Abstract
Long non-coding RNAs are associated with cancer progression. Long intergenic non-protein coding RNA (linc)-regulator of reprogramming (ROR) enhances tumor development in hepatocellular carcinoma (HCC). However, the effect of chemoresistance and its underlying mechanisms in HCC are not completely understood. The present study aimed to identify the effect of ROR on sensitivity to doxorubicin (DOX) in HCC cells. In the present study, Cell Counting Kit-8 and EdU assays were performed to assess cell viability and proliferation, respectively. In addition, E-cadherin and vimentin protein expression levels were assessed via western blotting and immunofluorescence. The results of the present study demonstrated that HCC cells with high linc-ROR expression levels were more resistant to DOX, and linc-ROR knockdown increased HCC cell DOX sensitivity compared with the control group. The results indicated that compared with the NC siRNA group, linc-ROR knockdown notably suppressed epithelial-mesenchymal transition by downregulating twist family bHLH transcription factor 1 (TWIST1) expression. TWIST1 knockdown displayed a similar effect on HCC cell DOX sensitivity to linc-ROR knockdown. Moreover, linc-ROR knockdown-induced HCC cell DOX sensitivity was inhibited by TWIST1 overexpression. The present study provided evidence that linc-ROR promoted HCC resistance to DOX by inducing EMT via interacting with TWIST1. Therefore, linc-ROR might serve as a therapeutic target for reducing DOX resistance in HCC.
Keywords: long intergenic non-protein coding RNA-regulator of reprogramming, hepatocellular carcinoma, doxorubicin, twist family bHLH transcription factor 1, epithelial-mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and is the second leading cause of cancer-related death worldwide (1). Despite the development of chemotherapy and active treatments, the 5-year survival rate of HCC (5–10%) remains low due to late diagnosis, tumor recurrence and drug resistance (2–4). Chemoresistance is the primary reason for cancer therapy failure and remains a big challenge in clinical treatment (5). At present, doxorubicin (DOX) is the first-line chemotherapy drug for transcatheter arterial embolic therapies (TACE) in HCC (6). However, the intrinsic or acquired resistance of HCC cells to DOX attenuates the effects of TACE (7). Therefore, understanding the mechanisms underlying DOX resistance and developing suitable therapeutic targets for DOX resistance in HCC is important.
Previous studies have demonstrated that most human genome transcripts are transcribed into non-coding RNAs (ncRNAs), including small ncRNAs and long ncRNAs (lncRNAs) (8,9). lncRNAs are a class of ncRNA transcripts that are >200 nucleotides in length (10). Increasing evidence has indicated that lncRNAs serve important roles in the occurrence and development of tumors, such as HCC, breast cancer cells and gastric cancer (10,11). The effects and functions of lncRNAs in chemotherapy resistance have also been extensively studied. Wu et al (12) reported that Keap1 regulation-associated lncRNA, as a competitive endogenous RNA, enhanced sensitivity to 5-fluorouracil in HCC by binding to microRNA (miR)-141 and regulating kelch like ECH associated protein 1. Moreover, downregulation of lncRNA NR2F1 antisense RNA 1 increased oxaliplatin sensitivity in HCC by targeting the miR-363/ATP binding cassette subfamily C member 1 signaling pathway (13). lncRNA ribosomal protein L13a pseudogene 20 knockdown markedly inhibited cell proliferation, enhanced apoptosis, suppressed tumor growth and increased sensitivity to DOX (14). Moreover, Li et al (15) reported that lncRNA arsR promotes resistance to DOX in HCC by modulating the PTEN/PI3K/Akt signaling pathway.
Long intergenic non-protein coding RNA (linc)-regulator of reprogramming (ROR) was first discovered in induced pluripotent stem cells (iPSCs). linc-ROR contributes to epigenetic regulators involved in pluripotency and lineage commitment (16,17). linc-ROR is frequently increased in different types of cancer, such as, breast cancer, endometrial cancer stem cells, pancreatic cancer and gastric cancer stem cells; it is also correlated to poor prognosis and progression in cancer (18–21). linc-ROR upregulation could promote cell invasion and metastasis by regulating miR-145 and zinc finger E-box binding homeobox 2, and inducing epithelial-mesenchymal transition (EMT) in HCC (22). Extracellular transfer of linc-ROR can promote cell survival during hypoxic stress and chemoresistance (23,24). However, the roles and functions of linc-ROR in drug resistance in HCC are not completely understood.
The present study investigated the potential effect of linc-ROR on DOX resistance in HCC, as well as the underlying mechanisms.
Materials and methods
Cell culture
HCC cell lines (Hep3B, Huh7, SNU387 and SNU449) were purchased from American Type Culture Collection. Huh7 and Hep3B cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) and MEM (Gibco; Thermo Fisher Scientific, Inc.), respectively. SNU387 and SNU449 cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.). All culture medium was supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C with 5% CO2. DOX was purchased from Selleck Chemicals.
