Abstract
Hydrogen sulfide (H2S) exerts an anti-atherosclerotic effect and decreases foam cell formation. Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a key factor involved in foam cell formation. However, the association between H2S and Lp-PLA2 expression levels with respect to foam cell formation has not yet been elucidated. The present study investigated whether H2S can affect foam cell formation and potential signalling pathways via regulation of the expression and activity of Lp-PLA2. Using human monocytic THP-1 cells as a model system, it was observed that oxidized low-density lipoprotein (ox-LDL) not only upregulates the expression level and activity of Lp-PLA2, it also downregulates the expression level and activity of Cystathionine γ lyase. Exogenous supplementation of H2S decreased the expression and activity of Lp-PLA2 induced by ox-LDL. Moreover, ox-LDL induced the expression level and activity of Lp-PLA2 via activation of the p38MAPK signalling pathway. H2S blocked the expression levels and activity of Lp-PLA2 induced by ox-LDL via inhibition of the p38MAPK signalling pathway. Furthermore, H2S inhibited Lp-PLA2 activity by blocking the p38MAPK signaling pathway and significantly decreased lipid accumulation in ox-LDL-induced macrophages, as detected by Oil Red O staining. The results of the present study indicated that H2S inhibited ox-LDL-induced Lp-PLA2 expression levels and activity by blocking the p38MAPK signalling pathway, thereby improving foam cell formation. These findings may provide novel insights into the role of H2S intervention in the progression of atherosclerosis.
Keywords: atherosclerosis, lipoprotein-associated phospholipase A2, p38MAPK, hydrogen sulfide
Introduction
Coronary heart disease (CHD) is one of the most common causes of death worldwide. Between 2000 and 2012, the death rate of CHD rose by 33.8% in men, and by 22.8% in women (1). Atherosclerosis (AS) is the basis of CHD and can cause coronary plaque formation, vascular stenosis or obstruction, resulting in myocardial ischemia, hypoxia or necrosis (2). Lipoprotein accumulation in macrophages causes cells to become foam cells; this process is one of the leading causes of arterial plaque formation (3,4). Previous studies have demonstrated that oxidized low-density lipoprotein (ox-LDL) is a key factor in the initiation and progression of AS as it induces vascular cells to recruit monocytes and promotes their differentiation into macrophages, which then transform into foam cells (5,6).
The PLA2G7 gene in humans encodes lipoprotein-associated phospholipase A2 (Lp-PLA2), also known as platelet-activating factor acetylhydrolase, which is an independent risk factor for the initiation and progression of cardiovascular disease (7). Lp-PLA2 catalyses the hydrolysis of oxidized phospholipids in ox-LDL, leading to the release of downstream inflammatory mediators, such as lysophosphatidylcholine and oxidized fatty acids (8). Oxidized phospholipids in ox-LDL can also promote the conversion of macrophages into foam cells and further increase the expression level of Lp-PLA2 (9,10). Finally, lysophosphatidylcholine induces the production of reactive oxygen species, thereby exacerbating the formation of atherosclerosis and destabilizing plaques (11,12). Therefore, Lp-PLA2 serves a crucial role in the pathogenesis of AS.
Hydrogen sulfide (H2S) is a key gas signaling molecule in the cardiovascular system and exerts notable cardiovascular protective effects (13). H2S has been shown to exhibit anti-inflammatory and antioxidative stress effects, as well as blocks the development of AS by protecting the vascular endothelium, inhibiting vascular smooth muscle cell proliferation and foam cell formation (14,15) and decreasing endothelial dysfunction by decreasing p38MAPK expression levels.
To the best of our knowledge, no studies have yet investigated the association between H2S and ox-LDL-induced Lp-PLA2 expression levels and its potential underlying mechanisms. The present study used ox-LDL to induce THP1 macrophage to establish a foam cell model. The aims of the present study were to determine whether H2S can decrease the expression level of Lp-PLA2 in THP1 cells induced by ox-LDL; to determine whether H2S improves the formation of foam cells caused by ox-LDL by decreasing the expression level of Lp-PLA2; and to identify potential signalling pathways that promote H2S to decrease Lp-PLA2 expression levels.
Materials and methods
Reagents
THP-1 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (cat. no. SCSP-567). RPMI-1640 cell culture medium (cat. no. 72400120; Thermo Fisher Scientific, Inc.) and FBS were obtained from Gibco (cat. no.12483020; Thermo Fisher Scientific, Inc.). ox-LDL, the p38MAPK specific inhibitors SB203580 (cat. no. S8307) and SB202190 (cat. no. S7067), NaHS (cat. no. 161527) and DL-propargylglycine (PPG; cat. no. P7888) were purchased from Sigma-Aldrich (Merck KGaA). An Lp-PLA2 primary antibody (cat. no. 160603) and enzyme activity detection kit (cat. no. 760901) were obtained from Cayman Chemical Company. ox-LDL was purchased from BIOSS (cat. no. bs-1698P). The total (t)-p38MAPK primary antibody (cat. no. ab31828) and phosphorylated (p)-p38MAPK primary antibody (cat. no. ab4822) were obtained from Abcam. Secondary antibodies [HRP-labeled Goat Anti-Rabbit IgG(H+L); cat. no. A0208] were purchased from Beyotime Institute of Biotechnology. Cystathionine γ-lyase (CSE) primary antibody was purchased from BIOSS (cat. no. bs-9515R). β-actin primary antibody was purchased from Beyotime Institute of Biotechnology (cat. no. AF5006).
Cell transfection
Cells were seeded in 6-well plates (4×105 cells/well) and transfected the following day with human Lp-PLA2 small interfering (si)RNA (30 nM; cat. no. AM16708; Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine® 2000 (cat. no. 11668019; Invitrogen; Thermo Fisher Scientific, Inc.), according to the following method: 24 h prior to transfection, the cells were seeded in 6-well plates and ~2 ml RPMI-1640 medium was added into each well, so that the cell density was 4×105 cells/well at the time of transfection, and 2 ml antibiotic-free medium was replaced. Then, Lipofectamine® 2000 (4 µl/well) was shaken gently and diluted with 245 µl opti-MEM (cat. no. 51985034; Thermo Fisher Scientific, Inc.), and the mixture was incubated at room temperature for 5 min. A total of 30 nM Lp-PLA2 siRNA/negative control (NC) siRNA (cat. no. AM16708; Thermo Fisher Scientific, Inc.) was diluted with 245 µl opti-MEM and mixed gently. The two mixtures with mixed together and allowed to stand at room temperature for 20 min in order to form a mixture of Lp-PLA2 siRNA/NC siRNA transfection reagents. This mixture (500 µl) was added to the well containing cells, along with 2 ml antibiotic-free medium. Cells were incubated in 5% CO2 at 37°C for 6 h and the medium was replaced with RPMI-1640 complete medium-containing serum. After transfection for 24 h, the cells were exposed to 50 µM ox-LDL at 37°C in a humidified atmosphere with 5% CO2 for 24 h. The Lp-PLA2 siRNA transfection and validation, followed the method of Zheng et al (16).
