Abstract
Background:
Sepsis and septic shock kill over 270,000 patients per year in the United States. Sepsis transitions from a hyper-inflammatory to a hypo-inflammatory phase. Alcohol dependence is a risk factor for mortality from sepsis. Ethanol exposure impairs pathogen clearance through mechanisms that are not fully understood. Sirtuin 2 (SIRT2) interferes with pathogen clearance in immune cells but its role in the effects of ethanol on sepsis is unknown. We studied the effect of ethanol exposure on hyper- and hypo-inflammation and the role of SIRT2 in mice.
Methods:
We exposed C57Bl/6 (WT) mice to ethanol via drinking water and used intraperitoneal cecal slurry (CS)-induced sepsis to study 1) 7-day survival, 2) leukocyte adhesion (LA) in the mesenteric microcirculation during hyper- and hypo-inflammation, 3) peritoneal cavity bacterial clearance, and 4) SIRT2 expression in peritoneal macrophages. Using ethanol-exposed and lipopolysaccharide (LPS)-stimulated RAW 264.7 (RAW) cell macrophages for 4h or 24h, we studied 1) tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10) and SIRT2 expression, and 2) the effect of the SIRT2 inhibitor AK-7 on inflammatory response at 24h. Lastly, we studied the effect of ethanol on sepsis in whole body Sirt2 knock out (SIRT2KO) mice during hyper- and hypo-inflammation, bacterial clearance and 7-day survival.
Results:
WT ethanol-sepsis mice showed: 1). Decreased survival, 2). Muted LA in the microcirculation, 3). Lower plasma TNF-α and IL-6 expression, 4). Decreased bacterial clearance, and 5). Increased SIRT2 expression in peritoneal macrophages vs. vehicle-sepsis. Ethanol-exposed LPS-stimulated RAW cells showed: 1). Muted TNF-α, IL-6 and increased IL-10 expression at 4h, 2). Endotoxin tolerance at 24h, and 3). Reversal of endotoxin tolerance with the SIRT2 inhibitor AK-7. Ethanol-exposed SIRT2KO-sepsis mice showed greater 7-day survival, LA, and bacterial clearance than WT ethanol-sepsis mice.
Conclusion:
Ethanol exposure decreases survival and reduces the inflammatory response to sepsis via increased SIRT2 expression. SIRT2 is a potential therapeutic target in ethanol with sepsis.
Introduction:
Sepsis burden is rising in the United States and around the world (Buchman et al., 2020, Rudd et al., 2020). Sepsis is the most expensive condition in the US (Torio and Moore, 2006). Alcohol dependence, reported by one in eight ICU-patients, increases sepsis-related mortality even further (O’Brien et al., 2007). Immune response in sepsis transitions from an early/hyper-inflammatory to a late/hypo-inflammatory and immunosuppressive phase within hours (Vachharajani et al., 2014). Hyper-inflammation, intended for pathogen clearance, cannot be sustained since it attacks the host tissue and organs indiscriminately. The compensatory anti-inflammatory response (CARS) helps immune cells activated during hyper-inflammation transition to a deactivated hypo-inflammatory phase, characterized by increased anti-inflammatory and decreased pro-inflammatory mediators (Osuchowski et al., 2006, Vachharajani and McCall, 2019, Wang et al., 2018b). While nearly one-third of sepsis-related deaths occur during hyper-inflammation, majority of sepsis-mortality occurs during late sepsis (Otto et al., 2011). Evidence suggests, alcohol abuse increases mortality and represses inflammatory response to sepsis (Barros et al., 2012, Klingensmith et al., 2018, Klingensmith et al., 2017, Yoseph et al., 2013). Impaired chemotaxis in immune cells and bacterial clearance due to ethanol are reported, but the mechanisms are not fully understood (Jin et al., 2017, Parlet et al., 2015).
Microcirculation, placed at a strategic interface between systemic circulation and local tissue, reflects interactions between the two. Leukocyte adhesion prior to extravasation (intended for pathogen clearance) is the rate determining step in inflammation (Jung et al., 1998). While excessive leukocyte adhesion is implicated in systemic inflammatory response (Vachharajani et al., 2005, Lerman and Kim, 2015, Sessler et al., 1995, Abrams et al., 2013, Vachharajani et al., 2006, Vachharajani et al., 2010, Wang et al., 2015, Liu et al., 2015), muted inflammatory response is implicated in hypo-inflammation and inability to clear pathogen (Miwa et al., 1997, Ren et al., 2010). Using leukocyte adhesion in the mesenteric microcirculation as a marker for inflammation and endotoxin tolerance as a marker for hypo-inflammation and immunosuppression (Biswas and Lopez-Collazo, 2009), we reported phases of sepsis in vivo in mice (Vachharajani et al., 2014, Wang et al., 2016). Similar to cell models in vitro (Chan et al., 2005, Chen et al., 2009), the early/hyper-inflammatory and endotoxin-sensitive phase of sepsis transitions to an endotoxin-tolerant-hypo-inflammatory phase with decreased bacterial clearance in vivo (Vachharajani et al., 2014, Wang et al., 2016). Ethanol attenuates inflammatory response and pathogen clearance in sepsis, however, whether and how it affects microvascular function/leukocyte adhesion in sepsis is not well understood.
Sirtuins (SIRTs), the NAD+ sensors, known for their anti-inflammatory and anti-oxidant properties, are a link between inflammation and metabolism (Vachharajani et al., 2016). Seven SIRTs (SIRT1–7), dispersed among cell compartments, have distinct functions of NAD+-dependent deacetylation and de-ribosylasation (Nakagawa and Guarente, 2011). SIRTs 1, 6 and 7 are primarily nuclear; SIRTs 3, 4 and 5 mitochondrial; and SIRT2 predominantly cytosolic. Under cellular stress, SIRT2 translocates to the nucleus (Korner et al., 2013, Feldman et al., 2015, Haigis and Guarente, 2006, Haigis and Sinclair, 2010, North and Verdin, 2007). All SIRTs have their own targets that determine their unique biological functions (Feldman et al., 2015).
Emerging evidence supports a crucial role for immuno-metabolic regulation of immune response to sepsis (Venet et al., 2017, Kumar, 2018). Immune cells use aerobic glycolysis to support phagocytosis/pathogen clearance during hyper- and fatty acid oxidation during hypo-inflammation as an energy source (Arts et al., 2017, Vachharajani and McCall, 2019). SIRTs, the metabolic sensors of cells, promote fatty acid oxidation (Purushotham et al., 2012, Li et al., 2011, Purushotham et al., 2009) during hibernation (Rouble and Storey, 2015). SIRTs are crucial in the immuno-metabolic re-programming in human monocytes and mouse macrophages by switching the phenotype from hyper- to hypo-inflammation (Vachharajani et al., 2014, Liu et al., 2015, Liu et al., 2012, Wang et al., 2016). SIRT1 plays a crucial role and is a therapeutic target in lean, while SIRT2 in obese mice with sepsis (Wang et al., 2016). During hyper-inflammation in obesity with sepsis, SIRT2 expression and activity decrease via direct oxidation of SIRT2(Chen et al., 2018, Wang et al., 2018a) while during hypo-inflammation, the levels of oxidized SIRT2 drop, total SIRT2 expression increases and SIRT2 deacetylates and deactivates NFκB p65 to contribute to immune repression (Wang et al., 2018a). Reports suggest enhanced pathogen clearance in SIRT2KO mice (Ciarlo et al., 2017).
Thus, the metabolic phenotype of the host is an important determinant in immune response during sepsis, SIRTs modulate this response. Alcohol consumption related metabolic dysregulation is dependent upon dose and duration of alcohol consumption (Waszkiewicz et al., 2012, Addolorato et al., 1998, Souza-Smith et al., 2017). Ethanol exposure causes immuno-metabolic dysregulation in immune cells(Slovinsky et al., 2020). However, the role of SIRTs in the transition of hyper- to hypo-inflammation in ethanol exposure with sepsis is not well understood. In this project, we studied the effect of ethanol exposure on immune response to sepsis. Using mouse and cell culture models, we determined the role of SIRT2 in modulation of inflammatory response in ethanol exposure with sepsis. We showed, for the first time, that SIRT2 contributes to ethanol-mediated immune-suppression in macrophages and increased mortality in mice during sepsis.
