). Second, we applied a two-sample t test to assess differences in brain activation between patients and control volunteers. To assess these positive or negative differences throughout the whole brain, we applied a P value that was corrected for multiple comparisons (based on the random field theory, P < .05) (28, 29). Further, focusing on our hypothesis regarding a decrease of brain function in the MTL in patients with Alzheimer's disease, we restricted our analysis to the MTL. On the T1 template in standard space, we manually drew a 3D region of interest by selecting the hippocampus and parahippocampal gyrus to define the MTL region of interest. In this MTL region of interest, we again applied a two-sample t test to assess differences in brain activation between patients and control volunteers. In this analysis, we applied an uncorrected P value (P < .05). Anatomic locations of significant signal change were determined by two investigators independently, using an atlas (30).
Results
Task 1
One patient had to be excluded from the analysis because of motion artifact, leaving data from 11 patients with Alzheimer's disease and 10 control volunteers for analysis. These patients (six male and five female patients) had a mean age of 64.6 years and a mean Mini-Mental State Examination score of 21.4. Control volunteers had an average recognition score of 0.63 (range, 0.42–0.86), whereas patients with Alzheimer's disease had an average score of 0.13 (range, 0.00–0.38). Mean atrophy scores were 0.9 (range, 0–2) and 2.5 (range, 1–4) for control volunteers and patients, respectively.
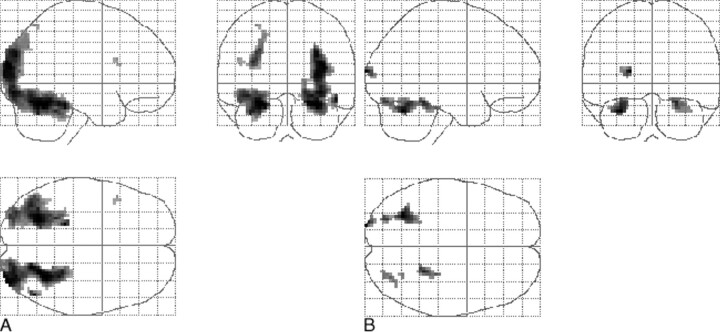
Local maxima of signal increase in the 10 control volunteers (P < .001; uncorrected; minimal cluster size, 108 mm3), as determined with a one-sample t test random effects analysis, were noted in the occipital cortex (Brodmann area 19), fusiform gyri (BA 36/37), left parahippocampal gyrus (BA 36), parietal lobe (BA 7), and left inferior frontal gyrus (BA 44) (Fig 1A). In patients, the main effect of signal increase was observed in the occipital cortex (BA 18), right parahippocampal gyrus (BA 36), fusiform gyrus (BA 20), and cerebellum (Fig 1B).
fig 1.
Projection of average brain activation during encoding of color pictures in sagittal, coronal, and transverse directions in the control group and in the patients.
A, Control group (n = 10, left in picture is left in brain). A one-sample t test random effects analysis (P < .001; uncorrected; minimal cluster size, 108 mm3) was applied. Significant activation is observed in the occipital cortex, fusiform gyri, left parahippocampal gyrus, parietal lobe, and left inferior frontal gyrus.
B, Patients (n = 11). The main effect of signal increase is seen in the occipital cortex, right parahippocampal gyrus, fusiform gyrus, and cerebellum.
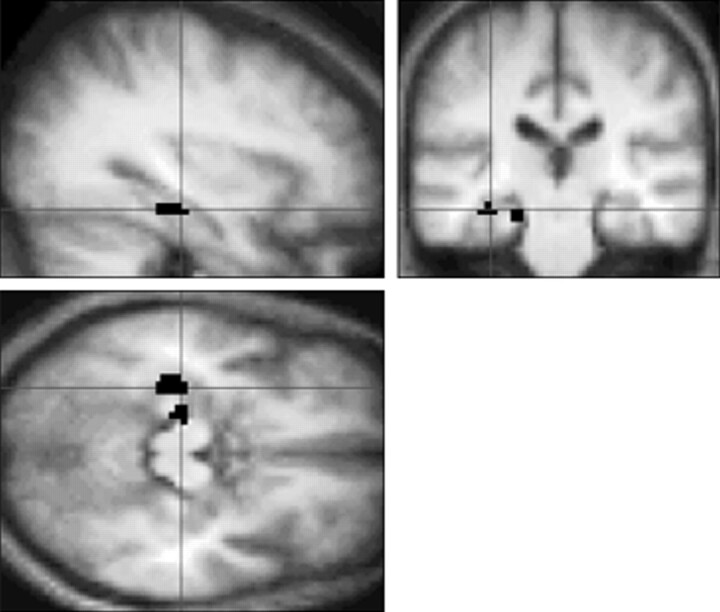
A two-sample t test, revealing areas in which brain activation in patients was different from that of control volunteers, did not reach significance in any brain area (P < .05, corrected for multiple comparisons). However, the region of interest analysis in the MTL showed that brain activation was significantly greater in the left parahippocampal gyrus (BA 36, z = 2.15), left hippocampus (z = 2.05), and right parahippocampal gyrus (BA 36, z = 1.84) in control volunteers (P < .05, uncorrected) (Fig 2). There were no areas in the MTL in which activation was increased in patients compared with control volunteers.
