SUMMARY
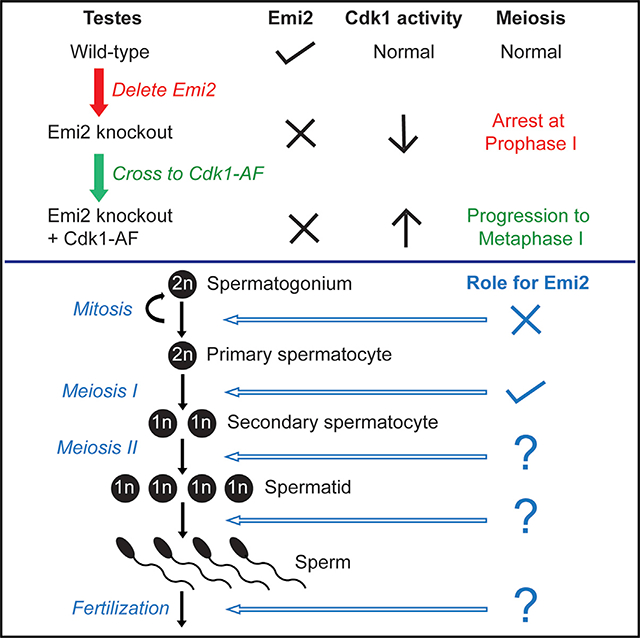
The meiotic functions of Emi2, an inhibitor of the APC/C complex, have been best characterized in oocytes where it mediates metaphase II arrest as a component of the cytostatic factor. We generated knockout mice to determine the in vivo functions of Emi2—in particular, its functions in the testis, where Emi2 is expressed at high levels. Male and female Emi2 knockout mice are viable but sterile, indicating that Emi2 is essential for meiosis but dispensable for embryonic development and mitotic cell divisions. We found that, besides regulating cell-cycle arrest in mouse eggs, Emi2 is essential for meiosis I progression in spermatocytes. In the absence of Emi2, spermatocytes arrest in early diplotene of prophase I. This arrest is associated with decreased Cdk1 activity and was partially rescued by a knockin mouse model of elevated Cdk1 activity. Additionally, we detected expression of Emi2 in spermatids and sperm, suggesting potential post-meiotic functions for Emi2.
In Brief
Gopinathan et al. use mouse genetics to characterize the in vivo functions of Emi2, a meiotic inhibitor of APC/C. Emi2 knockout mice are sterile, revealing that Emi2 is essential for oocytes and spermatocytes to complete meiotic divisions. Impaired Cdk1 activity upon loss of Emi2 contributes to spermatogenic defects.
Graphical Abstract
INTRODUCTION
Emi2 (early meiotic inhibitor 2), also called XErp1 (Xenopus Emi1-related protein 1) or Fbxo43, was originally identified in a yeast two-hybrid screen as a novel Plx-1 (Xenopus polo-like kinase)-interacting protein and was shown to mediate cytostatic factor (CSF) arrest in Xenopus eggs (Schmidt et al., 2005; Tung et al., 2005). The mechanism of CSF arrest and the essential role of Emi2 have been well characterized in frogs (Bhatt and Ferrell, 1999; Gross et al., 1999; Haccard et al., 1993; Masui, 2000; Masui and Markert, 1971; Sagata et al., 1989; Schmidt et al., 2006; Shibuya and Masui, 1988; Tunquist et al., 2002, 2003), and the role of Emi2 as a CSF component that is required for maintenance of metaphase II (MII) arrest appears to be similarly conserved in mouse oocytes (Madgwick et al., 2006; Shoji et al., 2006). Emi2 is phylogenetically closely related to Emi1 (early mitotic inhibitor 1) or Fbxo5 (Reimann et al., 2001; Shoji et al., 2006), and both proteins are inhibitors of the ubiquitin ligase activity of the APC/C complex. Essential to their APC/C inhibitory functions is the zinc-binding region (ZBR) that both proteins contain in addition to their C-terminal F-box domains (Reimann et al., 2001; Schmidt et al., 2005; Suzuki et al., 2010). While Emi1 is ubiquitously expressed, Emi2 is restricted to testis and ovary in mice (Shoji et al., 2006), suggesting that the functions of Emi2 may be specific to meiosis. The functions of Emi2 in mouse testis have not yet been studied. Interestingly, in Xenopus, Emi2 also has mitotic functions and is essential for early embryonic divisions (Tischer et al., 2012). In order to ascertain the in vivo functions of Emi2 in the mouse, we generated conditional Emi2 knockout mice, which were viable but sterile. Our analyses of the knockout mice reveal that, in addition to regulating cell-cycle arrest in oocytes, Emi2 is essential for spermatogenesis.