Materials and Methods
Reagents and antibodies
Antibodies were obtained from the following vendors: SIRT2 (Cell signaling, D4050),GAPDH (Santa Cruz Biotech, sc-322336C5), Anti rabbit IgG, HRP- linked antibody (Cell signaling, 7074), Anti mouse IgG, HRP-linked antibody (Cell signaling, 7076), Alexa Fluor 488 from Invitrogen (Carlsbad, CA, USA). Chemicals were purchased from the following vendors: AK-7 from TOCRIS Bioscience (Minneapolis, MN, USA), Ethyl alcohol from Pharmco by Greenfield global and Lipopolysaccharide (LPS) from Sigma-Aldrich (St. Louis, MO, USA). TNF-α, IL-6 and IL-10 ELISA kit were obtained from BioLegends (San Diego, CA, USA). RAW264.7 (ATCC1 TIB-71™: RAW) cell macrophages were obtained from ATCC.
Animals:
Study was approved by the Institutional Animal Care and Use Committee (IACUC) of Lerner Research Institute (LRI) and experiments were performed according to the NIH guidelines. The wild type (WT: C57Bl/6; 4–6 weeks old and 16 weeks old for cecal slurry preparation) and B6.129-SIRT2tm1.1Fwa/J (SIRT2 whole body knock out: SIRT2KO; 4–6 weeks old) breeding pairs were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and mice were bred in the AAALAC approved animal facility of LRI.
Ethanol exposure in mice:
Male and Female C57BL/6 mice (5–6 weeks old) were housed in standard animal care facilities (5 mice per cage). Age- and gender-matched mice (50% male and 50% female) were randomized and allowed free access to ethanol or water-containing bottles. Mice were exposed to increasing dose of ethanol via drinking water; 5% ethanol vol/vol 2 days, followed by 10% ethanol vol/vol for 2 days followed by 30% ethanol vol/vol for 7 days. Control mice had free access to water alone. On day 11, mice were subjected to experimental conditions as indicated.
Cecal slurry preparation:
We used cecal slurry (CS) injection model of rodent sepsis described in literature (Starr et al., 2014). Briefly, male (WT: C57Bl/6; 16 weeks old male) donor mice were euthanized by cervical dislocation under isoflurane anesthesia (1–3% Isoflurane- O2 mixture via nose cone) and cecal contents were collected using sterile technique. Pooled (from a n=20 mice) cecal contents were weighed and completely suspended with 10% glycerol-PBS (Phosphate buffer saline) at a ratio of 1-mL for every 100mg cecal contents in a sterile beaker and continually stirred on stir plate with a magnetic bar. The cecal slurry was sequentially filtered through sterile meshes (860-, 380-, 190-, and 74-μm, Bellco Glass, Inc.) to remove debris. While continuously stirring, 1–2 ml of CS stock was aliquoted into cryovials. The CS stock aliquots were placed in a pre-chilled (−80° C freezer) sterile cryogenic freezing containers (Biocision, Larkspur, CA) and kept at −80°C freezer.
Cecal slurry injection model of sepsis:
Mice were anesthetized using isoflurane anesthesia (1–3% Isoflurane- O2 mixture via nose cone) and were injected with 250 μl CS or vehicle (glycerol-PBS) intraperitoneally to induce sepsis. We used 250 μl glycerol-PBS solution to inject intraperitoneally as vehicle-control as indicated. All mice were received broad spectrum antibiotic Meropenem (25mg/Kg body weight) subcutaneously twice daily for five doses, starting at 18h post CS injection. Mice were monitored at least twice a day.
In separate cohorts of mice, we studied 1). Leukocyte adhesion using intravital microscopy during hyper-inflammatory (4h post-CS/control) and hypo-inflammatory (24h post-injury) sepsis phases (Vachharajani et al., 2014). 2). Plasma cytokine expression, and 3). Peritoneal cavity bacterial clearance and SIRT2 expression described below.
Intravital fluorescent video microscopy (IVM):
We studied leukocyte adhesion in ethanol/water exposed sepsis (CS injection) vs. vehicle (glycerol-PBS) groups at 4h (hyper-inflammatory phase) or 24h (hypo-inflammatory phase) post-CS/vehicle injections; the time points were based on our previous work (Vachharajani et al., 2014, Wang et al., 2016). We used intraperitoneal injection of ketamine (150mg/kg) +xylazine (7.5 mg/kg) to anesthetize mice and performed intravital microscopy procedures described previously (Vachharajani et al., 2014, Wang et al., 2016).In anesthetized mice, we performed jugular venous cannulation (to inject Rhodamine 6G intravenously). We performed laparotomy to expose and exteriorize small intestine (jejunum) to study mesenteric microcirculation. To visualize leukocytes, we injected Rhodamine 6G (0.005 % solution 100 microliter intravenously). The post-capillary venules (n=3–5/mouse; 3–5 mice per group) were recorded (for 1 min 10 seconds each) and leukocyte adhesion quantified. A leukocyte was considered adherent if stationary for at least 30 consecutive seconds of one minute recording analyzed. The mean of the average values of leukocyte adhesion per venule (number of adherent leukocytes/mm2 in each venule) was used to generate the mean value for each mouse which was then used to generate a group mean using GraphPad Prism described in statistical methods.
Survival study:
We studied 7-day survival in Ethanol/vehicle (water) -fed wild type mice using cecal slurry (CS) model of sepsis. Mice were injected with CS or equal volume of vehicle (glycerol-PBS) as indicated. All mice were received Meropenem (25mg/Kg body weight) subcutaneously twice daily for three days. Mice were monitored at least twice a day. Pain and distress were scored using pain scoring system and if necessary humane end points by euthanasia were followed as described in detail previously (Wang et al., 2016).
Plasma ALT, cytokine, peritoneal lavage bacterial colony forming unit (CFU):
Plasma cytokine, plasma alanine aminotransferase (ALT), and bacterial CFU in the peritoneal lavage of CS injected mice were determined at 4h and 24h post-injury (Vachharajani et al., 2014) and compared with control (glycerol-PBS injection). Briefly, mice were anesthetized using isoflurane anesthesia (1–3% Isoflurane- O2 mixture via nose cone) and whole blood collected using cardiac puncture to study plasma TNF-α and IL-6 expression. Subsequently, we euthanized mice using cervical dislocation under anesthesia (1–3% Isoflurane- O2 mixture via nose cone). Anterior abdominal wall was cleaned using 70% ethanol solution. Using aseptic precautions, sterile PBS (3 ml) was used to perform peritoneal lavage. This fluid was then used to quantify peritoneal bacterial colony forming unit (CFU) count and macrophage isolation (below). Serially diluted peritoneal lavage fluids were plated on LB agar plates using aseptic precautions and incubated overnight at 37°C. The number of aerobic bacterial colonies were counted and expressed as CFU. CFU was calculated using the following formula. CFU/ml = (Number of colonies*dilution factor)/volume of culture plated.
Peritoneal cell immunocytochemistry:
Using cytospin centrifugation, cells were transferred to a glass slide. Cells were fixed using 4% paraformaldehyde, washed with phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton-X-100 for 10 minutes. Cells were then washed with PBS, blocked for 1 hour and incubated overnight with SIRT2 primary antibody (1:250 dilution in blocking buffer) at 4 °C. Cells were then washed with PBS and incubated in the dark with secondary antibodies for 1 hour at room temperature. Cells were again washed with PBS and mounted with a DAPI-containing mounting media. Images were acquired using a Leica-confocal microscope using 63X objectives.
RAW 264.7 cells with ethanol exposure:
Using aseptic precautions, RAW 264.7 cell macrophages (RAW cells, 1X 105 cells/ml) were cultured in Dulbecco’s modified Eagle’s media (DMEM) containing 10% fetal bovine serum, penicillin and streptomycin at 37° C and 5% CO2. Cells were exposed to phosphate buffered saline (PBS, vehicle) or ethanol (final concentration 25 mM) overnight, followed by further treatment with LPS (final 100ng/mL) for 4h (Hyper-inflammation). For Hypo-inflammation, cells were exposed to vehicle or ethanol (final concentration 25 mM) in presence of LPS for 24h. Supernatant were collected and cells were lysed subsequently for cytokine ELISA and Western blot analysis. Following ethanol/vehicle treatments for 4h and 24h, cells were fixed and immunostained for SIRT2 as detailed above in peritoneal macrophage section.