fig 2.
Sagittal, coronal, and transverse sections showing a significant increase in brain activation in control volunteers compared with patients in the left hippocampus and parahippocampal gyrus (black areas) during the first task, after application of the region of interest analysis (P < .05, uncorrected). The same effect is seen in the right parahippocampal gyrus (not shown). Activation is projected on the average brain of the 10 control volunteers (3D gradient-echo, T1-weighted sequence with parameters 15/7/1). Left in the figure is left in the brain
Task 2
One patient with Alzheimer's disease had to be excluded from the analysis because of motion artifacts. This patient had also been excluded from task 1 for the same reason. For another patient, the experiment was terminated halfway because the patient was no longer willing to continue, leaving eight patients with Alzheimer's disease and 10 control volunteers for analysis. These patients (five male and three female patients) had a mean age of 67.6 years and a mean Mini-Mental State Examination score of 20.9. The control volunteers had a mean recognition score of 0.79 (range, 0.35–1.00), and the patients with Alzheimer's disease had a mean score of 0.01 (range, 0.00–0.10).
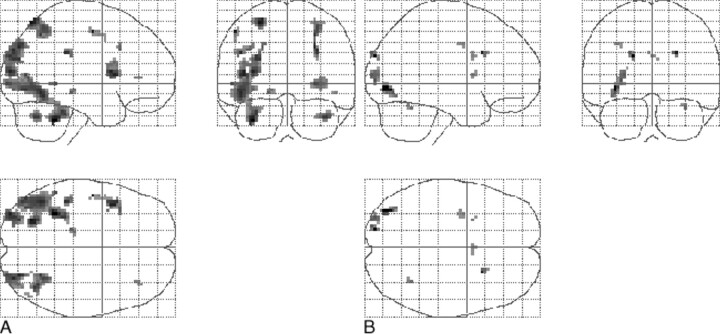
Local maxima of signal increase in the 10 control volunteers (P < .001; uncorrected; minimal cluster size, 108 mm3), as determined with a one-sample t test random effects analysis, was noted in the cerebellum, occipital cortex (BA 18/19), parietal cortex (BA 7/40), inferior frontal gyrus (left and right, BA 44/45), and left parahippocampal gyrus (BA 35/36) (Fig 3A). In patients, the main effect of signal increase was observed in the occipital (BA 18/19) and parietal (BA 7) cortex, right precentral gyrus (BA 6/44), left insula, left middle frontal gyrus (BA 9), and cingulate sulcus (BA 24) (Fig 3B).
fig 3.
Projection of average brain activation during encoding of line drawings in sagittal, coronal, and transverse directions in the control group and in patients.
A, Control group (n = 10, left in the figure is left in the brain). A one-sample t test random effects analysis (P < .001; uncorrected; minimal cluster size, 108 mm3) was applied. Significant activation is observed in the cerebellum, occipital cortex, parietal cortex, inferior frontal gyrus, and left parahippocampal gyrus.
B, Patients (n = 8). The main effect of signal increase is seen in the occipital and parietal cortex, right precentral gyrus, left insula, left middle frontal gyrus, and cingulate sulcus.
A two-sample t test, revealing areas in which brain activation in patients was different from that in control volunteers, did not reach significance in any brain region (P < .05, corrected for multiple comparisons). The region of interest analysis in the MTL did not reveal any areas of significant difference in signal change between patients and control volunteers.
Discussion
We applied functional MR imaging with two learning tasks to study memory-related brain activation in 12 patients with mild to moderate forms of Alzheimer's disease and 10 age-matched control volunteers. An important finding was that functional MR imaging seemed to be feasible in elderly control volunteers and in patients with Alzheimer's disease. All patients except one were able to complete both parts of the investigation. One patient requested that the experiment be terminated during task 2. For a second patient, motion artifacts interfered with analysis. Thus, 11 of 12 patients were studied successfully with task 1 and eight of 10 with task 2. To perform both tasks, patients had to stay in the MR imager for 45 to 55 min, including determination of recognition score for each task and acquisition of anatomic reference data. Because the task order was not randomized, we cannot exclude the possibility of deteriorating performance during the second half of the investigation due to fatigue. This factor might be assumed to relate to disease severity and may thus become more important when cases with more severe dementia are studied.