RESULTS
Emi2 Knockout Mice Are Viable
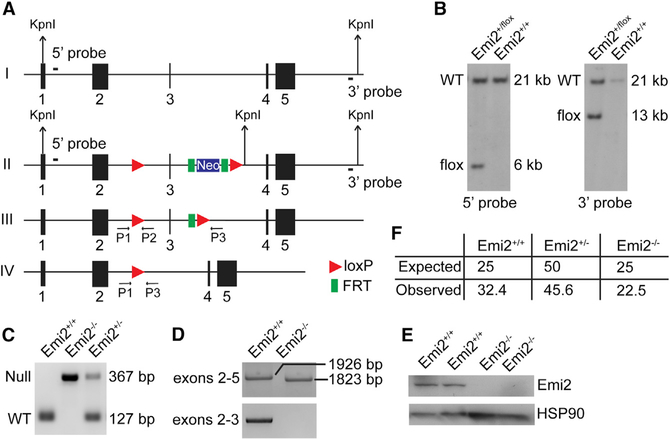
Conditional Emi2 knockout mice were generated by inserting loxP sites flanking the third exon of the Emi2 gene (Figure 1A). The resulting targeting vector was electroporated into embryonic stem cells (ESCs) to obtain heterozygous Emi2+/flox ESCs by homologous recombination (Figure 1B). Emi2+/flox ESCs were injected into mouse blastocysts and homozygous Emi2flox/flox mice were obtained through standard procedures. Emi2flox/flox mice were phenotypically normal, demonstrating that the floxed Emi2 allele is functionally wild-type. Emi2flox/flox mice were then crossed with β-actin-Cre transgenic mice (Lewandoski et al., 1997) to obtain Emi2+/null mice that were intercrossed to obtain homozygous knockout (KO) Emi2null/null (Emi2KO) mice. Genotyping of Emi2KO mice revealed the presence of a “null” allele band of the expected size (Figure 1C). Analysis of mRNA in Emi2KO testes by RT-PCR revealed the presence of a truncated transcript, the size matching that expected upon loss of the targeted exon 3 (Figure 1D, top panel). No mRNA was detected by RT-PCR in Emi2KO testes with primers encompassing the targeted exon 3 (Figure 1D, bottom panel), and western blot analysis using antibodies specific for Emi2 (Figure S6) revealed a complete absence of Emi2 protein in KO testes (Figure 1E). Emi2KO mice were obtained at the expected Mendelian frequencies (Figure 1F) and displayed a normal lifespan (up to 2 years) that was comparable to that of wild-type and heterozygous (Emi2+/null) littermates, indicating that Emi2 is not an essential gene in the mouse, thus precluding an essential function in mammalian development or mitotic cell division. To ensure that the observed dispensability of Emi2 for mouse viability was not due to developmental compensation that genetic KOs are prone to (Barbaric et al., 2007; Rossi et al., 2015), we deleted Emi2 in adult mice using tamoxifen administration in the ROSA-CreERT2 strain. Adult male mice (Emi2flox/null ROSA-CreERT2) lacking Emi2 appeared healthy, with no overt defects for up to 6 months following tamoxifen administration (data not shown).
Figure 1. Generation of Emi2KO Mice.
(A) The Emi2 genomic locus (I) was modified in ESCs using the targeting vector (described in Supplemental Experimental Procedures). LoxP recombination sites (red triangles) along with an FRT (green rectangles)-flanked neomycin-selection cassette were introduced flanking exon 3 (II). Expression of FLP recombinase results in removal of the neomycin cassette (III). Expression of Cre recombinase results in the excision of exon 3 (IV) and a frameshift, generating a null allele. FRT, flippase recognition target sites; FLP, flippase.
(B) Following KpnI digestion, 5′ [PKO1253, PKO1254] and 3′ [PKO1365, PKO1366] probes located outside of the targeting vector were used for Southern blot analysis of genomic DNA extracted from wild-type (Emi2+/+) and Emi2+/flox ES cell clones, resulting in 21 kb for wild-type or 6 kb for the floxed locus with the 5′ probe and 21 kb for wild-type or 13 kb for the floxed locus with the 3′ probe.
(C) PCR genotyping with primers P1 (PKO2025), P2 (PKO2026), and P3 (PKO2028) demonstrates absence of the Emi2 wild-type band in testis of Emi2KO mice.
(D) RT-PCR of mRNA isolated from wild-type and Emi2KO testes indicated the presence of a truncated transcript in KO testis (top panel, primers PKO2144 and PKO2147) and absence of targeted exon 3 (bottom panel, primers PKO2145 and PKO2147). Sequences for primers used are listed in Table S1. Results are representative of two independent experiments.
(E) Western blot analysis of Emi2 in testis extracts from wild-type and Emi2KO adult (P60) mice, indicating absence of Emi2 protein in KO testis; two mice per genotype were used. Results are representative of over six independent experiments. HSP90, heat shock protein 90.
(F) Expected and observed frequency (%) of P21 pups (200 pups counted in total) obtained from heterozygous Emi2 intercrosses.
See also Supplemental Experimental Procedures and Table S1.
Emi2KO Mice Are Sterile
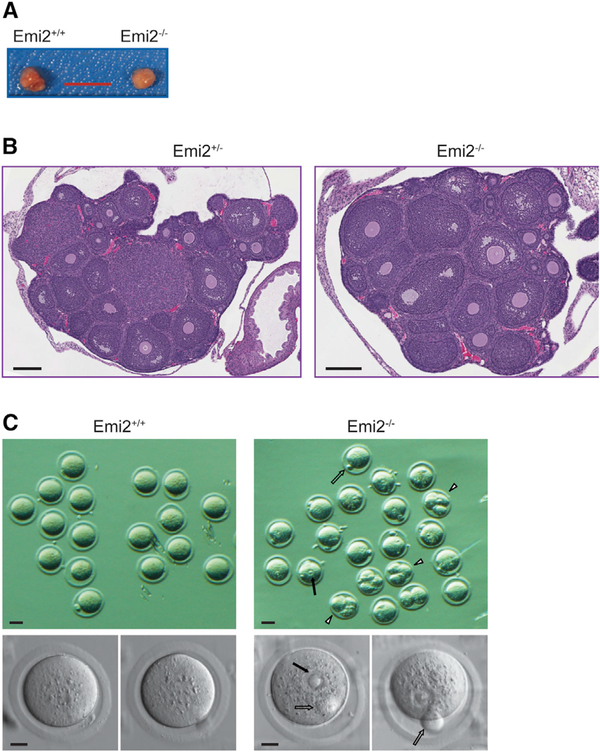
Attempted breedings of adult male and female Emi2KO mice with fertile wild-type mice did not result in pregnancies or produce any pups, indicating sterility and an essential role for Emi2 in meiosis. Emi2 heterozygous (Emi2+/null) mice were fertile and phenotypically indistinguishable from wild-type mice. Histopathological analysis revealed healthy ovaries in KO mice (Figures 2A and 2B); however, eggs collected from oviducts following superovulation displayed abnormal morphology that was distinct from wild-type oocytes arrested in metaphase of meiosis II. In addition to normal protrusion of the first polar body, Emi2KO oocytes exhibited a germinal vesicle-like structure with chromatin that is likely to be decondensed (Figure 2C). Additionally, some Emi2KO oocytes also displayed parthenogenetic divisions. This indicates successful completion of the first meiotic division by Emi2KO oocytes but defects in entry into meiosis II. These results are comparable to earlier studies that used anti-sense morpholinos or small interfering RNA (siRNA) to silence Emi2 in mouse oocytes (Madgwick et al., 2006; Shoji et al., 2006).