Cell transfection
linc-ROR small interfering RNA (siRNA), TWIST1 siRNA (20 µM), TWIST1 overexpression (OE) vector (OE-TWIST1) (2 µg) and empty vector, negative control (NC) siRNA (non-targeting) were synthesized by Qiagen Benelux B.V. The control was the blank control. HCC cells (2×105 cells/well) were transfected with linc-ROR siRNA, TWIST1 siRNA, OE-TWIST1 or NC siRNA using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol at 37°C for 6 h. The primers for linc-ROR siRNA, TWIST1 siRNA and NC siRNA were as follows: linc-ROR forward, 5′-GAUGGCACUAUGACUACAATT−3′ and reverse, 5′-UUGUAGUCAUAGUGCCAUCTT−3′; TWIST1 forward, 5′-GGUGUCUAAAUGCAUUCAUTT−3′ and reverse, 5′-AUGAAUGCAUUUAGACACCTT−3′; and NC siRNA forward, 5′-UUCUCCGAACGUGUCACGUTT−3′ and reverse, 5′-ACGUGACACGUUCGGAGAATT−3′.
Cell viability assay
Cells were seeded (5×103 cells/well) into 96-well plates. Following incubation for 12 h at 37°C, cells were incubated in fresh culture medium containing different concentrations of DOX (0–5 µg/ml) for 48 h at 37°C. Cell viability was determined using the Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.). Then, 10 µl CCK-8 solution was added, the cells were incubated for an additional 3 h at 37°C, absorbance was measured at a wavelength of 450 nm using an MRX II microplate reader (Dynex Technologies, Inc.).
EdU assay
To assess cell proliferation, HCC cells were seeded (5×103 cells/well) into 96-well plates for 2 days. Subsequently, cells were incubated with EdU for 60 min at 37°C, followed by 4% paraformaldehyde for fixation and staining at room temperature for 15–30 min for EdU-incorporated cells using a ClickiT EdU Assay kit (Invitrogen; Thermo Fisher Scientific, Inc.). Hoechst 33342 (Invitrogen; Thermo Fisher Scientific, Inc.) was used to stain cell nuclei at room temperature for 30 min in the dark. The proportion of EdU-positive cells was determined by randomly counting cells under a fluorescent microscope in 5–10 fields of view (magnification, ×100).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using the PrimeScript RT Reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol. Subsequently, qPCR was performed using Premix Ex Taq II (Takara Biotechnology Co., Ltd.) and an ABI 7500 Fast system. The PCR conditions were as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 30 sec, 60°C for 34 sec and 72°C for 30 sec. linc-ROR expression levels were quantified using the 2−ΔΔCq method (25) and normalized to the internal reference gene β-actin. RT-qPCR was performed in triplicate. The following primers were used for qPCR: linc-ROR forward, 5′-ACCTGCAACACTCCAGCTAT-3′ and reverse, 5′-TGACCTGTTGACCCACCTTT-3′; TWIST1 forward, 5′-GGUACAUCGACUUCCUCUATT−3′ and reverse, 5′-UAGAGGAAGUCGAUGUACCTT−3′; and β-actin forward, 5′-ATCAAGGAGAAGCTCTGCTACATC−3′ and reverse, 5′-TCAGACTCGGCTGGAAGAGA-3′.
Western blotting
Total protein was isolated from HCC cells using RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein concentrations were determined using the Bradford protein assay method (Thermo Fisher Scientific, Inc.). Proteins (40 µg) were separated via 10% SDS-PAGE and transferred onto PVDF membranes (Thermo Fisher Scientific, Inc.). After blocking with 5% TBS-Tween 20 (TBST; 0.1% Tween 20 containing 5% BSA (Sangon Biotech Co., Ltd.), for 1 h at 37°C. The membranes were incubated at 4°C overnight with primary antibodies (all 1:1,000; Abcam) targeted against the following: TWIST1 (cat. no. ab49254), E-cadherin (cat. no. ab40772), vimentin (cat. no. ab92547). Following washing three times with TBST, the membranes were incubated with a HRP-conjugated secondary antibody (cat. no. ab7090; 1:2,000; Abcam) for 1 h at 37°C. Protein bands were visualized using an enhanced chemiluminescence kit [Roche Diagnostics (Shanghai) Co., Ltd.]. GAPDH (Cell Signaling Technology, Inc.; cat. no. 5174S; 1:2,000) was used as the loading control.