Western blot analysis:
For western blot analysis, raw cells were exposed with either ethanol or vehicle and induced with LPS as mentioned previously. Post treatment, cells were washed with PBS and were lysed using RIPA lysis buffer (Thermo Fisher Scientific) along with phosphatase and protease inhibitors mixture (Roche Diagnostics). Cell debris were removed by centrifugation at 10,000 × g for 15 min and the total protein in the supernatant was estimated by BCA method (Pierce). Approximately 50μg of protein samples were denatured and reduced with SDS sample buffer and BME at 95ºC for 10 mins. Denatured samples were subjected to SDS-PAGE (4–15% gel) followed by transfer to 0.2 μm PVDF membrane. The membrane was blocked with 5% skimmed milk in Tris-buffered saline TBS with tween (0.05%) (TBST) for 90 mins at room temperature. Blot was incubated with anti SIRT2 primary antibody (1:1000dilution) overnight at 4ºC. The blot was washed thrice with TBST and incubated with anti-rabbit IgG, HRP-linked secondary antibody for 1h at room temperature. ECL (BioRad) was used for detection and images were captured using Chemi Doc Imaging system (Bio-Rad, USA).
ELISA:
Following ethanol/vehicle treatments for 4h and 24 h, supernatant collected and cells were homogenized with lysis buffer and protein content was determined. TNF-α, IL-6 and IL-10 were measured from the supernatant and cell lysates according to manufacturer’s protocol (catalogue no. # 430901). For SIRT2 inhibitor treatment, cells were treated with AK-7 (final conc. 25μm) along with 1st LPS exposure.
Statistical Analysis:
All data were expressed as the mean ± standard error of the mean (SEM) with n = 3–4 data point per experimental groups. Statistical analyses were performed using GraphPad Prism software Version 5.02 (GraphPad Software, San Diego, CA, USA). For comparing multiple groups, analysis of variance was used with a NewMann-Koyle post hoc test. Survival analysis was conducted using the Kaplan-Meier method with censoring at 7 days (168 hours). Survival curves between groups were compared with the log-rank test. Statistical significance was defined as p < 0.05.
Results:
Effect of ethanol exposure on body weight and markers of liver injury:
We studied the effect of ethanol vs. vehicle exposure on body weight, plasma ALT to assess liver injury and cytochrome P450 2E1 (CYP2E1) expression in the liver tissue as a biomarker for ethanol metabolism under three conditions: control (glycerol-PBS injection), hyper-inflammation (4h post-CS) and hypo-inflammation (24h post-CS) based on our previous reports(Wang et al., 2016, Vachharajani et al., 2014). Ethanol exposure significantly increased the expression of CYP2E1 in the liver under all three conditions versus respective vehicle-exposed groups. There were no differences in body weights between ethanol vs. vehicle in either of the three groups. While we observed a trend towards increased plasma ALT levels during hyper- and hypo-inflammatory phases vs. respective controls in vehicle and ethanol-exposed mice (indicating sepsis-effect), there were no significant differences between ethanol vs. vehicle exposure in any groups at any point (Table 1), suggesting that ethanol in the drinking itself did not result in acute liver injury.
Table 1.
Body Weights, alanine aminotransferase (ALT) and CYP2E1 protein expression in vehicle- and ethanol-fed sepsis mice.
| Vehicle |
Ethanol |
|||||
|---|---|---|---|---|---|---|
| Control | Sepsis 4h | Sepsis 24h | Control | Sepsis 4h | Sepsis 24h | |
| CYP2E1/GAPDH | 0.068±0.06 | 0.72±0.06 | 0.65±0.04 | 1.4±0.08* | 1.57±0.06* | 1.21±0.07* |
| Body weight (g) | 18.0 ± 0.7 | 18.9 ± 0.3 | 17.4 ± 0.4 | 18.8 ± 1.5 | 18.5 ± 1.2 | 17.7 ± 1.0 |
| ALT (U/ml) | 27.9 ± 1.5 | 78.4±20.0 | 54.6±7.5 | 38.2±10.0 | 60.2±21.0 | 60.0±8.4 |
p< 0.05 versus respective vehicle-exposed group
Ethanol exposure lowers survival and mutes inflammatory response in mice with sepsis:
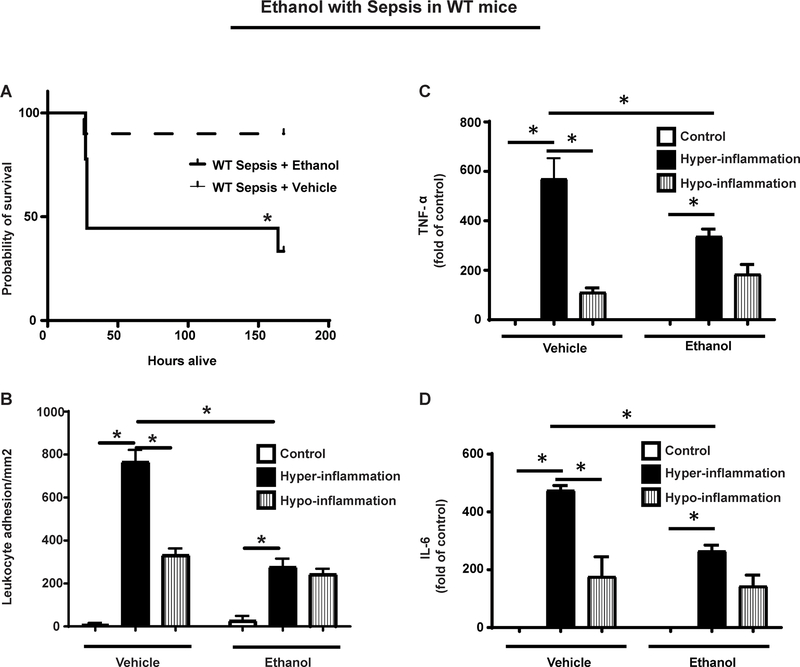
We studied 7-day survival in vehicle vs. ethanol-exposed mice with sepsis. The Kaplan-Meier curve shows that ethanol exposed mice showed lower (45%) 7-day survival vs. vehicle-exposed (90%) mice with sepsis (p<0.05) (Figure 1A).
Figure 1: Effect of ethanol on sepsis survival and leukocyte adhesion in ethanol with sepsis.
A. Ethanol with sepsis WT mice (n=10) show significantly decreased survival compared to sepsis alone (WT Sepsis; n=10). *p<0.05 vs. WT Sepsis+ Vehicle. B. Leukocyte adhesion in WT ethanol with sepsis mice was significantly lower vs. WT vehicle with sepsis (n=4) during hyper- (4h post-CS) and hypo- (24h post-CS) inflammation. Leukocyte adhesion in sepsis (CS-injected) groups was significantly higher vs. respective control (glycerol-PBS injected) groups during hyper- and hypo-inflammation. (n=4–5 in each group). C and D. Plasma TNF-α (C) and IL-6 (D) protein levels were lower in ethanol vs. vehicle-exposed WT mice with sepsis during hyper-inflammatory phase (4h post-CS). TNF-α were higher and IL-6 levels were not significantly different in ethanol vs. vehicle-exposed mice at 24h post-CS. *p<0.05
Leukocyte adhesion in the microcirculation is an early rate determining factor of inflammatory response in vivo (Jung et al., 1998). Using leukocyte adhesion in the mesenteric microcirculation, we studied in vivo inflammatory response in ethanol vs. vehicle-exposed mice as sepsis progressed through hyper- to hypo-inflammatory phase. During hyper-inflammation, the leukocyte adhesion in ethanol-exposed mice was significantly lower compared to vehicle-exposed mice. During hypo-inflammation however, there was no difference in leukocyte adhesion between ethanol versus vehicle exposed sepsis-mice (Figure 1B).Leukocyte adhesion was significantly higher in sepsis vs. respective control (vehicle or ethanol) during hyper-inflammation and lower (vs. hyper-inflammation) during the hypo-inflammatory phase. There was no difference in leukocyte adhesion between ethanol vs. vehicle control (glycerol-PBS) groups.