Figure 2. Loss of Emi2 Leads to Defects in Meiosis II Entry of Eggs upon Ovulation.
(A and B) Overall size (A) and histology (B; H&E) of ovaries from 4-week-old Emi2KO mice are phenotypically normal.
(C) Upon superovulation, eggs collected from Emi2KO mice displayed first polar body (open arrows), germinal vesicle-type structures (solid arrows), and parthenogenetic division (arrowheads).
Scale bars: 5 mm in (A); 200 μm in (B); 50 μm in (C), top panels; and 20 μm in (C), bottom panels. Results are representative of at least three mice per genotype.
Of the 11 Emi2KO mice examined, oocytes from only two KO mice displayed parthenogenetic divisions (Figure 2C). In anticipation of ovarian cancer that can result from parthenogenetic divisions (Colledge et al., 1994; Eppig et al., 1996; Hashimoto et al., 1994; Vanderhyden et al., 2003; Yoshida et al., 2007), we aged female Emi2KO mice (n = 7). However, these mice had a normal lifespan (around 2 years) and, upon necropsy, displayed normal gross morphology of ovaries (data not shown). Our results suggest that parthenogenesis is infrequent or rare in Emi2KO oocytes and may not impact ovary health.
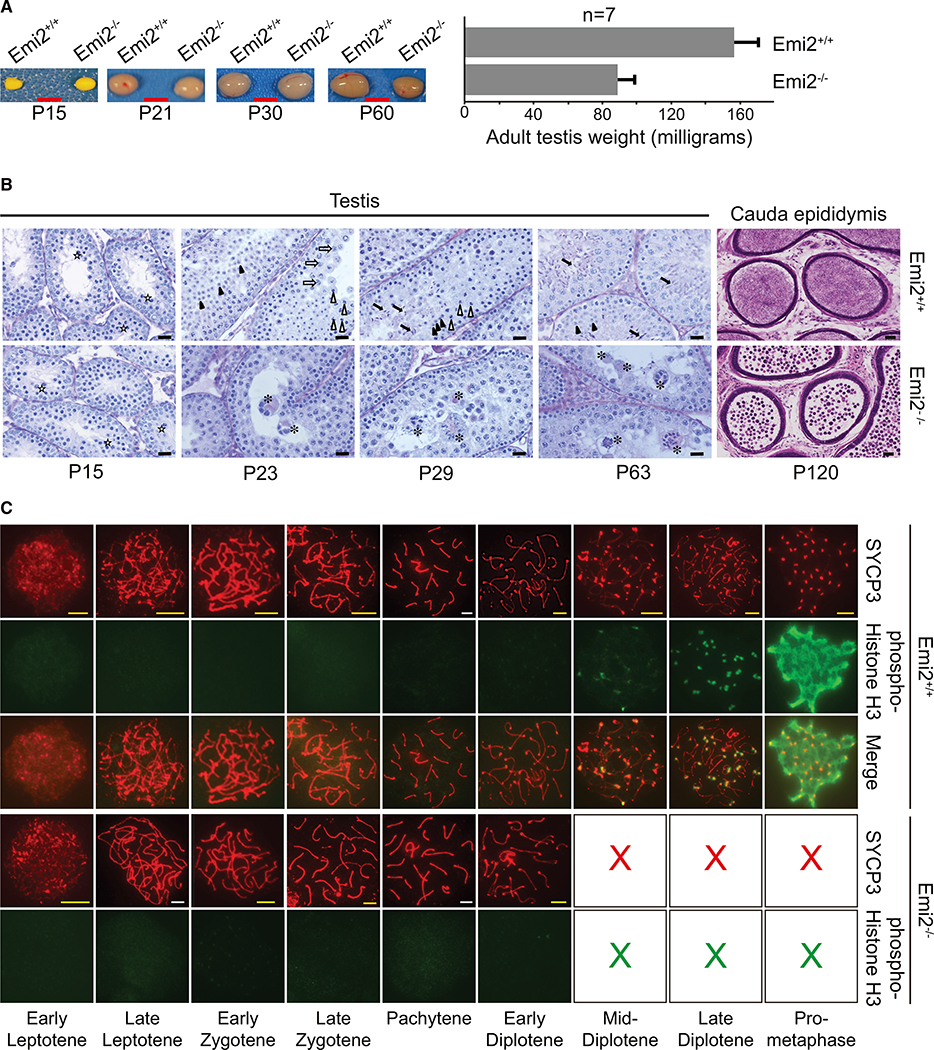
Testes from adult Emi2KO mice displayed decreased size and weight compared to wild-type littermates (Figure 3A). Histopathological analysis revealed a complete absence of spermatids in Emi2KO testes (Figure 3B, P63 testis panels) and an absence of sperm in Emi2KO epididymis (Figure 3B, P120 cauda epididymis panels), suggesting that spermatocytes are unable to complete meiotic divisions in the absence of Emi2.