Cell culture
THP-1 cells were maintained in RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Before performing the experiments, the medium was replaced with RPMI-1640 medium containing fresh serum unless otherwise indicated. Cells were divided into the following groups: Control (THP-1 cells treated with RPMI-1640 medium supplemented with 10% FBS); ox-LDL [THP-1 cells treated with ox-LDL (50 µg/ml) for 24 h]; ox-LDL + SB203580 [THP-1 cells pretreated with SB203580 (20 µM) for 30 min before being treated with ox-LDL (50 µg/ml) for 24 h]; ox-LDL + SB202190 [THP-1 cells pretreated with SB202190 (20 µM) for 30 min before being treated with ox-LDL (50 µg/ml) for 24 h]; ox-LDL + NaHS [THP-1 cells pretreated with the exogenous H2S donor, NaHS, at different concentrations (0, 50, 100 or 200 µM) for different times (0, 6, 12 or 24 h) in the presence of ox-LDL (50 µg/ml)]; ox-LDL + PPG [THP-1 cells pretreated with PPG (3 mM) for 2 h before being treated with ox-LDL (50 µg/ml) for 24 h]; and ox-LDL + Lp-PLA2 siNRA [THP-1 cells pretreated with Lp-PLA2 siNRA (30 nM) for 48 h before being treated with ox-LDL (50 µg/ml) for 24 h].
Western blot analysis
Following treatment, cells were collected by centrifugation (300 × g for 10 min at 4°C), then resuspended with appropriate volume of PBS buffer, centrifuged at 300 × g for 10 min at 4°C, and the supernatant removed. The above operations were repeated twice to collect cell precipitates. The cells were lysed in mammalian cell lysis buffer (cat. no. AS1004; Aspen Biotechnology Co., Ltd.) on ice for 30 min. A pipette was used to blow repeatedly and ensure that the cells were completely lysed (8). The resulting cell lysates were clarified by centrifugation at 12,000 × g for 15 min at 4°C. BCA protein concentration assay kit (cat. no. AS1086; Aspen Biotechnology Co., Ltd.) was used to determine the protein concentration of samples. According to the concentration of the sample, the loading amount was determined to ensure that the total protein loading amount of each sample was 40 µg. The appropriate amount of 5X protein loading buffer was added to the protein sample, which was placed in a boiling water bath at 95–100°C for 5 min. The supernatants were subjected to 10% SDS-PAGE and then transferred onto nitrocellulose membranes. The membranes were blocked with 3% non-fat milk in TBS-Tween-20 buffer (50 mM Tris, 250 mM NaCl, and 0.1% Tween-20; pH 7.5) and then probed with antibodies against β-actin (1:2,500), CSE (1:400), Lp-PLA2 (1:200), t-p38MAPK (1:500) and p-p38MAPK (1:1,000) in a sealed plastic bag on a shaker at room temperature for 4 h, during which the bag was turned frequently. After three washes in TBST, the membranes were incubated with the appropriate secondary antibodies for 1 h at room temperature. The Developer and Fixer kit for Black and White Film and Papers (cat. no. P0019; Beyotime Institute of Biotechnology) was used to prepare the developer and fixing solution and the film was finally exposed to X-rays. The results were analyzed using Quantity One software (version 4.6.6; Bio-Rad Laboratories, Inc.) to determine the ratio of the grey value, and the β-actin represent the corresponding protein expression level.
Reverse transcription (RT)-PCR
Cells were collected by centrifugation at 300 × g for 6 min at 4°C, an appropriate volume of cold PBS buffer added to resuspend, centrifuged at 300 × g for 10 min at 4°C and the supernatant aspirated. This operation was repeated twice to collect the cell pellet. TRIzol® solution (1 ml; cat. no. 15596-026; Thermo Fisher Scientific, Inc.), was pipetted repeatedly to fully pipette the cells into TRIzol, 250 µl of chloroform added and the mixture stood on ice for 5 min. The mixed solution was centrifuged at 10,000 × g for 10 min at 4°C. Supernatant (500 µl) was pipetted into a 1.5 ml EP tube, an equal volume of 4°C pre-cooled isopropanol added and the mixture stood at −20°C for 15 min. The solution was centrifuged at 4°C and 10,000 × g for 10 min, the liquid discarded, 1 ml of 75% ethanol pre-cooled added at 4°C, the RNA precipitate washed and centrifuged at 4°C and 10,000 × g for 5 min and the supernatant discarded. RNase-free water (10 µl) was added to fully dissolve the RNA. First-strand cDNA synthesis was performed using PrimeScript™ RT reagent kit (cat. no. RR047A; Takara Biotechnology Co., Ltd.) with gDNA Eraser. The reaction solution (5X gDNA Eraser Buffer 2 µl, gDNA Eraser 1 µl and RNA 10 µl) was prepared on ice to remove genomic DNA and configure the reaction The PCR machine was placed at 42°C for 2 min and then cooled to 4°C. The reverse transcription reaction was performed again and the reaction solution configured on ice (5X gDNA Eraser Buffer 2 µl, gDNA Eraser 1 µl, RNA 10 µl, PrimeScript RT Enzyme Mix I 1 µl, RT Primer Mix 1 µl, 5X PrimeScript Buffer 2 4 µl and RNase Free dH2O 4 µl). Following configuration of the reaction system, it was placed on the PCR machine at 37°C for 15 min, 85°C for 5 sec and then cooled to 4°C. PCR was completed on the StepOne Real-Time PCR instrument from Thermo Fisher Scientific, Inc., each sample was placed into 3 replicate wells and the SYBR® Premix Ex Taq™ kit (cat. no. RR420A; Takara Biotechnology Co., Ltd.) was used as follows: Pre-denaturation 95°C, 1 min, 40 cycles, 95°C for 15 sec, 58°C for 20 sec, 72°C for 45 sec and extension at 72°C for 5 min. The cDNA for Lp-PLA2, CSE and β-actin was amplified using specific primers. The PCR products were subjected to electrophoresis on 1% agarose gels and visualized with ethylene bromide. The specific primers used were as follows: CSE, forward 5′-GAGGGAAGTCTTGGAAATGGC-3′ and reverse 5′-CGCAACATTTCATTTCCCG-3′; Lp-PLA2, forward 5′-CGTAAGATCTCCAGACTTCCTACTGCAATCAG-3′ and reverse 5′-GACTTAGATCTTCTCGCCGACAGCACTG-3′; and β-actin, forward 5′-ATCCTCACCCTGAAGTACC-3′ and reverse 5′-CTCCTTAATGTCACGCACG-3′.