To further evaluate the pro-inflammatory response in sepsis, we measured plasma TNF-α and interleukin-6 (IL-6) levels in ethanol or vehicle-exposed mice during hyper-inflammatory and hypo-inflammatory phase using ELISA. We observed that the TNF-α (Figure 1C) and IL-6 (Figure 1D) levels increased during hyper-inflammatory phase vs. control and decreased during hypo-inflammation vs. hyper-inflammatory phase in both, ethanol and vehicle exposed mice. Moreover, we observed that the TNF-α and IL-6 levels in ethanol-exposed mice were lower during hyper-inflammation vs. vehicle exposure. During hypo-inflammation, TNF-α or IL-6 levels were not significantly different between ethanol vs. vehicle- exposed mice.
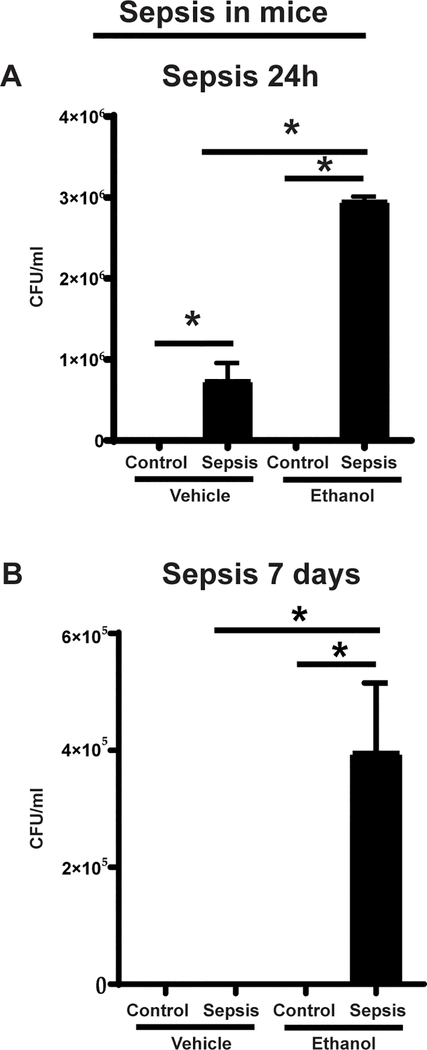
Next, to evaluate the functional significance of leukocyte adhesion/extravasation from the mesenteric microcirculation, we studied the peritoneal cavity-bacterial clearance using peritoneal lavage fluid from ethanol vs. vehicle exposed mice at 24h and 7 days (in surviving mice) post-CS injection. We observed that the bacterial growth in ethanol-exposed mice was significantly higher than that in the vehicle-exposed mice at 24h post-sepsis induction. Similarly, the bacterial growth was significantly higher in sepsis vs. respective control in both ethanol and vehicle-exposed mice (Figure 2A). Moreover, at 7-day time point, while we didn’t detect bacterial growth in vehicle-exposed group, we observed continued bacterial growth in surviving ethanol-exposed sepsis mice (Figure 2B). These data suggest impaired bacterial clearance in ethanol vs. vehicle-exposed mice with sepsis.
Figure 2: Bacterial clearance in the peritoneal cavity.
Peritoneal lavage from vehicle vs. ethanol exposed WT sepsis mice at 24h (A) and 7-days (B), post-cecal slurry (Sepsis) vs. control (glycerol-PBS) injection. Bacterial colony forming units (CFU) are presented (n=4–7 each group)* p<0.05.
Together, these data show that decreased survival in ethanol-exposed sepsis mice was accompanied by significant immune dysfunction.
Ethanol exposure mutes inflammatory response and increases SIRT2 expression in macrophages:
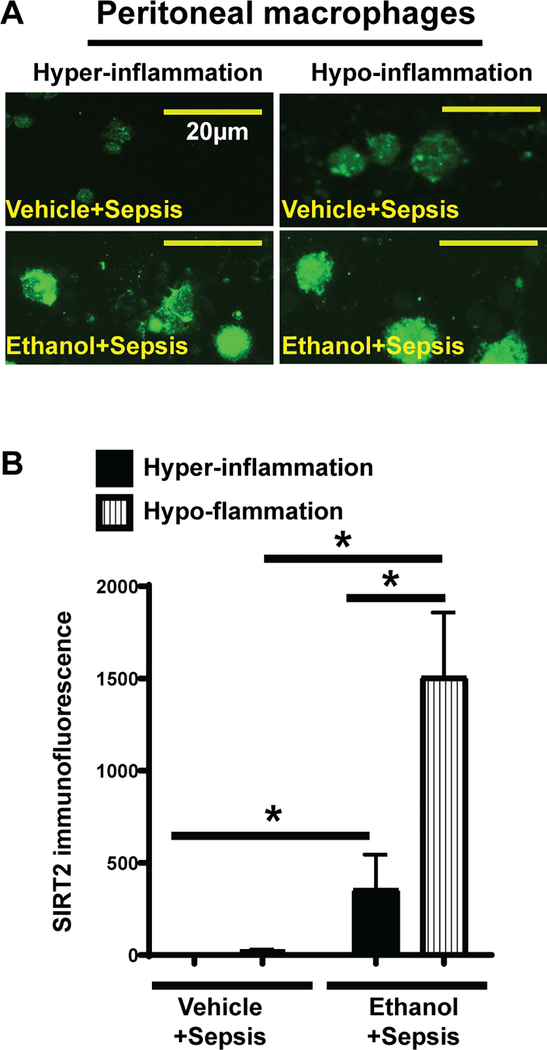
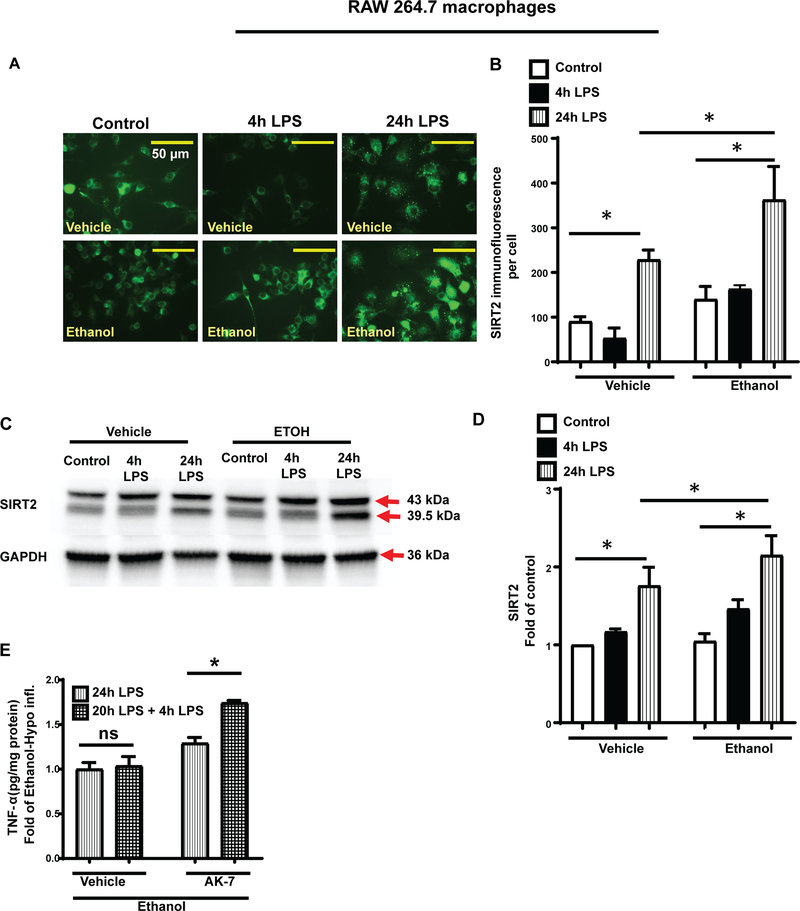
To investigate the role of SIRT2 in impairment of bacterial clearance in peritoneal cavity, we studied SIRT2 expression in peritoneal macrophages from ethanol and vehicle-exposed mice during hyper- and hypo-inflammatory phases using immunocytochemistry. SIRT2 expression in peritoneal macrophages from ethanol-exposed mice was higher during both, hyper- and hypo-inflammatory phases compared to vehicle mice shown in the images and immunofluorescence quantification (Figure 3A and B).
Figure 3: SIRT2 expression in peritoneal macrophages.
Representative image of SIRT2 expression in WT mouse peritoneal macrophages (A) and mean fluorescence intensity (B) from peritoneal macrophages (n=10 cells/ group) show increased SIRT2 expression in ethanol with sepsis (lower panels) vs. vehicle with sepsis (upper panels) during hyper- and hypo-inflammation. * p<0.05.