Figure 3. Emi2 Is Essential for Mouse Spermatogenesis.
(A) Testes were excised from control (Emi2+/+ or Emi2+/−) or Emi2KO (Emi2−/−) mice at different ages; testes from adult Emi2KO mice are smaller in size (n = 5). P15 testes were fixed in Bouin’s fixative before images were taken. Scale bars: 5 mm; error bars represent mean ± SD. Images are representative of testes extracted from at least three mice per age per genotype.
(B) Testes sections were stained by the Periodic Acid Schiff (PAS) technique. Cauda epididymis sections were stained with H&E. Spermatids are absent in Emi2KO testes, and mature sperm are absent in Emi2KO epididymis. In the testis, open stars indicate normal pachytene spermatocytes, open arrows indicate normal diplotene spermatocytes, open arrowheads indicate meiotic metaphases in spermatocytes, closed arrowheads indicate round spermatids, closed arrows indicate elongating spermatids, asterisks indicate mono- or multi-nuclear apoptotic pachytene/diplotene spermatocytes. The round cells in the epididymis are most likely apoptotic immature germ cells. Scale bars: 20 μm for testis; 50 μm for epididymis. Results are representative of testes/cauda epididymis extracted from at least three mice per age per genotype.
(C) Chromosome spreads were prepared from testes of 3-week-old mice and immunostained for SYCP3 and phospho-S10-histone H3; spermatocytes were from Emi2KO mice arrest at early diplotene of prophase I (n = 6). Scale bars: yellow, 5 μm; and white, 10 μm. Images are representative of at least three mice per genotype.
Emi2 Is Essential for Completion of Prophase in Meiosis I of Spermatogenesis
To determine the exact stage of meiosis where Emi2KO spermatocytes arrest, we performed detailed histopathological analysis of testes from KO mice of various ages (Figure 3B). Testes collected from Emi2KO mice at P15 were indistinguishable from those of wild-type and heterozygote littermates. This was further supported by gene expression analysis, where no differentially regulated genes were seen at P15 in Emi2KO testes, in comparison to wild-type testes (Figure S1). Since by P15, primary spermatocytes enter into the first meiotic divisions, normalcy at this stage in KO testes indicates that Emi2 is not required for mitotic spermatogonial divisions or entry into meiosis I. While the most advanced cells in wild-type and heterozygote testes were round spermatids at P23 and elongated spermatids at P29, the most advanced cells in Emi2KO testes at P23 and P29 were pachytene/diplotene spermatocytes that underwent apoptosis at stage XII of the cycle of seminiferous epithelium. Adult P63 testes from wild-type and heterozygote littermates displayed complete spermatogenesis, while adult testes from Emi2KO mice contained apoptotic pachytene/diplotene spermatocytes (Figure 3B). Similar results were seen upon tamoxifen administration to Emi2flox/null ROSA26-CreERT2 adult male mice (Figure S2).
To verify that the sterility seen in Emi2KO males is specifically due to loss of Emi2 in germ cells, we crossed Emi2flox mice to Stra8-Cre transgenic mice. The expression of Cre-recombinase in Stra8-Cre transgenic male mice is first detected at P3 (postnatal day 3) in early-stage spermatogonia and persists through P7 in pre-leptotene spermatocytes (Sadate-Ngatchou et al., 2008). Stra8-Cre recombinase-mediated deletion of Emi2 in pre-meiotic cells resulted in the sterility of male mice, with spermatocytes arresting and undergoing apoptosis at pachytene/diplotene, indicating that Emi2 expression in male germ cells is indispensable for meiotic progression (Figures S3A and S3B).