Lp-PLA2 enzyme activity assay
Following treatment, cells were collected according to the manufacturer's instructions of the PAF Acetylhydrolase Assay kit (cat. no. 760901; Cayman Chemical Company). The samples were loaded as follows: Blank wells (no-enzyme control) contained 10 µl Ellman's reagent (DTNB), 15 µl assay buffer and 200 µl substrate solution; positive control wells (human Lp-PLA2) contained 10 µl DTNB, 5 µl assay buffer, 10 µl PAF-AH and 200 µl substrate solution; and sample wells contained 10 µl DTNB, 5 µl assay buffer, 10 µl sample and 200 µl substrate solution. The absorbance was read once every min at 405 nm using a plate reader to obtain ≥5 time points.
Measurement of CSE activity
Following treatment, THP-1 cells were collected and homogenized in 50 mM ice-cold potassium phosphate buffer (pH 6.8). Each 1 ml of the reaction mixture contained potassium phosphate buffer (100 mM; pH 7.4), L-cysteine (10 mM), pyridoxal 5′-phosphate (2 mM) and cell lysis solution. In the central pool, 1% zinc acetate (400 µl) was added to trap the evolved H2S. The reaction was performed in tightly stoppered cryovial test tubes in a shaking water bath at 37°C. After incubating for 120 min, the zinc acetate was collected, and N,N-dimethyl-p-phenylenediamine sulfate (20 mM; 40 µl) in 7.2 mol/l HCl was added, which was immediately followed by the addition of FeCl3 (30 mM; 40 µl) in 1.2 mol/l HCl. Subsequently, the absorbance at 670 nm was measured using a microplate reader. According to the standard curve, the protein concentrations of control group and ox-LDL group were calculated, and the release of endogenous H2S was calculated. As CSE is the synthetase of endogenous H2S in THP-1, the determination of H2S content is also a direct comparison of CSE activity. The experiment was repeated ≥3 times, consistent with previous studies (17).
Oil Red O (ORO) staining
THP-1 cells were pretreated with phorbol-12-myristate-13-acetate (PMA, 100 nM, cat. no. P1585; Sigma-Aldrich; Merck KGaA.) at 37°C in a humidified atmosphere with 5% CO2 for 72 h to differentiate cells into macrophages, which were then washed with PBS three times and fixed with 10% formalin at 37°C in a humidified atmosphere with 5% CO2 for 10 min. After being washed for a further three times with PBS, the cells were incubated with ORO solution (0.6 g/l in 60% isopropanol) for at 37°C in a humidified atmosphere with 5% CO2 for 30 min and washed with 70% methanol for 10 min. The number of ORO-positive cells was observed under a microscope (Olympus Corporation) at a magnification ×40. Red areas from six random fields were analysed using ImageJ v1.41o software (National Institutes of Health), and the positive area of ORO (%) was used to represent the lipid accumulation.
Statistical analysis
Each assay was performed ≥3 times, and all data are presented as the mean ± SEM. Statistical analysis was performed using unpaired Student's t-test for comparisons between two groups. One-way ANOVA followed by post hoc Tukey's test was used to evaluate the expression levels of Lp-PLA2 and p38MAPK and the positive area of ORO (%). P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using GraphPad Prism 6 (v6.01; GraphPad Software, Inc.). The number of repeats performed for each assay is indicated in the figure legends.
Results
ox-LDL increases the expression level of Lp-PLA2 but decreases the expression of CSE
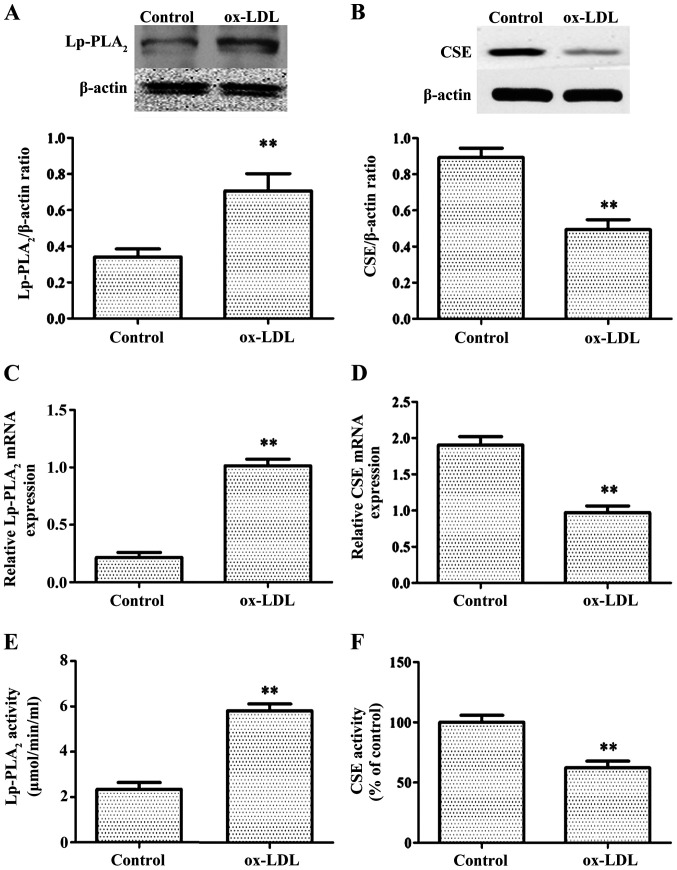
First, to determine whether ox-LDL can increase the levels of Lp-PLA2, RT-PCR, western blotting and Lp-PLA2 activity assays were used to examine mRNA levels, protein expression levels and Lp-PLA2 activity, respectively, in THP-1 cells treated with or without ox-LDL. Control THP-1 cells only exhibited weak expression levels and decreased activity of Lp-PLA2, whereas treatment with ox-LDL for 24 h significantly increased the expression level and activity of Lp-PLA2 (Fig. 1A, C and E).
Figure 1.
ox-LDL induces Lp-PLA2 expression and inhibits CSE expression in THP-1 cells. Cells were treated with 50 µg/ml ox-LDL for 24 h. Relative protein expression levels of (A) Lp-PLA2 and (B) CSE were determined via western blot analysis. Relative mRNA expression levels of (C) Lp-PLA2 and (D) CSE were determined via reverse transcription-PCR. (E) Activity levels of Lp-PLA2 were determined using a Lp-PLA2 activity kit. (F) CSE activity was determined with a methylene blue spectrophotometric assay. The data are presented as the mean ± SEM (n=5). **P<0.01 vs. control. ox-LDL, oxidized low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; CSE, cystathionine γ-lyase.