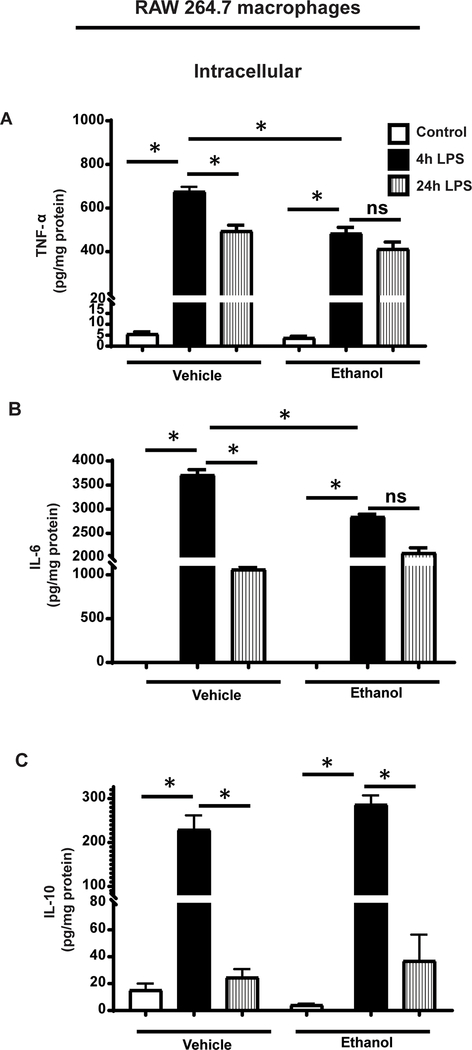
To further elucidate the effect of ethanol exposure on immune dysfunction, we used the murine macrophage-like RAW264.7 (RAW) cell line. We exposed RAW cells to ethanol/vehicle, stimulated with lipopolysaccharide (LPS) or normal saline (control). During hyper- (4h LPS) and hypo-inflammation (24h LPS)(Wang et al., 2016), we measured tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and interleukin-10 (IL-10) protein expression in cell lysates (intracellular expression) and supernatants (cell media) using ELISA. We observed that at 4h LPS stimulation, TNF-α, IL-6 and IL-10 expressions were significantly higher in ethanol and vehicle-exposed groups vs. respective control (vehicle/ethanol) in both, cell lysates (Figure 4) and supernatant (Supplemental Figure 1). With 4h LPS stimulation, the intracellular TNF-α and IL-6 expression (in the cell lysates) in ethanol-exposed cells were significantly lower vs. vehicle-exposure, indicating muted pro-inflammatory response (Figure 4A and B). Intracellular IL-10 levels were numerically higher in ethanol vs. vehicle-exposed cells, but this difference was not statistically significant (Figure 4C). In cells with 24h LPS stimulation (hypo-inflammation), the intracellular TNF-α (Figure 4A), IL-6 (Figure 4B) and IL-10 (Figure 4C) expressions decreased in both, vehicle and ethanol-exposed cells vs. respective 4h LPS groups.
Figure 4: TNF-α, IL-6 and IL-10 expression in ethanol-exposed RAW cells.
Phosphate buffered saline (Vehicle) or ethanol (25mM, Ethanol) exposed RAW264.7 cell macrophages (RAW) were stimulated with LPS and intracellular TNFα, IL-6 and IL-10 proteins were detected in cell lysates during hyper-inflammation (4h post-LPS) or hypo-inflammation (24h post-LPS) using ELISA Intracellular TNF-α (A) and IL-6 (B) levels were lower and IL-10 (C) protein levels were higher in ethanol vs. vehicle-exposed macrophages during hyper-inflammation. There was no difference in TNF-α and IL-6 levels between ethanol vs. vehicle-exposed macrophages during hypo-inflammation while IL-6 levels were higher in ethanol vs. vehicle-exposed cells. * p<0.05.
All three cytokines continued to accumulate in the supernatants from ethanol and vehicle-exposed cells at 24h post-LPS, there were no significant differences in TNF-α, IL-6 or IL-10 levels between ethanol vs. vehicle-exposed control groups. (Supplemental Figure 1). We did not find significant differences in supernatant TNF-α levels between 4h vs. 24h TNF-α in either ethanol of vehicle-exposed cells, also no significant difference between ethanol vs. vehicle-exposed cells at either 4h or 24h time point (Supplemental Figure 1A). Supernatant IL-6 levels were significantly higher in ethanol vs. vehicle-exposed cells at 24h (Supplemental Figure 1B), IL-10 levels were higher in ethanol vs. vehicle-exposed cells at 4h time point (Supplemental Figure 1C).
Next, we studied the effect of ethanol exposure on SIRT2 expression in RAW cells with LPS stimulation for 4h and 24h using immunocytochemistry and western blot analysis. Ethanol-exposed macrophages exhibited increased SIRT2 expression during at 4h and 24h LPS stimulation (Figure 5 A–D). In vehicle-exposed cells, SIRT2 expression decreased at 4h LPS and increased at 24h LPS vs. control with immunocytochemistry (Figure 5A and B) consistent with previous reports(Wang et al., 2016). We did not appreciate the decreased SIRT2 expression during hyper-inflammation with western blot analysis in vehicle-exposed cells (Figure 5C and D). We feel this discrepancy may be due to the fact that the immunocytochemistry is quantitative while western blot analysis is a qualitative assay.
Figure 5: SIRT2 expression is increased in ethanol-exposed RAW cells.
Phosphate buffered saline (Vehicle) or ethanol (25mM, Ethanol) exposed RAW264.7 cell macrophages (RAW) were stimulated with LPS and TNF-α and SIRT2 proteins detected 4h post-LPS or 24h post-LPS. A and B. Increased SIRT2 protein expression using immunocytochemistry in r ethanol vs. vehicle-exposed RAW cells during hyper- and hypo-inflammation. Representative images acquired using 63X objective shown in A and immunofluorescence analysis using Image Pro Plus software shown in B. C and D. Representative image of SIRT2 protein expression (C) and image quantification using image J software (D) detected by Western Blot analysis with GAPDH loading control revealed increased SIRT2 expression in ethanol vs. vehicle-exposed macrophages during hypo-inflammation. *p<0.05). E. TNF-α protein levels detected by ELISA in vehicle (normal saline) or AK-7-treated ethanol- exposed hypo-inflammatory (20h post-1st LPS) RAW cells stimulated with or without a 2nd LPS exposure for additional 4h revealed that vehicle-treated cells remained endotoxin tolerant while AK-7 treatment reversed endotoxin tolerance.
We and others have shown that SIRT2 is an immune repressor (Eskandarian et al., 2013, Wang et al., 2016) and SIRT2 inhibition during hypo-inflammation reverses this effect. Endotoxin tolerance is a marker for immune repression. We tested endotoxin response in ethanol vs. vehicle-exposed RAW cells treated with AK-7/vehicle (DMSO). Specifically, we treated ethanol/vehicle-exposed RAW cells with AK-7/vehicle after 1st LPS and stimulated with 2nd LPS/vehicle at 20h post-1st LPS for additional 4h. We observed that while the vehicle-exposed cells remained endotoxin tolerant (no further increase in TNF-α protein expression), AK-7 treated cells showed a significant response to 2nd LPS stimulation (Figure 5E) indicating at least partial reversal of endotoxin tolerance.