Immunofluorescence
HCC cells were seeded into 48-well plates at 3×103 cells/well. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, washed three times with PBS (3 min per wash), permeated with 0.5% Triton X-100 (prepared in PBS) for 20 min at room temperature, washed three times with PBS (3 min per wash). Following absorption of PBS using absorbent paper, blocked using 1% BSA for 30 min in the room temperature, cells were incubated with FITC-conjugated primary antibodies targeted against E-cadherin (cat. no. ab40772; 1:50; Abcam) and vimentin (cat. no. ab92547; 1:50; Abcam) overnight at 4°C. The cells were incubated with FITC-conjugated secondary antibody (cat. no. ab6785; 1:100; Abcam) at 4°C for 2 h. Subsequently, cells were incubated with DAPI for 5 min at room temperature in the dark to stain cell nuclei. Following washing four times with PBST (0.1% Tween 20 containing 5% BSA) (5 min per wash), stained cells were observed and imaged using a confocal fluorescence microscope (magnification, ×100).
Statistical analysis
Comparisons between two groups were analyzed using the Student's t-test followed by unpaired t-test. Comparisons among multiple groups were analyzed using one-way ANOVA followed by Tukey's post hoc test. StarBase (version 3; starbase.sysu.edu.cn/index.php) was used to analyze the level of linc-ROR in liver HCC (LIHC). P<0.05 was considered to indicate a statistically significant difference. All experiments were performed in triplicate.
Results
linc-ROR expression is negatively associated with sensitivity to DOX in HCC cells
To investigate the relationship between linc-ROR expression and DOX sensitivity, HCC cell viability following DOX treatment was assessed. Among the HCC cell lines used in the present study, the Huh7 cell line was the most DOX-sensitive, whereas the SNU449 cell line was the most DOX-resistant (Fig. 1A). The IC50 of DOX in the HCC cell lines, from highest to lowest, was SNU449, SNU387, Hep3B and Huh7 cells (Fig. 1B). Subsequently, linc-ROR expression levels in the four HCC cell lines were measured. Among the HCC cell lines, SNU449 cells and Huh7 cells displayed the highest and lowest linc-ROR expression levels, respectively (Fig. 1C), which indicated that linc-ROR expression was negatively associated with DOX sensitivity. Furthermore, starBase was used to analyze linc-ROR expression levels in LIHC. linc-ROR expression levels were higher in the 374 LIHC samples compared with the 50 healthy samples (Fig. S1A).
Figure 1.
linc-ROR expression levels in HCC cell lines and the relationship between linc-ROR expression and DOX sensitivity. (A) HCC cell viability following exposure to 0, 0.3125, 0.625, 1.25, 2.5 or 5 µg/ml DOX. ***P<0.001 vs. Huh7. (B) IC50 values of DOX in HCC cells. **P<0.01 and ***P<0.001 vs. Huh7. (C) linc-ROR expression levels in HCC cell lines. **P<0.01 vs. Huh7. linc-ROR, long intergenic non-protein coding RNA-regulator of reprogramming; HCC, hepatocellular carcinoma; DOX, doxorubicin; ACTB, β-actin.
linc-ROR knockdown increases sensitivity to DOX in HCC cells
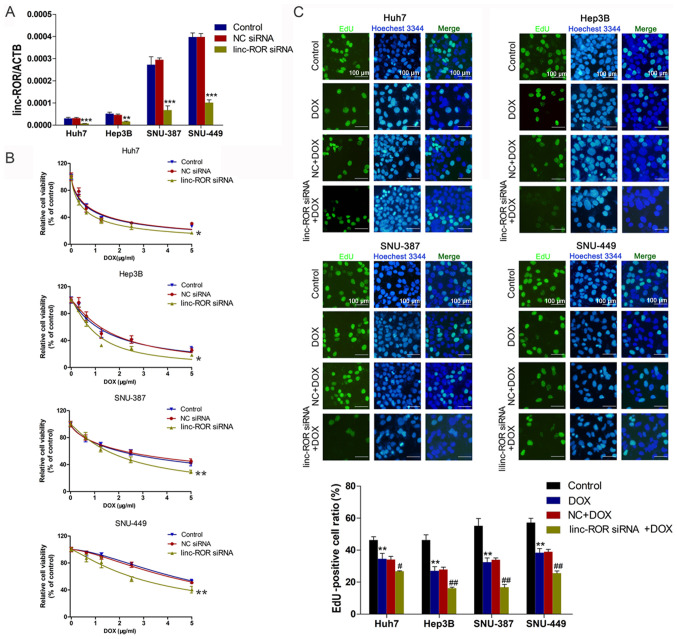
To investigate the effect of linc-ROR on DOX sensitivity in HCC, interference oligonucleotides were synthesized and transfected into HCC cells to knock down linc-ROR (Fig. 2A). Cell viability was assessed by performing the CCK-8 assay. The results indicated that the linc-ROR knockdown group was significantly more sensitive to DOX compared with the control group (Fig. 2B). The EdU assay results for cell proliferation were similar to the CCK-8 assay results, indicating that linc-ROR knockdown significantly enhanced DOX sensitivity compared with the DOX group (Fig. 2C).
Figure 2.