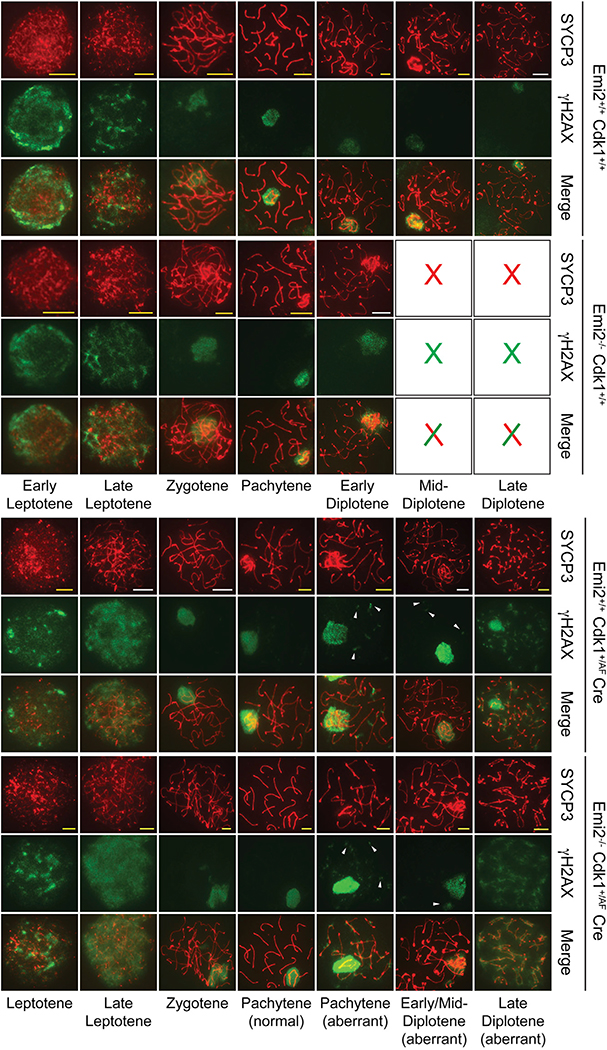
To validate the aforementioned histopathological findings, we analyzed synaptonemal complex formation in chromosome spreads from the P21 testes of control and Emi2KO mice by immunofluorescent staining for SYCP3, one of the lateral elements of the synaptonemal complex (Dobson et al., 1994; Heyting et al., 1985, 1987), in conjunction with other markers as described previously. Chromosome spreads from wild-type testes stained with antibodies against SYCP3 displayed complete prophase I. This was observed as the redistribution of SYCP3 from the lateral element to centromeric regions at diakinesis/pro-metaphase that is, in turn, associated with strong phosphorylation of histone H3 (Figure 3C). Phosphorylation of histone H3, a marker of chromosome condensation that is a characteristic feature of diplotene-to-metaphase transition (Hendzel et al., 1997; Sun and Handel, 2008), was first detected in early to mid-diplotene and increased in intensity in late diplotene, as expected in wild-type chromosomes. In contrast, the most advanced step observed in Emi2KO chromosome spreads was early to mid-diplotene (Figure 3C). Phosphorylation of histone H3 was undetectable in Emi2KO spermatocytes. Of the six Emi2KO mice analyzed, chromosome spreads from only one KO mouse testis displayed mid- to late diplotene-like structures when stained with anti-SYCP3 antibodies; however, phosphorylation of histone H3 was completely absent in these chromosomes (data not shown), indicating the inability of meiosis I chromosomes to undergo condensation and progress beyond diplotene in the absence of Emi2. The arrest of Emi2KO spermatocytes at pachytene/diplotene was corroborated by the decreased expression of cyclin A1 (see Figure 7C), which has previously been shown to be necessary for the progression of spermatocytes beyond diplotene (Liu et al., 1998; Nickerson et al., 2007).
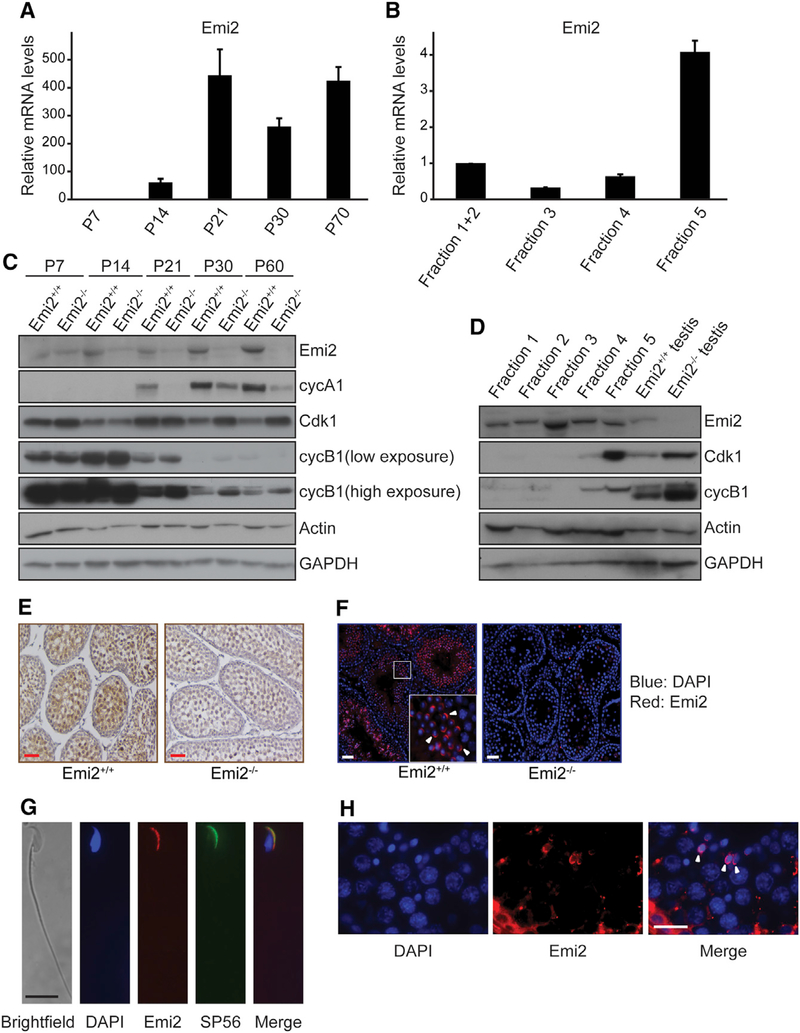
Figure 7. Emi2 Is Expressed Post-meiotically.
(A and B) Real-time PCR analysis was performed to analyze mRNA levels of genes at different ages
(A) and in elutriation fractions (B) of testes. Expression values were normalized to house-keeping gene Elongation Factor 2 (EF2) and expressed relative to P7 (A) or Fractions 1 + 2 (B). Sequences of primers used are listed in Table S2. Mean values obtained from testes extracted from three mice per age group are reported in (A). For (B), results are representative of two independent elutriation experiments, with testes from three mice used per experiment. Error bars represent mean ± SD.