The production of H2S primarily involves three constitutively expressed enzymes: CSE, cystathionine β-synthase and 3-mercaptopyruvate sulfurtransferase (18). CSE is present specifically in the tissues of the cardiovascular system (19). It was then determined whether ox-LDL treatment of THP-1 cells affects CSE expression levels. Under normal conditions, THP-1 exhibited high CSE expression levels and activity, whereas after ox-LDL treatment for 24 h, CSE expression levels and activity were significantly decreased (Fig. 1B, D and F).
Role of H2S in the expression level of Lp-PLA2 induced by ox-LDL
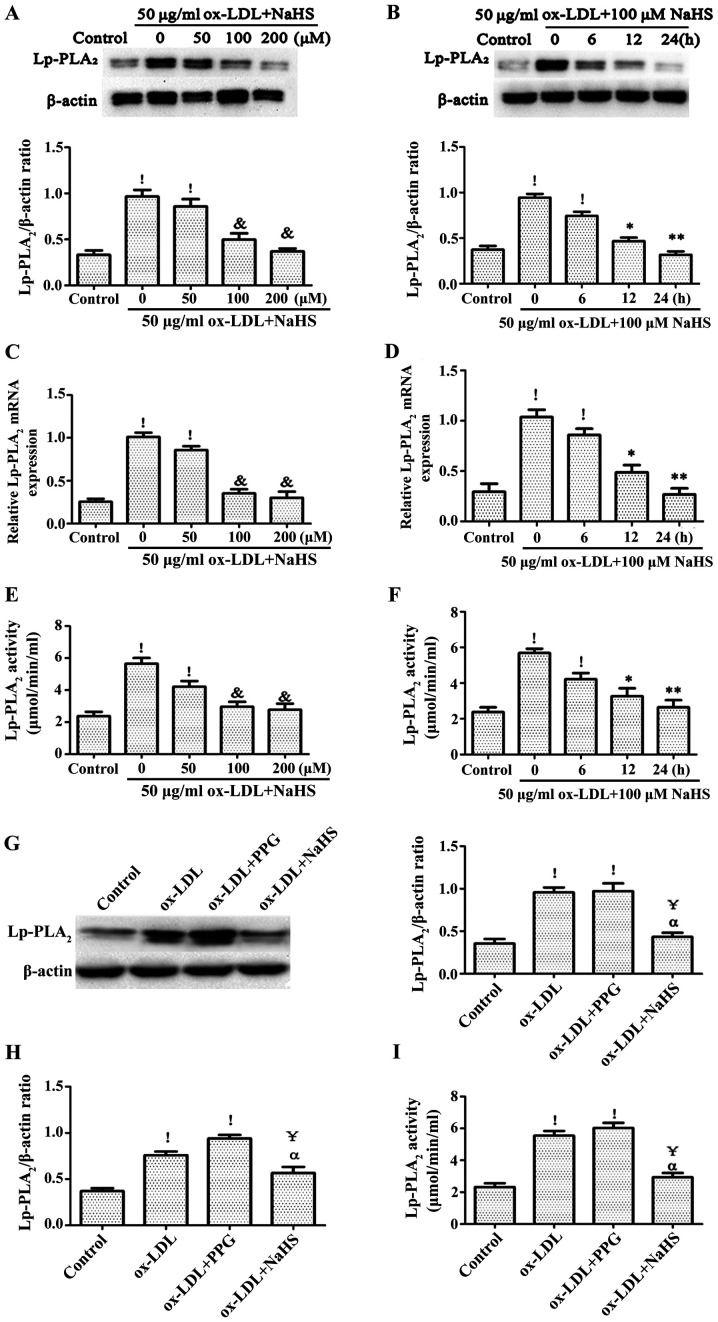
In order to confirm the effect of H2S on Lp-PLA2 expression levels and activity in ox-LDL-induced THP-1 cells, THP-1 cells were pretreated with NaHS from exogenous H2S donors for different time points (0, 6, 12 and 24 h) or different concentration (0, 50, 100 and 200 µM), followed by treatment with ox-LDL for an additional 24 h. The expression level and activity of Lp-PLA2 was detected via RT-PCR, western blotting and Lp-PLA2 activity assays. It was found that pretreatment of THP-1 cells with NaHS decreased the expression level and activity of ox-LDL-induced Lp-PLA2 in a time- and concentration-dependent manner (Fig. 2A-F). In addition, pretreatment with PPG for 2 h significantly increased ox-LDL-induced Lp-PLA2 expression levels and activity compared with the control group (Fig. 2G-I).
Figure 2.
Role of H2S in ox-LDL-induced Lp-PLA2 expression levels in THP-1 cells. Relative Lp-PLA2 protein expression levels were determined via western blotting in (A) cells pretreated with NaHS (exogenous H2S donor) for 24 h prior to incubation with 50 µg/ml ox-LDL for 24 h and (B) cells pretreated with 100 µM NaHS for 24 h prior to incubation with 50 µg/ml ox-LDL for the indicated times. Relative mRNA expression levels of Lp-PLA2 were determined via reverse transcription-PCR in (C) cells pretreated with NaHS for 24 h prior to incubation with 50 µg/ml ox-LDL for 24 h and (D) cells pretreated with 100 µM NaHS for 24 h prior to incubation with 50 µg/ml ox-LDL for the indicated times. Levels of Lp-PLA2 activity were determined with a Lp-PLA2 activity kit in (E) cells pretreated with NaHS for 24 h prior to incubation with 50 µg/ml ox-LDL for 24 h and (F) cells pretreated with 100 µM NaHS for 24 h prior to incubation with 50 µg/ml ox-LDL for the indicated times. Relative (G) protein and (H) expression levels, as well as (I) activity levels of Lp-PLA2 were determined in cells pretreated with 100 µM NaHS for 24 h or 3 mM PPG for 2 h prior to incubation with 50 µg/ml ox-LDL for 24 h. The data are presented as the mean ± SEM (n=5). !P<0.01 vs. control; &P<0.01 vs. 50 µg/ml ox-LDL + 0 µM NaHS; *P<0.05, **P<0.01 vs. 100 µM NaHS pretreated for 0 h + 50 µg/ml ox-LDL; ҰP<0.01 vs. ox-LDL; αP<0.01 vs. ox-LDL + PPG. ox-LDL, oxidized low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; PPG, DL-propargylglycine.