Effect of linc-ROR on DOX sensitivity in HCC cells. (A) Transfection efficiency of linc-ROR siRNA. **P<0.01 and ***P<0.001 vs. control. (B) HCC cell viability following transfection with linc-ROR siRNA or NC siRNA and treatment with 0, 0.3125, 0.625, 1.25, 2.5 or 5 µg/ml DOX. *P<0.05 and **P<0.01 vs. control (C) HCC cell proliferation following treatment with DOX (IC50), NC siRNA + DOX or linc-ROR siRNA + DOX (magnification, ×100). **P<0.01 vs. control; #P<0.05 and ##P<0.01 vs. DOX. linc-ROR, long intergenic non-protein coding RNA-regulator of reprogramming; DOX; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; NC, negative control; ACTB, β-actin.
linc-ROR knockdown inhibits the EMT signaling pathway by downregulating TWIST1
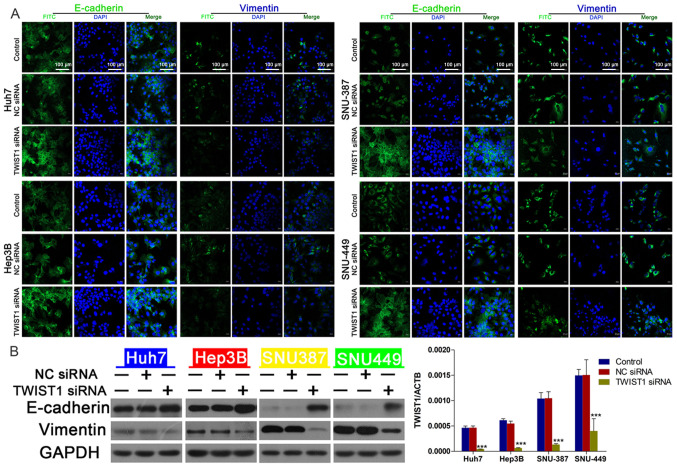
To explore whether the EMT signaling pathway mediated linc-ROR-induced DOX resistance in HCC, the expression of EMT-related proteins was assessed. The transfection efficiency of linc-ROR siRNA was examined via RT-qPCR (Fig. 3A). Compared with NC siRNA, linc-ROR knockdown markedly downregulated the expression levels of TWIST1, an important promoter of EMT (26) (Fig. 3B). E-cadherin (an epidermal marker) (26) expression was notably upregulated, whereas vimentin (a mesenchymal marker) (26) expression was clearly downregulated by linc-ROR knockdown compared with the NC siRNA group (Fig. 3C). To explore the effects of linc-ROR knockdown on the EMT signaling pathway, immunofluorescence assays were performed to examine E-cadherin and vimentin expression. The immunofluorescence assay results were similar to the western blotting results, as linc-ROR knockdown notably increased E-cadherin expression, but markedly decreased vimentin expression compared with the NC siRNA group (Fig. 3D).
Figure 3.
linc-ROR knockdown regulates the expression of epithelial-mesenchymal transition-related proteins. (A) Transfection efficiency of linc-ROR siRNA. (B) linc-ROR knockdown downregulated TWIST1 protein expression. **P<0.01 and ***P<0.001 vs. control. (C) linc-ROR knockdown increased E-cadherin expression and decreased vimentin expression. (D) Immunofluorescence detection of E-cadherin and vimentin expression following transfection with linc-ROR siRNA (magnification, ×100). linc-ROR, long intergenic non-protein coding RNA-regulator of reprogramming; siRNA, small interfering RNA; TWIST1, twist family bHLH transcription factor 1; NC, negative control; ACTB, β-actin.