(C) Protein extracts of testes collected from control or Emi2KO mice at various ages were subjected to SDS-PAGE and western blotting with the indicated antibodies. Results are representative of three independent experiments.
(D) Protein extracts of fractions collected from testicular cell elutriation were subjected to SDS-PAGE and western blotting with the indicated antibodies. Fraction 1: residual bodies + elongated spermatids; Fraction 2: residual bodies + elongated spermatids; Fraction 3: round spermatids; Fraction 4: round spermatids + spermatocytes; and Fraction 5: spermatocytes. Results are representative of three independent experiments.
(E) Testes were isolated from control or Emi2KO mice (n = 3) at P20 and processed for immunohistochemistry with antibodies against Emi2. Scale bars: 100 μm.
(F) Testes from control or Emi2KO mice (n = 3) at P60 were processed for immunofluorescent staining with antibodies against Emi2.
(G) Cauda epididymal sperm from wild-type mice (n = 3) were immunostained for Emi2 and SP56.
(H) Human testicular tissues were immunostained for Emi2.
White arrowheads point to spermatids with acrosomal staining. Results are representative of three independent experiments.
To verify that spermatocytes are able to complete the stages preceding diplotene normally in the absence of Emi2, we examined the phosphorylation of H2AX histone variant as a marker for recombination initiation (Mahadevaiah et al., 2001; Paull et al., 2000) and the expression of SYCP1, the central element of the synaptonemal complex as a marker for pachytene synapsis (de Vries et al., 2005; Dobson et al., 1994; Meuwissen et al., 1992). Similar to chromosomes from wild-type testis, strong γH2AX staining was seen in Emi2KO chromosomes until zygotene; after this, staining was confined to the XY sex body, indicating normal initiation of meiotic recombination and resolution of double-stranded breaks (DSBs) in the absence of Emi2 (Figure 4). Emi2KO chromosomes displayed normal SYCP1 staining at pachytene, and no SYCP1 was detected in diplotene (Figure S4), as is expected due to disassembly of the synaptonemal complex following pachytene (Moens, 1995). Thus, Emi2KO spermatocytes are able to progress normally through the steps preceding diplotene but are unable to complete diplotene to enter metaphase I.
Figure 4. Elevated Cdk1 Activity Results in Persistence of Double-Strand Breaks after Recombination.
Testes from Emi2+/+, Emi2−/−, Emi2+/+Cdk1+/AF ROSA26-CreERT2, and Emi2−/−Cdk1+/AFROSA26-CreERT2 mice around 3 weeks of age were used for preparing chromosome spreads that were immunostained with the indicated antibodies. For those mice expressing ROSA26-CreERT2, tamoxifen was injected intraperitoneally, and testes were excised 48 hr later. Scale bars: yellow, 5 μm; and white, 10 μm. Results are representative of at least three independent experiments.
Cdk1/Cyclin B1-Associated Kinase Activity Is Decreased in the Absence of Emi2
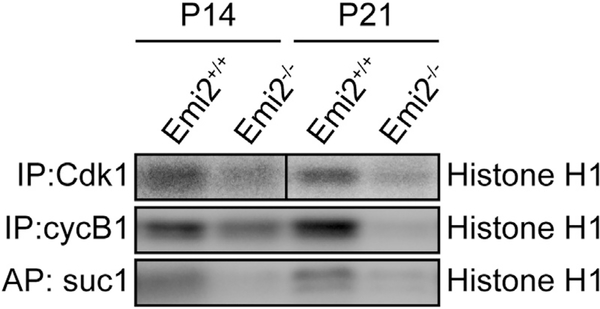
Since Cdk1/cyclin B complexes are believed to play a predominant role in the transition from prophase I to metaphase I in spermatogenesis, presumably by chromatin remodeling (Sun and Handel, 2008), we decided to analyze Cdk1/cyclin B1 expression levels and activity in Emi2KO testes at P14 and P21. At P14, wild-type and Emi2KO testes are histologically identical, whereas at later ages, apoptotic spermatocytes can be detected (Figure 3B). As a measure of Cdk1-associated activity, we performed kinase assays with immunoprecipitated Cdk1 and cyclin B1 complexes, as well as with Cdk1 complexes affinity purified using suc1 beads. When compared to wild-type testes, Emi2KO testes exhibited decreased Cdk1/cyclin B1-associated kinase activity (Figure 5) that was associated with increased inhibitory Tyr15 phosphorylation of Cdk1 (Figure 6A) at P14. Similar results were obtained at P21. No changes were detected in Cdk1 and cyclin B1 mRNA levels (data not shown) or protein expression (Figure 7C) in Emi2KO testes at P14.
Figure 5. Cdk1-Associated Activity Is Decreased in Emi2KO Testes.
Protein extracts from testes isolated from control (Emi2+/+ or Emi2+/−) or Emi2KO (Emi2−/−) mice at P14 and P21 were immunoprecipitated (IP) with antibodies against Cdk1 or cyclin B1 or affinity purified (AP) with suc1 beads, and kinase activity was measured using radiolabeled ATP and histone H1 as substrate. Kinase assay results are representative of at least two independent experiments.
Figure 6. Elevated Cdk1 Activity in Emi2KO Spermatocytes Allows for Aberrant Metaphase Entry.