H2S inhibits ox-LDL-induced expression levels of Lp-PLA2 by blocking p38MAPK
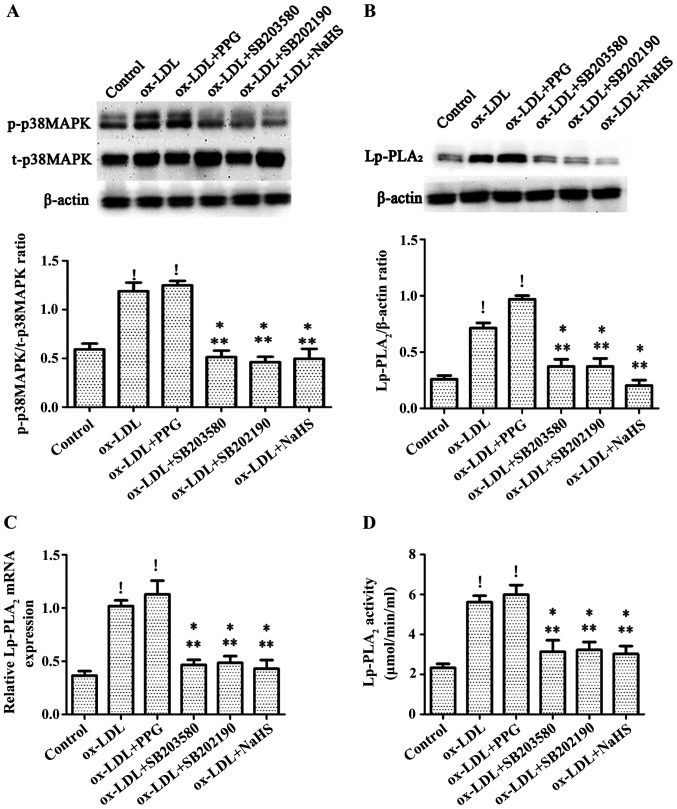
In order to determine whether H2S can decrease the expression of Lp-PLA2 in THP-1 cells by inhibiting the p38MAPK pathway, cells were treated with the p38MAPK specific inhibitors SB203580 and SB202190 for 30 min prior to incubation with 50 µg/ml ox-LDL for 24 h, following H2S preincubation for 24 h, or following PPG preincubation for 2 h then incubation with 50 µg/ml ox-LDL for 24 h. It was observed that treatment with ox-LDL and pretreatment with PPG followed by ox-LDL treatment significantly increased the expression level of p38MAPK compared with the control group (Fig. 3A). Pretreatment with NaHS significantly decreased the expression level of p38MAPK in THP-1 cells induced by ox-LDL to a level equivalent to that observed following pretreatment of cells with the p38MAPK specific inhibitors SB203580 and SB202190, which also decreased ox-LDL-induced p38MAPK effects (Fig. 3A). In addition, NaHS, SB203580 and SB202190 significantly decreased the expression level and activity of Lp-PLA2 in THP-1 cells induced by ox-LDL, whereas treatment with ox-LDL and pretreatment with PPG prior to ox-LDL treatment increased the expression level and activity of Lp-PLA2 in THP-1 cells induced by ox-LDL (Fig. 3B-D).
Figure 3.
H2S inhibits ox-LDL-induced expression levels of Lp-PLA2 by blocking p38MAPK. Cells were pretreated with the specific p38 inhibitors SB203580 (20 mM) and SB202190 (20 mM) for 30 min, with PPG (3 mM) for 2 h or with NaHS (100 µM) for 24 h prior to incubation with ox-LDL (50 µg/ml) for 24 h, after which (A) protein expression levels of p-p38MAPK and t-p38MAPK, and relative (B) protein and (C) mRNA Lp-PLA2 levels were determined via western blot analysis. (D) Lp-PLA2 activity was determined using a Lp-PLA2 activity kit. Data are presented as the mean ± SEM (n=5). !P<0.01 vs. control; *P<0.05 vs. ox-LDL; **P<0.01 vs. ox-LDL + PPG. ox-LDL, oxidized low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; PPG, DL-propargylglycine; t-, total; p-, phosphorylated.
H2S decreases lipid accumulation in macrophages by inhibiting the activity of Lp-PLA2
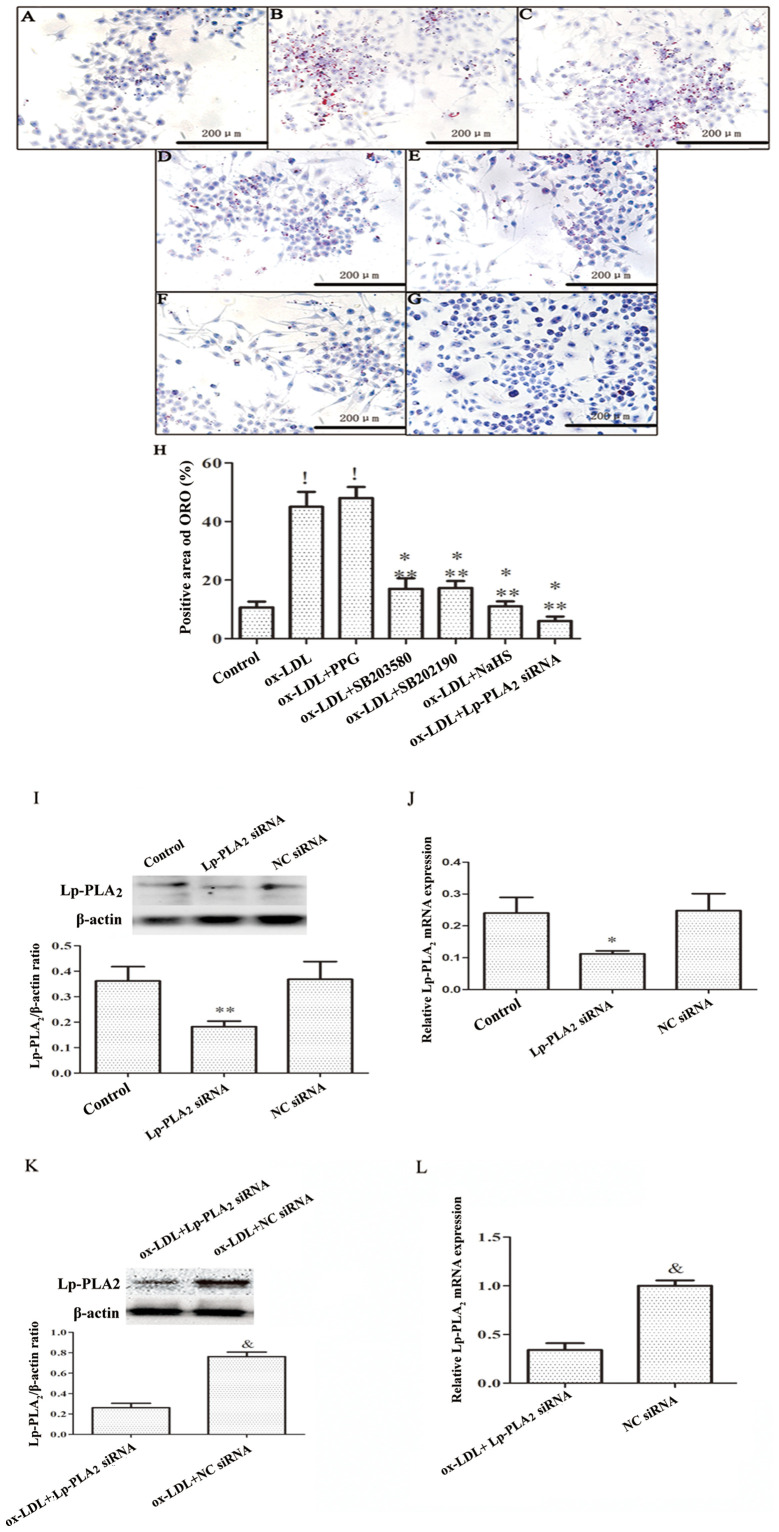
In order to determine whether H2S decreases lipid accumulation in macrophages by decreasing Lp-PLA2 expression levels, THP-1 cells were incubated with PMA for 72 h to establish a macrophage model, after which the cells were treated with ox-LDL + SB203580, ox-LDL + SB202190, ox-LDL + PPG or ox-LDL + Lp-PLA2 siRNA. The transfection efficiency of the Lp-PLA2 siRNA was verified in macrophages vs. the NC siRNA, both in the absence and presence of ox-LDL (Fig. 4I-L). It was found that Lp-PLA2 siRNA downregulated macrophage and ox-LDL-induced macrophage Lp-PLA2 expression. ORO staining was used to assess the uptake of ox-LDL by THP-1 cells. The results demonstrated that both the ox-LDL and ox-LDL + PPG treatments increased intracellular lipid accumulation (Fig. 4A-H). By contrast, NaSH, SB253580, SB202190 and Lp-PLA2 siRNA inhibited intracellular lipid accumulation. These results indicate that inhibition of Lp-PLA2 activity can decrease lipid deposition in macrophages (Fig. 4).