SIRT2 deficiency reverses repressed immune response and improves survival in ethanol exposed mice with sepsis:
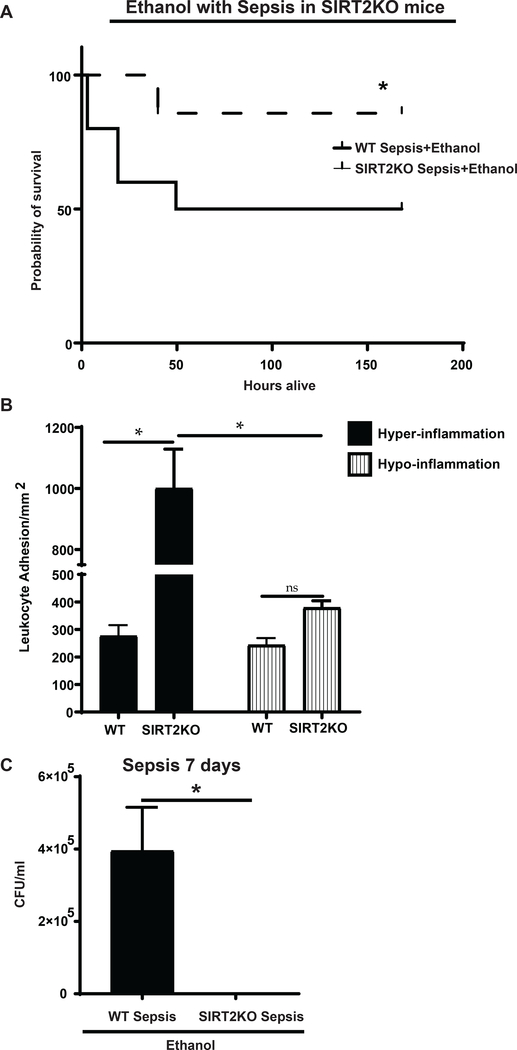
To further evaluate the effect of SIRT2 deficiency on ethanol with sepsis, we studied the effect of ethanol exposure on 7-day survival in WT vs. whole body SIRT2 knock out (SIRT2KO) mice with sepsis. We observed significantly higher survival in SIRT2KO vs. WT ethanol with sepsis mice (SIRT2KO: 90% WT: 50%; p<0.05) (Figure 6A).
Figure 6: Immune response in SIRT2KO ethanol with sepsis.
A. Effect of ethanol on sepsis survival in WT and SIRT2 KO mice. SIRT2KO ethanol with sepsis (n=8) mice show significantly higher survival vs. WT ethanol with sepsis (n=9). *p<0.05 vs. WT Sepsis.
B. Leukocyte adhesion in SIRT2KO ethanol with sepsis mice was significantly higher vs. WT ethanol with sepsis mice during hyper- (4h post-CS) and hypo- (24h post-CS) inflammation *p<0.05. C. Bacterial clearance in the peritoneal cavity of WT and SIRT2KO ethanol with sepsis mice. Peritoneal lavage from WT or SIRT2KO ethanol with sepsis mice at 7-days post-cecal slurry (CS) injection. Bacterial colony forming units (CFU) revealed that while SIRT2KO ethanol with sepsis mice cleared bacteria from peritoneal cavity, WT ethanol with sepsis mice showed continued growth (n=8–9 per group). * p<0.05.
To elucidate the effect of genetic SIRT2 deficiency on in vivo inflammatory response in ethanol with sepsis mice, we exposed WT and SIRT2KO mice to ethanol and studied leukocyte adhesion in the mesenteric microcirculation in response to CS-induced sepsis during hyper- and hypo-inflammatory phases. We observed that leukocyte adhesion in SIRT2KO groups was significantly higher than respective WT groups during hyper- inflammatory phases but not during hypo-inflammation (Figure 6B). Leukocyte adhesion decreased significantly in ethanol-exposed SIRT2KO mice during hypo- vs. hyper-inflammation indicating that the pro-inflammatory phenotype was not persistent.
To further elucidate the clinical significance of increased leukocyte adhesion in the mesenteric microcirculation, we studied peritoneal cavity-bacterial clearance in surviving ethanol-exposed SIRT2KO vs. WT sepsis mice at 7-days post-sepsis. We observed that the peritoneal cavity bacterial growth in ethanol exposed SIRT2KO mice was significantly lower (abolished) vs. WT, indicating improved bacterial clearance (Figure 6C).
Discussion:
The goal of this project was to characterize immune dysfunction and study the role of SIRT2 in ethanol with sepsis. Alcohol use disorder, a common co-morbid condition in the intensive care units, is an independent risk factor for death in sepsis patients (O’Brien et al., 2007). Using mouse and cell models of sepsis with ethanol exposure, we observed a muted immune response, impaired bacterial clearance and decreased survival in ethanol-exposed sepsis mice which was associated with increased SIRT2 expression in peritoneal macrophages. Moreover, we found that SIRT2 deficiency was associated with significantly improved immune function and greater bacterial clearance with higher 7-day survival in SIRT2KO- vs. WT ethanol with sepsis mice. Thus, we report, for the first time to our knowledge, that SIRT2, with anti-inflammatory and immune-repressor properties (Pereira et al., 2018, Eskandarian et al., 2013) plays a critical role in suppressed immune response in ethanol exposure with sepsis. While immune dysfunction in ethanol with sepsis is well described in literature, there is a relative paucity of information regarding mechanisms responsible and potential therapeutic targets (Klingensmith et al., 2018, Klingensmith et al., 2017, Yoseph et al., 2013).
To investigate the contribution of ethanol feeding during sepsis, mice were exposed to ethanol in drinking water for 11 days before induction of sepsis. Excessive ethanol consumption leads to liver injury, which itself modulates both local and systemic immune responses (Jaruga et al., 2004, Abrams et al., 2013, Shepard and Tuma, 2009). To elucidate the contribution of ethanol exposure per se (without liver injury as a confounding factor) during sepsis, we based our model on Meadows-Cook model, a well described rodent model of alcohol consumption not associated with liver injury (Meadows et al., 1993, Powers et al., 2012). Accordingly, while we report effect of ethanol exposure on immune response, ethanol itself did not affect plasma ALT levels or body weight which remained comparable to vehicle-exposed mice (Table 1) at any time points. The expression of ethanol metabolizing enzyme CYP2E1, a marker for ethanol exposure, was induced in the liver of ethanol-exposed mice (Table 1). We found that the ALT levels showed a trend toward increase in sepsis vs. respective control groups in ethanol and vehicle exposed mice indicating liver injury due to sepsis. However, the ALT values were not significantly different between ethanol vs. vehicle exposed control, hyper-inflammatory and hypo-inflammatory phases. Thus, we show that in this model of ethanol-induced immune dysfunction without liver injury.
We found a significant increase in SIRT2 expression in immune cells in ethanol with sepsis. The role of sirtuins in acute systemic inflammation is emerging; by far, SIRT1 is the most well studied sirtuin. So far, SIRT2 is a relatively unknown entity in acute inflammatory conditions. We have reported previously, that SIRT2 expression is decreased during hyper- inflammatory phase and induced during hypo-inflammation and that SIRT2 inhibition reverses hypo-inflammatory phase in obese mice with sepsis(Wang et al., 2016). Based on those data, targeted therapies focused on inducing SIRT2 during hyper- while SIRT2-inhibition during hypo-inflammatory phase of sepsis would seem intuitive. However, in this project, we report that SIRT2 expression increased early, during hyper- and was sustained during hypo-inflammatory phase, making SIRT2 inhibition a therapeutic target during both, hyper- and hypo-inflammatory phases in ethanol with sepsis. Thus, the role of SIRT2 in sepsis seems to be dependent upon the co-morbid condition of the host. Obesity and ethanol consumption, two of the most common co-morbidities (Schetz et al., 2019, O’Brien et al., 2007) in ICUs, are associated with completely different immuno-metabolic phenotypes (Vachharajani and Granger, 2009, Souza-Smith et al., 2017, Waszkiewicz et al., 2012, Addolorato et al., 1998). The role of SIRT2 in regulation of sepsis-inflammation under these two conditions reflects this difference. While it is too early to speculate, the context-dependent role of SIRT2 and SIRT2 as a therapeutic target in sepsis needs in-depth evaluation.
We show that ethanol exposure decreases 7-day survival in WT-sepsis and surviving mice with increased peritoneal cavity bacterial growth vs. vehicle-sepsis. It is possible that these mice are slow to clear infection or this was a resurgence of infection. However, these data suggest worse immune dysregulation in ethanol vs. vehicle with sepsis mice.
We show that along with muted leukocyte adhesion, the pro-inflammatory cytokine TNF-α and IL-6 levels were also lower in ethanol vs. vehicle exposure during hyper-inflammation. Similarly, we show lower intracellular TNF-α and IL-6 levels in ethanol-exposed macrophages during the hyper-inflammatory phase while both decreased significantly during hypo- vs. hyper-inflammatory phase. However, we observed continued rise in the supernatant TNF-α and IL-6 levels during hypo-inflammatory phase (supplementary figure 1). These differences may be due to the fact that the intracellular cytokine expression are reflective of current (at that point) status of the cellular inflammatory response while the increase in the supernatant for 24h reflective of ongoing secretion/degradation dynamic in the supernatant (cell culture medium).