The main effects for task 1 and 2 versus baseline are shown in Figures 1 and 3. Although we did not directly compare brain activation between young and elderly control volunteers in this study, the elderly volunteers showed activation in the same structures as those observed in young healthy individuals with the same paradigms, thus revealing brain activation in the occipital cortex, fusiform gyrus, MTL memory system, and frontal cortex (16, 17). However, signal changes in the MTL seem less pronounced in the current study compared with these previous findings (16). This might be related to our having applied a random-effects analysis in this study, which decreases statistical power (31).
Most important, during both tasks, much less activation was observed in patients than in control volunteers, although the activation pattern along the ventral route was still recognizable (Figs 1 and 3). When data regarding control volunteers and patients were compared statistically with a two-sample t test, no differences in brain activation were noted when considering the whole brain (ie, application of a stringent threshold, corrected for multiple comparisons). However, our hypothesis was that brain activation in the MTL was decreased in patients with Alzheimer's disease compared with control volunteers. Restricting our analysis to the MTL and applying an uncorrected threshold showed that activation in the left hippocampus and left and right parahippocampal gyri was significantly decreased in patients during the encoding of color pictures. This effect was not observed during the encoding of line drawings. This might be explained in that during this latter task, control volunteers showed less pronounced activation in the MTL than during task 1. Again, this difference could (partly) be explained by the nature of our analysis, in which strong variation between participants severely decreases statistical power. Activation patterns for patients and control volunteers and the differences between them might become more significant when the number of participants is increased.
By definition, patients with Alzheimer's disease had higher MTL atrophy scores than did control volunteers and, in addition, had a worse performance of recognition after undergoing imaging. Therefore, the significant decrease in brain activation in the MTL in patients is in agreement with atrophy scores and recognition scores. From the group activation in patients or control volunteers, we cannot conclude whether it relates to novelty assessment, the encoding process, or both. However, recent correlational and event-related functional MR imaging experiments showed strong evidence for encoding-related brain activation in the posterior MTL (32, 33). Our current data also showed decreased posterior MTL activation when encoding performance (as determined with the recognition score) was worse, suggesting that brain activation in the tasks we used reflects the encoding process.
We showed that functional MR imaging signal changes can differentiate brain activation in the MTL in elderly control volunteers from that in patients with mild to moderate forms of Alzheimer's disease (for task 1), and our results are in agreement with recent results showing dysfunction of the hippocampal system during face encoding in four patients with Alzheimer's disease compared with four control volunteers (34). In the current study, we could not distinguish between effect of group (Alzheimer's disease/no Alzheimer's disease), memory performance (low/high) and atrophy score (high/low), because these three factors are, by definition, strongly dependent. The final goal of our research is to determine whether differences in brain activation can be observed in earlier stages of the disease. To determine whether such functional MR imaging experiments have early diagnostic value for Alzheimer's disease research, large groups of elderly participants need to be studied longitudinally.
In the present study, there were at least two uncontrolled factors. First, it is possible that decreased global cerebral blood flow and/or decreased global vascular response in the Alzheimer's disease group might (partly) explain the differences. The application of simple paradigms, such as a simple visual stimulation and/or the method of varying difficulty levels in paradigms over a wide range, could serve as internal reference and might control for these effects in future experiments. Second, it can be argued that differences in attention and concentration during performance of the tasks may have biased our results. We took care to implement tasks that were very easy to perform (whether information is successfully encoded) and might even be performed without any instructions at all, especially when patients have a mild form of Alzheimer's disease. During the experiment, the lights in the imaging room were turned off and all that could be seen were the projected pictures on the screen. This diminished the possibility that patients were distracted during imaging. Further, we asked patients to describe what they saw on the screen with pictures projected before imaging started, and thus made sure that they saw pictures with enough detail and that attention was focused on the pictures. During the task, no feedback mechanism was used to monitor attention. Therefore, despite our precautions, we cannot exclude that attention was diminished in patients and that this attentional component (partly) explains the diminished MTL activation.