Figure 4.
H2S decreases lipid accumulation in macrophages by inhibiting the activity of Lp-PLA2. THP-1 cells were preincubated with PMA for 72 h to establish a macrophage model. THP-1 macrophages were pretreated with SB203580 (20 mM) and SB202190 (20 mM) for 30 min, PPG (3 mM) for 2 h, NaHS (100 µM) for 24 h or Lp-PLA2 siRNA (30 nM) for 48 h, prior to incubation with ox-LDL (50 µg/ml) for 24 h. Cells exhibiting lipid accumulation were observed and counted via light microscopy following ORO staining (magnification, ×40) in the (A) Control, (B) ox-LDL, (C) ox-LDL + PPG, (D) ox-LDL + SB203580, (E) ox-LDL + SB202190, (F) ox-LDL + NaHS and (G) ox-LDL + Lp-PLA2 siRNA groups. Images are representative of six independent repeats. (H) Quantitative analysis of the positive area of ORO (%) in each group. Detection of Lp-PLA2 siRNA transfection by (I) western blotting and (J) RT-PCR. THP-1 macrophages were pretreated with Lp-PLA2 siRNA (30 nM) or NC siRNA(30 nM) for 48 h. (K) Western blotting and (L) RT-PCR were conducted to detect the effect of Lp-PLA2 siRNA on inhibiting the expression of Lp-PLA2 in THP-1 macrophages induced by ox-LDL. !P<0.01 vs. control; *P<0.05 vs. ox-LDL; **P<0.01 vs. ox-LDL + PPG; &P<0.01 vs. ox-LDL + Lp-PLA2 siRNA. Lp-PLA2, lipoprotein-associated phospholipase A2; PMA, phorbol-12-myristate-13-acetate; PPG, DL-propargylglycine; ox-LDL, oxidized low-density lipoprotein; ORO, oil red O; RT, reverse transcription; siRNA, small interfering RNA; NC, negative control.
Discussion
Lp-PLA2 is an independent risk factor for the development of AS and is notably associated with the occurrence of numerous types of cardiovascular events, such as acute myocardial infarction (20) and unstable angina (21). Therefore, Lp-PLA2 is considered to be a potential therapeutic target for AS. In the vessel wall, the interaction between Lp-PLA2 and ox-LDL produces oxidized fatty acids and lysophosphatidylcholine, which are potent atherogenic factors (20). Moreover, H2S exerts notable cardiovascular protective effects, particularly in AS (15,22). The present study investigated the effects of H2S on the expression level of Lp-PLA2 in ox-LDL-treated THP-1 macrophages to elucidate its effect on the formation of foam cells.
Previous studies have demonstrated that a poor lipid profile (i.e., high levels of ox-LDL and triacylglycerol) in circulating blood can significantly increase the expression level and activity of Lp-PLA2 (23,24). The present study demonstrated that exposing THP-1 cells to ox-LDL significantly inhibited the expression level and activity of Lp-PLA2, which is consistent with the results of Wang et al (8). CSE is not only important for the vascular system but is also the primary H2S-producing enzyme in macrophages. Knocking out CSE can decrease plasma H2S levels and inhibit the CSE-H2S pathway in macrophages, accelerating the progression of AS in apoE−/− mice (25,26). Based on previous research, the present study demonstrated that ox-LDL can decrease the expression level and activity of CSE in THP-1 cells.
H2S is the third most crucial cardiovascular mediator after nitric oxide and carbon monoxide, and has been shown to prevent AS (27). When NaHS is dissolved in water, it acts as an exogenous H2S donor, releasing HS− and forming H2S with H+, with the H2S concentration ~33% of the initial mass of NaHS (28). Exogenous NaHS supplementation can prevent the conversion of macrophages into foam cells by increasing the level of H2S, and interfering with the production inflammatory cytokines and the occurrence of oxidative stress (29,30). Lp-PLA2 is involved in vascular inflammation and oxidative stress in macrophages, which contributes to macrophage-derived foam cell formation (16). In the present study, H2S downregulated the expression level and activity of Lp-PLA2 in THP-1 monocytes induced by ox-LDL, whereas PPG significantly upregulated the effect of ox-LDL on the expression level and activity of Lp-PLA2 in THP-1 monocytes.
Previous reports demonstrated that ox-LDL can upregulate Lp-PLA2 expression levels due to its oxidized phospholipids (oxPCs) (8), with ox-LDL/oxPCs increasing the generation of inflammatory cytokines in multiple types of inflammatory responses (31). oxPCs have been shown to activate numerous signaling pathways, such as the p38MAPK and JNK pathways (32). The p38MAPK pathway participates in a number of cellular processes, including inflammation, differentiation, cell growth, cell cycle and cell death (33). A number of studies have revealed that p38MAPK pathway activation mediates numerous mechanisms that enhance the pathogenesis of chronic inflammatory disease development, particularly in macrophages with respect to inhibiting the hardening of arteries in AS (34,35). The p38MAPK pathway has been shown to serve a key role in ox-LDL induction by upregulating Lp-PLA2 expression levels and decreasing the uptake of ox-LDL in THP-1 cells (8). Therefore, effective blocking of the p38MAPK pathway can decrease the activity of Lp-PLA2. H2S can also decrease cardiovascular inflammation by decreasing p38MPAK pathway activity (36). In addition, inflammation and apoptosis can result in the production of oxPCs by activating cAMP response elements (37); this effect can be blocked by H2S (38). The results of the present study demonstrate that H2S can decrease the expression levels and activity of Lp-PLA2 in THP-1 cells induced by ox-LDL by inhibiting the p38 MAPK pathway.