We show improved outcomes in SIRT2KO ethanol with sepsis mice. This is an important finding, SIRT2 inhibition using small molecular SIRT2 inhibitors (such as AK-7) during hyper- and hypo-inflammatory phase of sepsis needs further evaluation. While we report that SIRT2 modulates leukocyte adhesion, the molecular mechanisms involved in the role of SIRT2 in regulating the leukocyte and/or endothelial cell function in ethanol with sepsis need further elucidation. All SIRTs, including SIRT2 are NAD+ sensors. Alcohol abuse is a NAD+ deficient state. While we found no difference in NAD+ levels in RAW cells with or without ethanol exposure (data not shown), the exact mechanisms for early SIRT2 induction in ethanol exposed cells and mice needs further clarification as well. Similarly, the genetic/epigenetic mechanism for muted inflammatory response in ethanol with sepsis need to be investigated. Lastly, evidence suggests that SIRT2 is a major metabolic regulator in cancer cells (Hamaidi et al., 2020, Al-Azzam, 2020). The role of SIRT2 in metabolic perturbations in leukocytes and/ or endothelial cells in ethanol with sepsis needs to be evaluated.
In conclusion, we report that SIRT2 represses immune function in ethanol with sepsis. SIRT2 deficiency is associated with improved immune response and decreased mortality in ethanol exposed mice with sepsis. SIRT2 may be a potential therapeutic target in ethanol with sepsis.
Supplementary Material
Acknowledgement:
The authors appreciate the technical assistance provided by the Northeast Ohio Alcohol center (P50 AA024333) and Image Core Facility at the Lerner Research Institute. We utilized the Leica SP8 confocal microscope that was purchased with funding from National Institutes of Health SIG grant 1S10OD019972-01. This work was supported by the NIH grant R01GM099807 (VV).
Bibliography
- ABRAMS ST, ZHANG N, MANSON J, LIU T, DART C, BALUWA F, WANG SS, BROHI K, KIPAR A, YU W, WANG G & TOH CH 2013. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med, 187, 160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADDOLORATO G, CAPRISTO E, GRECO AV, STEFANINI GF & GASBARRINI G 1998. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med, 244, 387–95. [DOI] [PubMed] [Google Scholar]
- AL-AZZAM N 2020. Sirtuin 6 and metabolic genes interplay in Warburg effect in cancers. J Clin Biochem Nutr, 66, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARTS RJ, GRESNIGT MS, JOOSTEN LA & NETEA MG 2017. Cellular metabolism of myeloid cells in sepsis. J Leukoc Biol, 101, 151–164. [DOI] [PubMed] [Google Scholar]
- BARROS FR, CASTRO-FARIA-NETO HC, CASTRO CL, AGUIAR NEMER AS, ROCHA EM & SILVA FONSECA VA 2012. Effects of chronic ethanol consumption in experimental sepsis. Alcohol Alcohol, 47, 677–82. [DOI] [PubMed] [Google Scholar]
- BISWAS SK & LOPEZ-COLLAZO E 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol, 30, 475–87. [DOI] [PubMed] [Google Scholar]
- BUCHMAN TG, SIMPSON SQ, SCIARRETTA KL, FINNE KP, SOWERS N, COLLIER M, CHAVAN S, OKE I, PENNINI ME, SANTHOSH A, WAX M, WOODBURY R, CHU S, MERKELEY TG, DISBROW GL, BRIGHT RA, MACURDY TE & KELMAN JA 2020. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit Care Med, 48, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN C, LI L, MCCALL CE & YOZA BK 2005. Endotoxin tolerance disrupts chromatin remodeling and NF-kappaB transactivation at the IL-1beta promoter. J Immunol, 175, 461–8. [DOI] [PubMed] [Google Scholar]
- CHEN L, LI W, QI D, LU L, ZHANG Z & WANG D 2018. Honokiol protects pulmonary microvascular endothelial barrier against lipopolysaccharide-induced ARDS partially via the Sirt3/AMPK signaling axis. Life Sci, 210, 86–95. [DOI] [PubMed] [Google Scholar]
- CHEN X, EL GAZZAR M, YOZA BK & MCCALL CE 2009. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. The Journal of biological chemistry, 284, 27857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIARLO E, HEINONEN T, THEROUDE C, HERDERSCHEE J, MOMBELLI M, LUGRIN J, PFEFFERLE M, TYRRELL B, LENSCH S, ACHA-ORBEA H, LE ROY D, AUWERX J & ROGER T 2017. Sirtuin 2 Deficiency Increases Bacterial Phagocytosis by Macrophages and Protects from Chronic Staphylococcal Infection. Front Immunol, 8, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESKANDARIAN HA, IMPENS F, NAHORI MA, SOUBIGOU G, COPPEE JY, COSSART P & HAMON MA 2013. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science, 341, 1238858. [DOI] [PubMed] [Google Scholar]
- FELDMAN JL, DITTENHAFER-REED KE, KUDO N, THELEN JN, ITO A, YOSHIDA M & DENU JM 2015. Kinetic and Structural Basis for Acyl-Group Selectivity and NAD(+) Dependence in Sirtuin-Catalyzed Deacylation. Biochemistry, 54, 3037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAIGIS MC & GUARENTE LP 2006. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev, 20, 2913–21. [DOI] [PubMed] [Google Scholar]
- HAIGIS MC & SINCLAIR DA 2010. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol, 5, 253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMAIDI I, ZHANG L, KIM N, WANG MH, ICLOZAN C, FANG B, LIU M, KOOMEN JM, BERGLUND AE, YODER SJ, YAO J, ENGELMAN RW, CREELAN BC, CONEJO-GARCIA JR, ANTONIA SJ, MULE JJ & KIM S 2020. Sirt2 Inhibition Enhances Metabolic Fitness and Effector Functions of Tumor-Reactive T Cells. Cell Metab, 32, 420–436 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARUGA B, HONG F, KIM WH, SUN R, FAN S & GAO B 2004. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: alcohol disregulation of NF-kappaB and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol, 287, G471–9. [DOI] [PubMed] [Google Scholar]
- JIN L, BATRA S & JEYASEELAN S 2017. Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathog, 13, e1006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG U, NORMAN KE, SCHARFFETTER-KOCHANEK K, BEAUDET AL & LEY K 1998. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest, 102, 1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENSMITH NJ, FAY KT, LYONS JD, CHEN CW, OTANI S, LIANG Z, CHIHADE DB, BURD EM, FORD ML & COOPERSMITH CM 2018. Chronic Alcohol Ingestion Worsens Survival and Alters Gut Epithelial Apoptosis and Cd8+ T Cell Function after Pseudomonas Aeruginosa Pneumonia-Induced Sepsis. Shock. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENSMITH NJ, YOSEPH BP, LIANG Z, LYONS JD, BURD EM, MARGOLES LM, KOVAL M, FORD ML & COOPERSMITH CM 2017. Epidermal Growth Factor Improves Intestinal Integrity and Survival in Murine Sepsis Following Chronic Alcohol Ingestion. Shock, 47, 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNER S, BOSELT S, THAU N, RATH KJ, DENGLER R & PETRI S 2013. Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS? Neurodegener Dis, 11, 141–52. [DOI] [PubMed] [Google Scholar]
- KUMAR V 2018. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int Immunopharmacol, 58, 173–185. [DOI] [PubMed] [Google Scholar]
- LERMAN YV & KIM M 2015. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets, 15, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, XU S, GILES A, NAKAMURA K, LEE JW, HOU X, DONMEZ G, LI J, LUO Z, WALSH K, GUARENTE L & ZANG M 2011. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J, 25, 1664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU TF, VACHHARAJANI V, MILLET P, BHARADWAJ MS, MOLINA AJ & MCCALL CE 2015. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J Biol Chem, 290, 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU TF, VACHHARAJANI VT, YOZA BK & MCCALL CE 2012. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem, 287, 25758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEADOWS GG, ELSTAD CA, BLANK SE, GALLUCCI RM & PFISTER LJ 1993. Alcohol consumption suppresses metastasis of B16-BL6 melanoma in mice. Clin Exp Metastasis, 11, 191–9. [DOI] [PubMed] [Google Scholar]