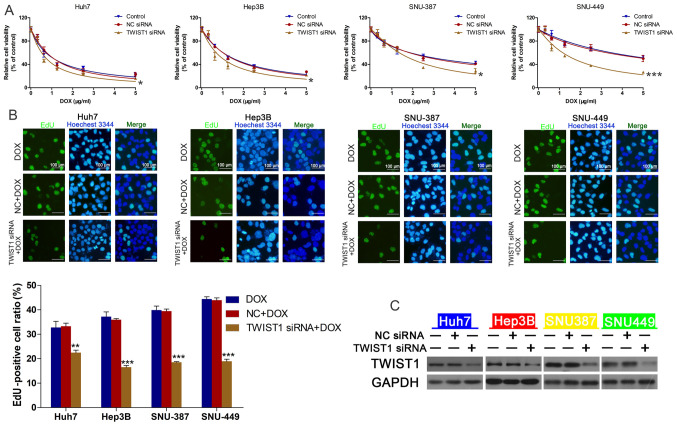
TWIST1 mediates linc-ROR knockdown-regulated DOX sensitivity
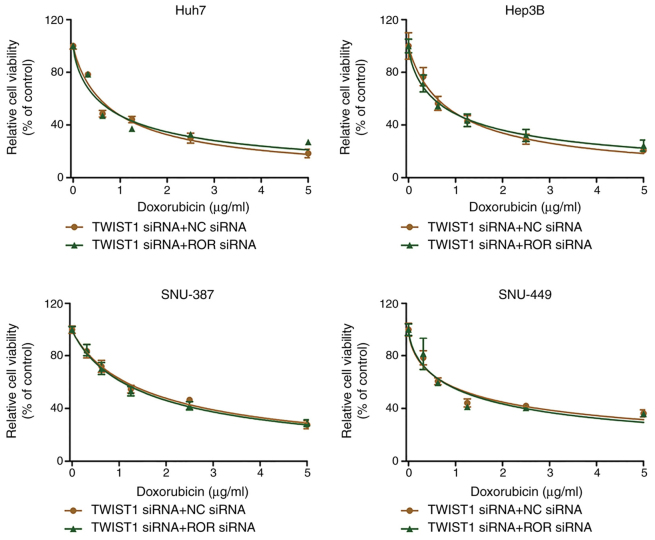
To further investigate the effect of TWIST1 on linc-ROR-mediated DOX resistance, the effect of TWIST1 knockdown on HCC cell viability was assessed. The transfection efficiency of TWIST1 knockdown was determined via western blotting (Fig. 4C). TWIST1-knockdown HCC cells were significantly more sensitive to DOX compared with the control group (Fig. 4A), EdU assay results demonstrated that TWIST siRNA combined with DOX could reduce cell proliferation compared with DOX group (Fig. 4B). Compared with the NC siRNA group, TWIST1 knockdown also notably upregulated E-cadherin expression and markedly downregulated vimentin expression, which was similar to the results obtained for linc-ROR knockdown (Fig. 5B). The western blotting (Fig. 5B) and immunofluorescence (Fig. 5A) results for E-cadherin and vimentin expression were consistent. Furthermore, there was no significant difference in cell viability between the TWIST1 siRNA + NC siRNA group and the TWIST1 siRNA + linc-ROR siRNA group (Fig. 6). In addition, linc-ROR knockdown-induced DOX sensitivity was inhibited by TWIST1 overexpression (Fig. S1B and C). The expression of TWIST was determined by RT-qPCR (Fig. S1D).
Figure 4.
TWIST1 knockdown regulates DOX sensitivity in HCC cells. (A) HCC cell viability following transfection with TWIST1 siRNA or NC siRNA and treatment with 0, 0.3125, 0.625, 1.25, 2.5 or 5 µg/ml DOX. *P<0.05 and ***P<0.001 vs. control. (B) HCC cell proliferation following treatment with DOX (IC50), NC siRNA + DOX or linc-ROR siRNA + DOX (magnification, ×100). **P<0.01 and ***P<0.001 vs. DOX. (C) Transfection efficiency of TWIST1 siRNA. TWIST1, twist family bHLH transcription factor 1; DOX, doxorubicin; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; NC, negative control; linc-ROR, long intergenic non-protein coding RNA-regulator of reprogramming.
Figure 5.
Effect of TWIST1 knockdown on E-cadherin and vimentin expression. (A) Immunofluorescence detection of E-cadherin and vimentin expression following TWIST1 knockdown (magnification, ×100). (B) TWIST1 knockdown increased E-cadherin expression and reduced vimentin expression. ***P<0.001 vs. control. TWIST1, twist family bHLH transcription factor 1; siRNA, small interfering RNA; NC, negative control; ACTB, β-actin.
Figure 6.
TWIST1 knockdown reverses linc-ROR knockdown-mediated effects on DOX sensitivity. HCC cells were co-transfected with TWIST1 siRNA and linc-ROR siRNA or NC siRNA. Subsequently, HCC cell viability was assessed following treatment with 0, 0.3125, 0.625, 1.25, 2.5 or 5 µg/ml DOX. TWIST1, twist family bHLH transcription factor 1; linc-ROR, long intergenic non-protein coding RNA-regulator of reprogramming; DOX, doxorubicin; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; NC, negative control.
Discussion
HCC is an aggressive malignant tumor, and although great progress in the treatment of HCC has been achieved, it still has a high recurrence rate and mortality rate (27,28). A primary pathological characteristic of HCC is chemoresistance against a series of chemotherapeutic drugs, including cisplatin, DOX and sorafenib (29,30). lncRNAs have been widely reported to participate in regulating tumorigenesis and chemoresistance in HCC cells (31,32). Moreover, linc-ROR participates in the regulation of malignant biological properties in HCC, such as proliferation and metastasis (22,33,34); however, the effects of linc-ROR in mediating HCC chemoresistance are not completely understood.