H2S can destroy lipid hydroperoxides (LOOHs) in ox-LDL, attenuate ox-LDL-induced oxidative stress, improve atherosclerosis and decrease the levels of ox-LDL (39). Previous studies have reported that LOOH is an essential component of oxPCs and serves a key role in the upregulation of Lp-PLA2 expression levels (8,40,41). The use of PPG to decrease endogenous H2S production can increase plasma lipid levels and oxidative stress, thereby aggravating the occurrence of AS (42). In the present study, ORO staining was performed to assess the lipid content in foam cells and it was observed that treatment with H2S and p38MAPK inhibitors, as well as with Lp-PLA2 siRNA, significantly decreased the lipid content in foam cells. The results of the present study, combined with those of Zhao et al (29) and Wang et al (43), indicate that H2S can decrease the activity of Lp-PLA2 in macrophages induced by ox-LDL by blocking the p38MAPK pathway, thereby decreasing lipid accumulation.
In summary, these results provide novel insights into the association between H2S and Lp-PLA2 in AS. H2S significantly decreased the expression level and activity of Lp-PLA2 in THP-1 cells induced by ox-LDL. Secondly, H2S may decrease lipid accumulation in ox-LDL-induced macrophages by decreasing Lp-PLA2 activity. Finally, H2S was shown to decrease the expression level and activity of Lp-PLA2 in THP-1 cells induced by ox-LDL by inhibiting the p38MAPK signalling pathway. Although the differential effects of H2S appear to be mediated by a typical signal cascade, the complexity of the signaling route has yet to be elucidated. Thus, the underlying mechanism of the anti-AS activity of H2S should be the focus of future investigations.
There were limitations to the present study; only cell changes were observed, and no animal experiments were performed. Therefore, it is not clear that the effect of hydrogen sulfide on atherosclerosis by regulating the expression of Lp-PLA2 in the overall model is worthy of further study.
Acknowledgements
Not applicable.
Funding Statement
The present work was supported by grants from the National Natural Science Foundation of China (grant no. 81700306), the Natural Science Foundation of Hunan Province (grant no. 2018JJ3469) and the China Postdoctoral Science Foundation (grant no. 2017M622588).
Funding
The present work was supported by grants from the National Natural Science Foundation of China (grant no. 81700306), the Natural Science Foundation of Hunan Province (grant no. 2018JJ3469) and the China Postdoctoral Science Foundation (grant no. 2017M622588).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HJH and ZSJ conceived and led the experimental design and participated in the writing of the manuscript. HJH, JQ, CZ, ZHT and SLQ performed the experiments. HJH and JQ conducted literature searches and completed the verification and revision of important knowledge content. HJH and ZSJ performed the final verification and proofreading of the article and are responsible for confirming the authenticity of the data in this section. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arroyo-Quiroz C, O'Flaherty M, Guzman-Castillo M, Capewell S, Chuquiure-Valenzuela E, Jerjes-Sanchez C, Barrientos-Gutierrez T. Explaining the increment in coronary heart disease mortality in Mexico between 2000 and 2012. PLoS One. 2020;15:e0242930. doi: 10.1371/journal.pone.0242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, Yang Y, Hu Y, Huang Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ. 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: Biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Robichaux WG, III, Mei FC, Yang W, Wang H, Sun H, Zhou Z, Milewicz DM, Teng BB, Cheng X. Epac1 (Exchange Protein Directly Activated by cAMP 1) upregulates LOX-1 (Oxidized Low-Density Lipoprotein Receptor 1) to promote foam cell formation and atherosclerosis development. Arterioscler Thromb Vasc Biol. 2020;40:e322–e335. doi: 10.1161/ATVBAHA.119.314238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Li Z, Liu B, Wu R, Gong H, Su Z, Zhang S. Isoborneol attenuates low-density lipoprotein accumulation and foam cell formation in macrophages. Drug Des Devel Ther. 2020;14:167–173. doi: 10.2147/DDDT.S233013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou L, Chen S, Yu H, Lu X, Chen J, Wang L, Huang J, Fan Z, Gu D. Associations of PLA2G7 gene polymorphisms with plasma lipoprotein-associated phospholipase A2 activity and coronary heart disease in a Chinese Han population: The Beijing atherosclerosis study. Hum Genet. 2009;125:11–20. doi: 10.1007/s00439-008-0587-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang WY, Li J, Yang D, Xu W, Zha RP, Wang YP. OxLDL stimulates lipoprotein-associated phospholipase A2 expression in THP-1 monocytes via PI3K and p38 MAPK pathways. Cardiovasc Res. 2010;85:845–852. doi: 10.1093/cvr/cvp367. [DOI] [PubMed] [Google Scholar]
- 9.Keleşoğlu M, Kızılay F, Barutçuoğlu B, Başol G, Saraç F, Mutaf I, Semerci B. The relationship between lipoprotein-associated phospholipase A2 with cardiovascular risk factors in testosterone deficiency. Turk J Urol. 2018;44:103–108. doi: 10.5152/tud.2017.30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nègre-Salvayre A, Augé N, Camaré C, Bacchetti T, Ferretti G, Salvayre R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic Biol Med. 2017;106:118–133. doi: 10.1016/j.freeradbiomed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Paapstel K, Kals J, Eha J, Tootsi K, Ottas A, Piir A, Jakobson M, Lieberg J, Zilmer M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr Metab Cardiovasc Dis. 2018;28:44–52. doi: 10.1016/j.numecd.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Uchida Y, Kameda N. Visualization of lipid components in human coronary plaques using color fluorescence angioscopy. Circ J. 2010;74:2181–2186. doi: 10.1253/circj.CJ-10-0451. [DOI] [PubMed] [Google Scholar]