- MIWA S, ISOBE M, SUZUKI J, MAKUUCHI M, MIYASAKA M, YAMAZAKI S & KAWASAKI S 1997. Effect of anti-intercellular adhesion molecule-1 and anti-leukocyte function associated antigen-1 monoclonal antibodies on rat-to-mouse cardiac xenograft rejection. Surgery, 121, 681–9. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA T & GUARENTE L 2011. Sirtuins at a glance. Journal of cell science, 124, 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH BJ & VERDIN E 2007. Mitotic regulation of SIRT2 by cyclin-dependent kinase 1-dependent phosphorylation. J Biol Chem, 282, 19546–55. [DOI] [PubMed] [Google Scholar]
- O’BRIEN JM JR., LU B, ALI NA, MARTIN GS, ABEREGG SK, MARSH CB, LEMESHOW S & DOUGLAS IS 2007. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med, 35, 345–50. [DOI] [PubMed] [Google Scholar]
- OSUCHOWSKI MF, WELCH K, SIDDIQUI J & REMICK DG 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol, 177, 1967–74. [DOI] [PubMed] [Google Scholar]
- OTTO GP, SOSSDORF M, CLAUS RA, RODEL J, MENGE K, REINHART K, BAUER M & RIEDEMANN NC 2011. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care, 15, R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARLET CP, KAVANAUGH JS, HORSWILL AR & SCHLUETER AJ 2015. Chronic ethanol feeding increases the severity of Staphylococcus aureus skin infections by altering local host defenses. J Leukoc Biol, 97, 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA JM, CHEVALIER C, CHAZE T, GIANETTO Q, IMPENS F, MATONDO M, COSSART P & HAMON MA 2018. Infection Reveals a Modification of SIRT2 Critical for Chromatin Association. Cell Rep, 23, 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWERS J, ZHANG H, BATTRELL L, MEADOWS GG & TROBRIDGE GD 2012. Establishment of an immunodeficient alcohol mouse model to study the effects of alcohol on human cells in vivo. J Stud Alcohol Drugs, 73, 933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURUSHOTHAM A, SCHUG TT, XU Q, SURAPUREDDI S, GUO X & LI X 2009. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab, 9, 327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURUSHOTHAM A, XU Q, LU J, FOLEY JF, YAN X, KIM DH, KEMPER JK, LI X 2012. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1alpha/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol Cell Biol, 32, 1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN G, ZHAO X, ZHANG L, ZHANG J, L’HUILLIER A, LING W, ROBERTS AI, LE AD, SHI S, SHAO C & SHI Y 2010. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol, 184, 2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUBLE AN & STOREY KB 2015. Characterization of the SIRT family of NAD(+)-dependent protein deacetylases in the context of a mammalian model of hibernation, the thirteen-lined ground squirrel. Cryobiology, 71, 334–43. [DOI] [PubMed] [Google Scholar]
- RUDD KE, JOHNSON SC, AGESA KM, SHACKELFORD KA, TSOI D, KIEVLAN DR, COLOMBARA DV, IKUTA KS, KISSOON N, FINFER S, FLEISCHMANN-STRUZEK C, MACHADO FR, REINHART KK, ROWAN K, SEYMOUR CW, WATSON RS, WEST TE, MARINHO F, HAY SI, LOZANO R, LOPEZ AD, ANGUS DC, MURRAY CJL & NAGHAVI M 2020. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet, 395, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHETZ M, DE JONG A, DEANE AM, DRUML W, HEMELAAR P, PELOSI P, PICKKERS P, REINTAM-BLASER A, ROBERTS J, SAKR Y & JABER S 2019. Obesity in the critically ill: a narrative review. Intensive Care Med, 45, 757–769. [DOI] [PubMed] [Google Scholar]
- SESSLER CN, WINDSOR AC, SCHWARTZ M, WATSON L, FISHER BJ, SUGERMAN HJ & FOWLER AA 3RD 1995. Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med, 151, 1420–7. [DOI] [PubMed] [Google Scholar]
- SHEPARD BD & TUMA PL 2009. Alcohol-induced protein hyperacetylation: mechanisms and consequences. World J Gastroenterol, 15, 1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOVINSKY WS, SHAGHAGHI H, PARA R, ROMERO F & SUMMER R 2020. Alcohol-induced lipid dysregulation impairs glycolytic responses to LPS in alveolar macrophages. Alcohol, 83, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA-SMITH FM, FORD SM JR., SIMON L & MOLINA PE 2017. Repeated Binge-Like Alcohol Intoxication: Depot-Specific Adipose Tissue Immuno-Metabolic Dysregulation. Shock, 48, 243–250. [DOI] [PubMed] [Google Scholar]
- STARR ME, STEELE AM, SAITO M, HACKER BJ, EVERS BM & SAITO H 2014. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS One, 9, e115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORIO CM & MOORE BJ 2006. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD). [PubMed] [Google Scholar]
- VACHHARAJANI V & GRANGER DN 2009. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life, 61, 424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACHHARAJANI V & MCCALL CE 2019. Epigenetic and metabolic programming of innate immunity in sepsis. Innate Immun, 25, 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACHHARAJANI V, RUSSELL JM, SCOTT KL, CONRAD S, STOKES KY, TALLAM L, HALL J & GRANGER DN 2005. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation, 12, 183–94. [DOI] [PubMed] [Google Scholar]
- VACHHARAJANI V, VITAL S, RUSSELL J, SCOTT LK & GRANGER DN 2006. Glucocorticoids inhibit the cerebral microvascular dysfunction associated with sepsis in obese mice. Microcirculation, 13, 477–87. [DOI] [PubMed] [Google Scholar]
- VACHHARAJANI V, WANG SW, MISHRA N, EL GAZZAR M, YOZA B & MCCALL C 2010. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation, 17, 407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACHHARAJANI VT, FU LIU T, BROWN CM, WANG X, BUECHLER NL, WELLS JD, YOZA BK & MCCALL CE 2014. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACHHARAJANI VT, LIU T, WANG X, HOTH JJ, YOZA BK & MCCALL CE 2016. Sirtuins Link Inflammation and Metabolism. J Immunol Res, 2016, 8167273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENET F, DEMARET J, BLAISE BJ, ROUGET C, GIRARDOT T, IDEALISOA E, RIMMELE T, MALLET F, LEPAPE A, TEXTORIS J & MONNERET G 2017. IL-7 Restores T Lymphocyte Immunometabolic Failure in Septic Shock Patients through mTOR Activation. J Immunol, 199, 1606–1615. [DOI] [PubMed] [Google Scholar]
- WANG X, BUECHLER NL, LONG DL, FURDUI CM, YOZA BK, MCCALL CE & VACHHARAJANI V 2018a. Cysteine thiol oxidation on SIRT2 regulates inflammation in obese mice with sepsis. Inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, BUECHLER NL, MARTIN A, WELLS J, YOZA B, MCCALL CE & VACHHARAJANI V 2016. Sirtuin-2 Regulates Sepsis Inflammation in ob/ob Mice. PLoS One, 11, e0160431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, BUECHLER NL, WOODRUFF AG, LONG DL, ZABALAWI M, YOZA BK, MCCALL CE & VACHHARAJANI V 2018b. Sirtuins and Immuno-Metabolism of Sepsis. Int J Mol Sci, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, BUECHLER NL, YOZA BK, MCCALL CE & VACHHARAJANI VT 2015. Resveratrol attenuates microvascular inflammation in sepsis via SIRT-1-Induced modulation of adhesion molecules in ob/ob mice. Obesity (Silver Spring), 23, 1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASZKIEWICZ N, SZAJDA SD, ZALEWSKA A, SZULC A, KEPKA A, MINAROWSKA A, WOJEWODZKA-ZELEZNIAKOWICZ M, KONARZEWSKA B, CHOJNOWSKA S, LADNY JR & ZWIERZ K 2012. Alcohol abuse and glycoconjugate metabolism. Folia Histochem Cytobiol, 50, 1–11. [DOI] [PubMed] [Google Scholar]
- YOSEPH BP, BREED E, OVERGAARD CE, WARD CJ, LIANG Z, WAGENER ME, LEXCEN DR, LUSCZEK ER, BEILMAN GJ, BURD EM, FARRIS AB, GUIDOT DM, KOVAL M, FORD ML & COOPERSMITH CM 2013. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One, 8, e62792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.