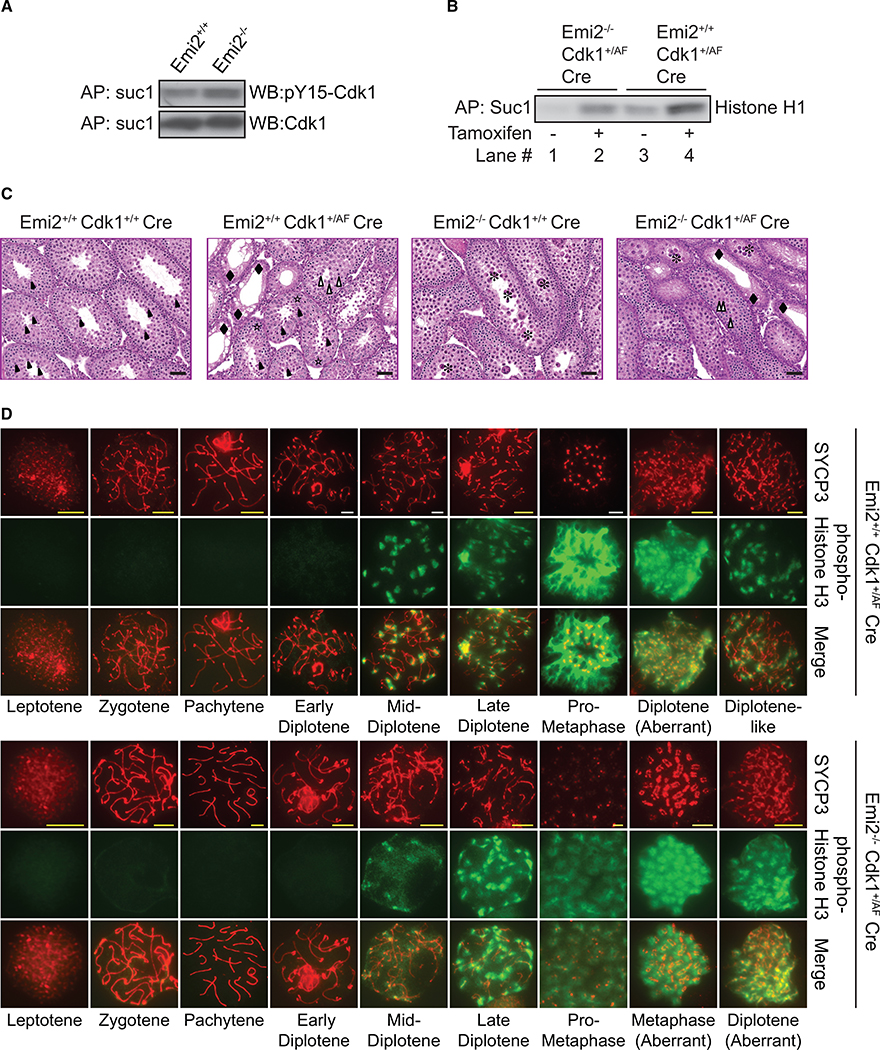
(A) Protein extracts from testes isolated from control (Emi2+/+ or Emi2+/−) or Emi2KO (Emi2−/−) mice at P14 were affinity purified (AP) with suc1 beads and subjected to SDS-PAGE and western blotting with the indicated antibodies. Cdk1 inhibitory phosphorylation was increased in Emi2KO (Emi2−/−) testes.
(B-D) Mice around 3 weeks of age expressing ROSA26-CreERT2 (n = 6) were administered tamoxifen intraperitoneally, and testes were isolated after 48 hr. (B) Protein extracts were affinity purified (AP) with suc1 beads, and kinase activity was measured using radiolabeled ATP and histone H1 as substrate. Suc1-associated activity was increased upon tamoxifen administration. Results are representative of two independent experiments. (C) Testes were fixed and processed for H&E staining. Open stars indicate normal pachytene spermatocytes, closed asterisks indicate apoptotic pachytene/diplotene spermatocytes, open arrowheads indicate meiotic metaphases in spermatocytes, closed arrowheads indicate round spermatids, and closed diamonds indicate Sertoli cells. Scale bars: 50 μm. Results are representative of testes extracted from at least five mice per genotype. (D) Chromosome spreads from testes were immunostained with the indicated antibodies. Metaphase spermatocytes were absent in Emi2KO testes but were detected upon increasing Cdk1 activity in Emi2KO testes. Scale bars: yellow, 5 μm; white, 10 μm. Results are representative of testes extracted from six mice per genotype.
Elevated Cdk1 Activity Enables Progression of Emi2KO Spermatocytes through Prophase I with Aberrant Entry into Metaphase I
Based on the aforementioned results of decreased Cdk1 activity in the absence of Emi2 (see Figure 5), we hypothesized that increasing Cdk1 activity by eliminating inhibitory phosphorylation sites would rescue the observed prophase I arrest in Emi2KO spermatocytes. Therefore, we generated a mouse model of elevated Cdk1 activity by replacing the inhibitory phosphorylation sites Thr14 and Tyr15 with non-phosphorylatable amino acids alanine and phenylalanine (Cdk1T14A/Y15F, henceforth referred to as Cdk1AF) (Adhikari et al., 2016). Heterozygous knockin mice (Cdk1+/AF) displayed early embryonic lethality and were maintained as conditional knockin mice crossed to Cre strains. The phenotypic characterization of testes from Cdk1+/AF Stra8-Cre mice is shown in Figures S3C–S3E.