linc-ROR is a 2.6-kb lncRNA comprised of four exons that was first described in iPSCs. linc-ROR is regulated by NANOG, SOX2 and OCT4 (16). linc-ROR is upregulated in numerous solid tumors, including ovarian, lung, esophagus and renal cancer (35–38). Furthermore, it has been reported that linc-ROR can regulate HCC cell migration, proliferation and hypoxia signaling pathways in HCC (22,23). Extracellular vesicle-mediated transfer of linc-ROR could promote chemoresistance in HCC cells (24). In the present study, the Huh7 cell line, which displayed the lowest linc-ROR expression levels among the HCC cell lines, was the most DOX-sensitive. By contrast, the SNU449 cell line displayed the highest expression levels of linc-ROR among the HCC cell lines and was the most DOX-resistant. Furthermore, linc-ROR expression levels in LIHC were analyzed using starBase. linc-ROR expression was higher in the 374 LIHC samples compared with the 50 healthy samples. However, the specimens were not used to construct survival curves, so this should be analyzed in a future study. Collectively, the present study demonstrated that compared with the control group, linc-ROR knockdown promoted sensitivity to DOX in HCC cells.
EMT is a complex process that is controlled by various transcriptional regulatory agencies via different signaling pathways, involving TWIST1, snail family transcriptional repressor 2 and snail family transcriptional repressor 1 (39). EMT also serves vital roles in the chemoresistance of cancer cells (40). The TWIST family, consisting of TWIST1 and TWIST2, serves a crucial role in the regulation of EMT (41). TWIST1 leads to reduced E-cadherin expression and increased mesenchymal marker vimentin expression, resulting in HCC cell invasion (42,43). TWIST is critically involved in drug resistance. For example, TWIST1 downregulation reduced drug resistance by regulating ATP binding cassette subfamily B member 1 and ATP binding cassette subfamily C member 1 expression in colon cancer cells (44). Mesenchyme homeobox 2 and TWIST1 upregulation are associated with lung cancer chemoresistance and prognosis (45). In HCC, the platelet-derived growth factor D/miR-106a/TWIST1 signaling pathway promotes gemcitabine resistance by regulating EMT (46). In the present study, linc-ROR knockdown notably downregulated the expression levels of TWIST1 and E-cadherin compared with the NC siRNA group. The human HCC cell lines Huh7 and Hep3B, which display an epithelial phenotype, displayed markedly higher E-cadherin expression levels compared with SNU387 and SNU449 cells, which display a mesenchymal phenotype (47). It has been previously reported that cells with the mesenchymal phenotype are more resistant compared with cells with the epithelial phenotype (47). Furthermore, the results of the present study indicated that linc-ROR regulated the expression of TWIST1, thereby promoting the EMT signaling pathway. Moreover, CCK-8 assays and western blotting were performed to examine cell viability and EMT-related protein expression levels following co-transfection of linc-ROR siRNA and OE-TWIST1, respectively. The results demonstrated that the effects of linc-ROR siRNA on HCC cell sensitivity to DOX were decreased after co-transfection of OE-TWIST1 and lin-ROR siRNA, indicating that linc-ROR regulated sensitivity to DOX via TWIST1 and EMT in HCC cells. A key limitation of the present study was that the mechanism underlying ROR-mediated regulation of TWIST1 was not investigated. It was hypothesized that linc-ROR might regulate TWIST1 via the PI3K/Akt and IL-6/STAT3 signaling pathways, but this should be investigated further in future studies.
In conclusion, the results of the present study provided novel mechanistic insight into chemotherapy resistance in HCC. To the best of our knowledge, the present study demonstrated linc-ROR/TWIST1 axis-mediated regulation of chemoresistance in HCC for the first time. Therefore, targeting the linc-ROR/TWIST1 axis might increase sensitivity and improve responses to traditional therapeutic agents used for HCC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Science and Technology Department Public Welfare Project of Zhejiang Province (grant no. LGF18H160023), the Administration of Traditional Chinese Medicine of Zhejiang Province (grant no. 2015ZA012), The National Natural Science Foundation of China (grant nos. 81572307, 81773096 and 81701630), the Major Project of Medical and Health Technology Development Program in Zhejiang Province (grant no. 7211902), the Zhejiang Provincial Medical and Health Research Project (grant nos. 2018KY126 and 2021KY030) and the Projects of Lishui Key Research and Development Plan in Zhejiang Province (grant no. 2017ZDYF12).