- 13.Teoh JP, Li X, Simoncini T, Zhu D, Fu X. Estrogen-mediated gaseous signaling molecules in cardiovascular disease. Trends Endocrinol Metab. 2020;31:773–784. doi: 10.1016/j.tem.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Mao Z, Huang Y, Zhang Z, Yang X, Zhang X, Huang Y, Sawada N, Mitsui T, Takeda M, Yao J. Pharmacological levels of hydrogen sulfide inhibit oxidative cell injury through regulating the redox state of thioredoxin. Free Radic Biol Med. 2019;134:190–199. doi: 10.1016/j.freeradbiomed.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Wen YD, Wang H, Zhu YZ. The Drug developments of hydrogen sulfide on cardiovascular disease. Oxid Med Cell Longev. 2018;2018:4010395. doi: 10.1155/2018/4010395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Cui D, Quan X, Yang W, Li Y, Zhang L, Liu E. Lp-PLA2 silencing protects against ox-LDL-induced oxidative stress and cell apoptosis via Akt/mTOR signaling pathway in human THP1 macrophages. Biochem Biophys Res Commun. 2016;477:1017–1023. doi: 10.1016/j.bbrc.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP, Yang ZL, Hu F, Chen PX, Liao XX, Feng JQ. Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol Cell Biochem. 2012;363:419–426. doi: 10.1007/s11010-011-1194-6. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H. Hydrogen sulfide: Its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 19.Olson KR. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011;301:R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 20.Chen BF, Deng Y, Xu X, Ma SC, Tang LQ, Chen JF, Sun WQ, Liu SF, Liang JR. Effect of selective thrombus aspiration on serum lipoprotein-associated phospholipase A2 in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention with high thrombus burden. Acta Cardiol Sin. 2018;34:233–241. doi: 10.6515/ACS.201805_34(3).20170227A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Cong HL, Wang SF, Liu T. AMP-activated protein kinase mediates the effects of lipoprotein-associated phospholipase A2 on endothelial dysfunction in atherosclerosis. Exp Ther Med. 2017;13:1622–1629. doi: 10.3892/etm.2017.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZJ, Wu J, Guo W, Zhu YZ. Atherosclerosis and the hydrogen sulfide signaling pathway-therapeutic approaches to disease prevention. Cell Physiol Biochem. 2017;42:859–875. doi: 10.1159/000478628. [DOI] [PubMed] [Google Scholar]
- 23.Nima B, Nasli-Esfahani E, Djafarian K, Qorbani M, Hedayati M, Mishani MA, Faghfoori Z, Ahmaripour N, Hosseini S. The beneficial effects of alpha lipoic acid supplementation on Lp-PLA2 mass and its distribution between HDL and apoB-containing lipoproteins in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Oxid Med Cell Longev. 2020;2020:5850865. doi: 10.1155/2020/5850865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayşegül KT, Sema U, Yalçin AU, Sahin G, Temiz G, Kara M, Temel HE, Demirkan ES, Colak E, Colak O. Effects of lipoprotein-associated phospholipase A2 on arginase/nitric oxide pathway in hemodialysis patients. Ren Fail. 2012;34:738–743. doi: 10.3109/0886022X.2012.681535. [DOI] [PubMed] [Google Scholar]
- 25.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhoták Š, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127:2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 26.Du HP, Li J, You SJ, Wang YL, Wang F, Cao YJ, Hu LF, Liu CF. DNA methylation in cystathionine-gamma-lyase (CSE) gene promoter induced by ox-LDL in macrophages and in apoE knockout mice. Biochem Biophys Res Commun. 2016;469:776–782. doi: 10.1016/j.bbrc.2015.11.132. [DOI] [PubMed] [Google Scholar]
- 27.Lv B, Chen S, Tang C, Jin H, Du J, Huang Y. Hydrogen sulfide and vascular regulation-An update. J Adv Res. 2020;27:85–97. doi: 10.1016/j.jare.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 29.Zhao ZZ, Wang Z, Li GH, Wang R, Tan JM, Cao X, Suo R, Jiang ZS. Hydrogen sulfide inhibits macrophage-derived foam cell formation. Exp Biol Med (Maywood) 2011;236:169–176. doi: 10.1258/ebm.2010.010308. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, et al. The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(−/-) mice. Br J Pharmacol. 2013;169:1795–1809. doi: 10.1111/bph.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 32.Loidl A, Sevcsik E, Riesenhuber G, Deigner HP, Hermetter A. Oxidized phospholipids in minimally modified low density lipoprotein induce apoptotic signaling via activation of acid sphingomyelinase in arterial smooth muscle cells. J Biol Chem. 2003;278:32921–32928. doi: 10.1074/jbc.M306088200. [DOI] [PubMed] [Google Scholar]
- 33.Kukkonen-Macchi A, Sicora O, Kaczynska K, Oetken-Lindholm C, Pouwels J, Laine L, Kallio MJ. Loss of p38gamma MAPK induces pleiotropic mitotic defects and massive cell death. J Cell Sci. 2011;124:216–227. doi: 10.1242/jcs.068254. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Cui X, Lee DS, Sohn JH, Yim JH, Kim YC, Oh H. Anti-inflammatory effect of neoechinulin a from the marine fungus eurotium sp. SF-5989 through the Suppression of NF-кB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules. 2013;18:13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai C, Cong H, Hou K, Hu Y, Zhang J, Zhang Y, Zhang Y, Zhang H. Effects of miR-124-3p regulation of the p38MAPK signaling pathway via MEKK3 on apoptosis and proliferation of macrophages in mice with coronary atherosclerosis. Adv Clin Exp Med. 2020;29:803–812. doi: 10.17219/acem/121926. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Peng H, Du Q, Lin W, Liu Y. GYY4137, a hydrogen sulfidereleasing molecule, inhibits the inflammatory response by suppressing the activation of nuclear factorkappa B and mitogenactivated protein kinases in Coxsackie virus B3infected rat cardiomyocytes. Mol Med Rep. 2015;11:1837–1844. doi: 10.3892/mmr.2014.2901. [DOI] [PubMed] [Google Scholar]
- 37.Kronke G, Bochkov VN, Huber J, Gruber F, Blüml S, Fürnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 38.Yong QC, Pan TT, Hu LF, Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J Mol Cell Cardiol. 2008;44:701–710. doi: 10.1016/j.yjmcc.2008.02.157. [DOI] [PubMed] [Google Scholar]
- 39.Muellner MK, Schreier SM, Laggner H, Hermann M, Esterbauer H, Exner M, Gmeiner BM, Kapiotis S. Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL. Biochem J. 2009;420:277–281. doi: 10.1042/BJ20082421. [DOI] [PubMed] [Google Scholar]
- 40.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, et al. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: Macrophage binding and activation. J Biol Chem. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibusuki D, Nakagawa K, Asai A, Oikawa S, Masuda Y, Suzuki T, Miyazawa T. Preparation of pure lipid hydroperoxides. J Lipid Res. 2008;49:2668–2677. doi: 10.1194/jlr.D800034-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Chen ZF, Zhao B, Tang XY, Li W, Zhu LL, Tang CS, DU JB, Jin HF. Hydrogen sulfide regulates vascular endoplasmic reticulum stress in apolipoprotein E knockout mice. Chin Med J (Engl) 2011;124:3460–3467. [PubMed] [Google Scholar]
- 43.Wang XH, Wang F, You SJ, Cao YJ, Cao LD, Han Q, Liu CF, Hu LF. Dysregulation of cystathionine gamma-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell Signal. 2013;25:2255–2262. doi: 10.1016/j.cellsig.2013.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.