Conclusion
Focusing on the main goals of our study, we can conclude that functional MR imaging is feasible in both elderly control volunteers and patients with Alzheimer's disease and is capable of showing significantly lower memory-related brain activation in patients with Alzheimer's disease compared with control volunteers in areas involved in memory function. Studying other disorders is necessary to draw conclusions regarding the specificity of our results to Alzheimer's disease. Taking into account the flexibility and noninvasive character of the functional MR imaging technique and its high individual spatial resolution, our results warrant further exploration of the (early) diagnostic usefulness of functional MR imaging in cases of Alzheimer's disease and other dementias.
Acknowledgments
The helpful and stimulating comments of Drs. Gary Small, Mony De Leon, and Jeffrey Cummings on earlier versions of the manuscript are greatly acknowledged.
Footnotes
Preliminary results of this study were presented at the Annual Meeting of the Society for Neuroscience, Miami, 1999; the Sixth International Conference on Alzheimer's Disease and Related Disorders, Amsterdam, 1998; and the Fourth International Conference on Functional Mapping of the Human Brain, Montreal, 1998.
This study was supported by a grant from the Vrije Universiteit Amsterdam (to S.A.R.B.R.). Additional funds were granted by the Stichting Alzheimer en Neuropsychiatry Foundation Amsterdam.
References
- 1.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology 1991;41:1006-1009 [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 1992;15:6-31 [DOI] [PubMed] [Google Scholar]
- 3.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol 1993;14:897-906 [PMC free article] [PubMed] [Google Scholar]
- 4.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain 1996;119:2001-2007 [DOI] [PubMed] [Google Scholar]
- 5.Jack CRJ, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 1992;42:183-188 [DOI] [PubMed] [Google Scholar]
- 6.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 1997;48:1297-1304 [DOI] [PubMed] [Google Scholar]
- 7.Laakso MP, Soininen H, Partanen K, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J Neural Transm 1995;9:73-86 [DOI] [PubMed] [Google Scholar]
- 8.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992;55:967-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grady CL, Mcintosh AR, Horwitz B, et al. Age-related reductions in human recognition memory due to impaired encoding. Science 1995;269:218-221 [DOI] [PubMed] [Google Scholar]
- 10.Haxby JV, Ungerleider LG, Horwitz B, et al. Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A 1996;93:922-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S. Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport 1994;5:2525-2528 [DOI] [PubMed] [Google Scholar]
- 12.Kopelman MD, Stevens TG, Foli S, Grasby P. PET activation of the medial temporal lobe in learning. Brain 1998;121:875-887 [DOI] [PubMed] [Google Scholar]
- 13.Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus 1997;7:249-256 [DOI] [PubMed] [Google Scholar]
- 14.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868-9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrieli JE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997;276:264-266 [DOI] [PubMed] [Google Scholar]
- 16.Rombouts SARB, Machielsen WCM, Witter MP, Barkhof F, Lindeboom J, Scheltens P. Visual association encoding activates the medial temporal lobe: a functional magnetic resonance imaging study. Hippocampus 1997;7:594-601 [DOI] [PubMed] [Google Scholar]
- 17.Stern CE, Corkin S, Gonzalez RG, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A 1996;93:8660-8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-944 [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-2414 [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-198 [DOI] [PubMed] [Google Scholar]
- 21.Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van , Gool WA. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol 1995;242:557-560 [DOI] [PubMed] [Google Scholar]
- 22.Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time series. Hum Brain Mapp 1994;1:153-171 [Google Scholar]
- 23.Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp 1995;3:165-189 [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995;2:189-210 [Google Scholar]
- 25.Talairach J, Tournoux P:, Co-Planar Stereotaxic Atlas of the Human Brain.. Stuttgart: Thieme Verlag; 1988
- 26.Friston KJ, Frith CD, Turner R, Frackowiak RSJ. Characterizing evoked hemodynamics with fMRI. Neuroimage 1995;2:157-165 [DOI] [PubMed] [Google Scholar]
- 27.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 1992;11:1685-1704 [DOI] [PubMed] [Google Scholar]
- 28.Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991;11:690-699 [DOI] [PubMed] [Google Scholar]
- 29.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992;12:900-918 [DOI] [PubMed] [Google Scholar]
- 30.Duvernoy H:, The Human Brain.. New York: Springer-Verlag; 1991
- 31.Woods RP. Modeling for intergroup comparisons of imaging data. Neuroimage 1996;4:S84-S94 [DOI] [PubMed] [Google Scholar]
- 32.Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JE. Making memories: brain activity that predicts how well visual experience will be remembered. Science 1998;281:1185-1187 [DOI] [PubMed] [Google Scholar]
- 33.Fernandez G, Weyerts H, Schraderbolsche M, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 1998;18:1841-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol 1999;45:466-472 [DOI] [PubMed] [Google Scholar]