Funding
The present study was supported by the Science and Technology Department Public Welfare Project of Zhejiang Province (grant no. LGF18H160023), the Administration of Traditional Chinese Medicine of Zhejiang Province (grant no. 2015ZA012), The National Natural Science Foundation of China (grant nos. 81572307, 81773096 and 81701630), the Major Project of Medical and Health Technology Development Program in Zhejiang Province (grant no. 7211902), the Zhejiang Provincial Medical and Health Research Project (grant nos. 2018KY126 and 2021KY030) and the Projects of Lishui Key Research and Development Plan in Zhejiang Province (grant no. 2017ZDYF12).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WLW and DZ conceptualized the study. YZ and WDW performed the experiments and confirmed the authenticity of the raw data. QS and LY analyzed the data. WLW drafted the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Song P, Cai Y, Tang H, Li C, Huang J. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends. 2017;11:389–398. doi: 10.5582/bst.2017.01202. [DOI] [PubMed] [Google Scholar]
- 3.Ozer Etik D, Suna N, Boyacioglu AS. Management of hepatocellular carcinoma: Prevention, surveillance, diagnosis, and staging. Exp Clin Transplant. 2017;15(Suppl 2):S31–S35. doi: 10.6002/ect.TOND16.L9. [DOI] [PubMed] [Google Scholar]
- 4.Kulik LM. Advancements in hepatocellular carcinoma. Curr Opin Gastroenterol. 2007;23:268–274. doi: 10.1097/MOG.0b013e3280ec5113. [DOI] [PubMed] [Google Scholar]
- 5.Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular carcinoma: Review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44:479–486. doi: 10.1067/j.cpradiol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa H, Kita R, Kimura T, Osaki Y. Transcatheter arterial embolic therapies for hepatocellular carcinoma: A literature review. Anticancer Res. 2014;34:6877–6886. [PubMed] [Google Scholar]
- 7.Pastorelli D, Cartei G, Zustovich F, Marchese F, Artioli G, Zovato S, Binato S, Ceravolo R, Cingarlini S, Salmaso F, et al. Gemcitabine and liposomal doxorubicin in biliary and hepatic carcinoma (HCC) chemotherapy: Preliminary results and review of the literature. Ann Oncol. 2006;17(Suppl 5):v153–v157. doi: 10.1093/annonc/mdj972. [DOI] [PubMed] [Google Scholar]
- 8.Yang JX, Rastetter RH, Wilhelm D. Non-coding RNAs: An introduction. Adv Exp Med Biol. 2016;886:13–32. doi: 10.1007/978-94-017-7417-8_2. [DOI] [PubMed] [Google Scholar]
- 9.Wei L, Wang X, Lv L, Liu J, Xing H, Song Y, Xie M, Lei T, Zhang N, Yang M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol Cancer. 2019;18:147. doi: 10.1186/s12943-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108:1927–1933. doi: 10.1111/cas.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weidle UH, Birzele F, Kollmorgen G, Ruger R. Long non-coding RNAs and their role in metastasis. Cancer Genomics Proteomics. 2017;14:143–160. doi: 10.21873/cgp.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, Pan C, Wei X, Shi Y, Zheng J, Lin X, Shi L. lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun Signal. 2018;16:47. doi: 10.1186/s12964-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H, Chen J, Ding CM, Jin X, Jia ZM, Peng J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J Cell Mol Med. 2018;22:3238–3245. doi: 10.1111/jcmm.13605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Xiao J, Lv Y, Jin F, Liu Y, Ma Y, Xiong Y, Liu L, Zhang S, Sun Y, Tipoe GL, et al. LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43:1926–1938. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Ye Y, Feng B, Qi Y. Long noncoding RNA lncARSR promotes doxorubicin resistance in hepatocellular carcinoma via modulating PTEN-PI3K/Akt pathway. J Cell Biochem. 2017;118:4498–4507. doi: 10.1002/jcb.26107. [DOI] [PubMed] [Google Scholar]
- 16.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, Wu M, Xiong J, Guo X, Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, Gao Q, Wang J, Zhang X, Liu K, Duan Z. Linc-RNA-RoR acts as a ‘sponge’ against mediation of the differentiation of endometrial cancer stem cells by microRNA-145. Gynecol Oncol. 2014;133:333–339. doi: 10.1016/j.ygyno.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Gao S, Wang P, Hua Y, Xi H, Meng Z, Liu T, Chen Z, Liu L. ROR functions as a ceRNA to regulate Nanog expression by sponging miR-145 and predicts poor prognosis in pancreatic cancer. Oncotarget. 2016;7:1608–1618. doi: 10.18632/oncotarget.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Liu F, Deng J, Cai X, Han J, Liu Q. Long noncoding RNA ROR regulates proliferation, invasion, and stemness of gastric cancer stem cell. Cell Reprogram. 2016;18:319–326. doi: 10.1089/cell.2016.0001. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Lu L, Feng B, Zhang K, Han S, Hou D, Chen L, Chu X, Wang R. The lincRNA-ROR/miR-145 axis promotes invasion and metastasis in hepatocellular carcinoma via induction of epithelial-mesenchymal transition by targeting ZEB2. Sci Rep. 2017;7:4637. doi: 10.1038/s41598-017-04113-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Odero-Marah V, Hawsawi O, Henderson V, Sweeney J. Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv Exp Med Biol. 2018;1095:101–110. doi: 10.1007/978-3-319-95693-0_6. [DOI] [PubMed] [Google Scholar]
- 27.Yee NS. Update in systemic and targeted therapies in gastrointestinal oncology. Biomedicines. 2018;6:34. doi: 10.3390/biomedicines6010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M. Systemic therapy for hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl 1):S135–S146. doi: 10.1159/000481245. [DOI] [PubMed] [Google Scholar]
- 29.Nishida N, Kitano M, Sakurai T, Kudo M. Molecular mechanism and prediction of sorafenib chemoresistance in human hepatocellular carcinoma. Dig Dis. 2015;33:771–779. doi: 10.1159/000439102. [DOI] [PubMed] [Google Scholar]
- 30.Lohitesh K, Chowdhury R, Mukherjee S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018;18:44. doi: 10.1186/s12935-018-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Chen J, Zhang K, Feng B, Wang R, Chen L. Progress and prospects of long noncoding RNAs (lncRNAs) in hepatocellular carcinoma. Cell Physiol Biochem. 2015;36:423–434. doi: 10.1159/000430109. [DOI] [PubMed] [Google Scholar]
- 32.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int J Biochem Cell Biol. 2013;45:1895–1910. doi: 10.1016/j.biocel.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Shen Z, Zhi Y, Zhou H, Zhang K, Wang T, Feng B, Chen Y, Song H, Wang R, Chu X. Long non-coding RNA ROR promotes radioresistance in hepatocelluar carcinoma cells by acting as a ceRNA for microRNA-145 to regulate RAD18 expression. Arch Biochem Biophys. 2018;645:117–125. doi: 10.1016/j.abb.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Pan Y, Li C, Chen J, Zhang K, Chu X, Wang R, Chen L. The emerging roles of long noncoding RNA ROR (lincRNA-ROR) and its possible mechanisms in human cancers. Cell Physiol Biochem. 2016;40:219–229. doi: 10.1159/000452539. [DOI] [PubMed] [Google Scholar]
- 35.Lou Y, Jiang H, Cui Z, Wang L, Wang X, Tian T. Linc-ROR induces epithelial-to-mesenchymal transition in ovarian cancer by increasing Wnt/β-catenin signaling. Oncotarget. 2017;8:69983–69994. doi: 10.18632/oncotarget.19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Y, Chen J, Tao L, Zhang K, Wang R, Chu X, Chen L. Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget. 2017;8:33144–33158. doi: 10.18632/oncotarget.16562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahebi R, Malakootian M, Balalaee B, Shahryari A, Khoshnia M, Abbaszadegan MR, Moradi A, Javad Mowla S. Linc-ROR and its spliced variants 2 and 4 are significantly up-regulated in esophageal squamous cell carcinoma. Iran J Basic Med Sci. 2016;19:1131–1135. [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Zhang W, Tian H, Zhang Q, Men T. lncRNA ROR promotes the proliferation of renal cancer and is negatively associated with favorable prognosis. Mol Med Rep. 2017;16:9561–9566. doi: 10.3892/mmr.2017.7775. [DOI] [PubMed] [Google Scholar]
- 39.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 40.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang Y, Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Sun B, Zhao X, Zhao N, Sun R, Zhu D, Zhang Y, Li Y, Gu Q, Dong X, et al. Twist1-related miR-26b-5p suppresses epithelial-mesenchymal transition, migration and invasion by targeting SMAD1 in hepatocellular carcinoma. Oncotarget. 2016;7:24383–24401. doi: 10.18632/oncotarget.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Han S, Wang X, Peng R, Li X. SOX5 promotes epithelial-mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32:461. doi: 10.1007/s12032-014-0461-2. [DOI] [PubMed] [Google Scholar]
- 44.Liu YR, Liang L, Zhao JM, Zhang Y, Zhang M, Zhong WL, Zhang Q, Wei JJ, Li M, Yuan J, et al. Twist1 confers multidrug resistance in colon cancer through upregulation of ATP-binding cassette transporters. Oncotarget. 2017;8:52901–52912. doi: 10.18632/oncotarget.17548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ávila-Moreno F, Armas-López L, Álvarez-Moran AM, López-Bujanda Z, Ortiz-Quintero B, Hidalgo-Miranda A, Urrea-Ramírez F, Rivera-Rosales RM, Vázquez-Manríquez E, Peña-Mirabal E, et al. Overexpression of MEOX2 and TWIST1 is associated with H3K27me3 levels and determines lung cancer chemoresistance and prognosis. PLoS One. 2014;9:e114104. doi: 10.1371/journal.pone.0114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Li Y, Hou Y, Yang Q, Chen S, Wang X, Wang Z, Yang Y, Chen C, Wang Z, Wu Q. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget. 2015;6:7000–7010. doi: 10.18632/oncotarget.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue F, Liu Y, Chu H, Wen Y, Yan L, Tang Q, Xiao E, Zhang D, Zhang H. eIF5A2 is an alternative pathway for cell proliferation in cetuximab-treated epithelial hepatocellular carcinoma. Am J Transl Res. 2016;8:4670–4681. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.