To understand the impact of elevated Cdk1 activity in the absence of Emi2, we bred Cdk1+/AF ROSA26-CreERT2 with Emi2+/− mice to obtain Emi2−/−Cdk1+/AF ROSA26-CreERT2 mice. Cdk1AF activity was induced by intraperitoneal (IP) administration of tamoxifen at around age P21. Since the induction of Cdk1AF activity resulted in morbidity within 4–5 days, we analyzed the testes 48–72 hr after tamoxifen administration. Cre-mediated recombination and Cdk1AF activation post-tamoxifen administration in testes were confirmed by genotyping (data not shown) and assessing suc1-associated Cdk1 kinase activity (Figure 6B). Kinase assays indicated that Cdk1AF resulted in elevated Cdk1 activity in both Emi2−/− and Emi2+/+ but that the activity of Emi−/−Cdk1+/AF ROSA26-CreERT2(+TAM) was comparable to that of Emi2+/+ (Figure 6B; compare lanes 2 and 3). Testes from control Emi2−/−Cdk1+/+ ROSA26-CreERT2 mice that were administered tamoxifen (+TAM) were phenotypically identical to Emi2KO testes (Figure 3B) and displayed apoptotic pachytene/diplotene spermatocytes (Figure 6C). Histopathological analysis of testes from Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) mice revealed a mixed phenotype derived from Cdk1+/AF and Emi2KO, i.e., tubules with only Sertoli cells (Figure S3E) as well as tubules with apoptotic pachytene/diplotene spermatocytes. Most significantly, Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) testes also displayed the presence of metaphase spermatocytes (Figure 6C), indicating progression beyond the diplotene arrest seen in Emi2KO testes.
We further verified the presence of metaphase spermatocytes in Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) testes by the immunostaining of chromosome spreads. Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) chromosome spreads looked similar to Emi2−/−Cdk1+/+ ROSA26-CreERT2(+TAM) spreads until early diplotene (Figure 6D). However, unlike Emi2−/−Cdk1+/+ ROSA26-CreERT2(+TAM) chromosomes that arrested in early diplotene with the absence of phospho-histone H3 staining (Figures 3C and 6D), Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) chromosomes started to display phospho-histone H3 signal at mid-diplotene and progressed to pro-metaphase (Figure 6D). Intriguingly, in addition to aberrant structures with discontinuous SYCP3 staining that were also observed in Emi2+/+Cdk1+/AF ROSA26-CreERT2(+TAM) chromosomes, we observed metaphase I-like chromosomes that stained for SYCP3 in Emi2−/− Cdk1+/AF ROSA26-CreERT2(+TAM) spermatocytes, suggesting aberrant entry into metaphase without relocalization of SYCP3 (Bisig et al., 2012; Parra et al., 2004). Similar to Emi2+/+Cdk1+/AF ROSA26-CreERT2(+TAM) chromosomes, Emi2−/−Cdk1+/AF ROSA26-CreERT2(+TAM) chromosomes displayed persistence of γH2AX foci at pachytene (Figure 4, bottom panels) and stained normally for SYCP1 (Figure S4). Similar results were seen using the Mvh-CreERT2 strain (data not shown) (Gallardo et al., 2007). Thus, the defects in chromosome condensation in Emi2KO chromosome could be rescued by induction of elevated Cdk1 activity, which allows for completion of prophase I and aberrant entry into metaphase I.
Emi2 Is Expressed Post-meiotically in Spermatids and Sperm
For a more detailed understanding of the role of Emi2 in testis development, we analyzed the expression of Emi2 in testes at various ages. The expression of Cdk1, cyclin B1, and cyclin A1 was also analyzed for a comparative assessment. Emi2 expression at mRNA and protein levels could not be detected at P7 and was first detected at P14 (Figures 7A and 7C). Notably, expression increased with age, and the highest expression of Emi2 was detected in adult testes. Cdk1 protein expression in wild-type mice was highest at P7 and P21 and declined thereafter (Figure 7C). Interestingly, Cdk1 expression in Emi2KO testes did not decline after P21 and remained elevated in comparison to wild-type testis, although this was still associated with decreased Cdk1 activity, as seen previously with P14 testes (Figures 5 and 7C; data not shown). Cyclin B1 protein expression was highest at P14 and declined from P21 onward (Figure 7C). While we are unaware of the reasons behind the decrease in expression of Cdk1 at P14, in contrast to the increased expression of cyclin B1 at this age, the decline in expression of Cdk1 and cyclin B1 after P21 correlates with predicted roles for Cdk1/cyclin B1 complexes as the metaphase or maturation-promoting factor (MPF) (Cobb et al., 1999; Godet et al., 2004; Handel et al., 1995; Sun and Handel, 2008; Wiltshire et al., 1995); metaphase onset is expected to occur between P14 and P21 during the first wave of spermatogenesis (Hermo et al., 2010).
Since increased expression of Emi2 in adult mice was suggestive of post-meiotic expression in spermatids, we performed centrifugal elutriation to isolate the various germ cell populations from adult testis (Barchi et al., 2009) and analyzed Emi2 mRNA and protein expression in each fraction. Real-time qPCR analysis revealed the highest expression of Emi2 in Fraction 5 (spermatocytes), followed by Fraction 1 (elongated spermatids) (Figure 7B). Descriptions of mRNA expression patterns of Cdk1, cyclin B1, SYCP3 (as a marker of spermatocyte expression), and Spata18 (as a marker of spermatid expression) in elutriation fractions can be found in the Supplemental Information (Figure S5). Emi2 protein expression was detected in all fractions (Figure 7D). This was in contrast to Cdk1 and cyclin B1, which were predominantly detected in Fraction 5 (spermatocytes). Expression of Emi2 in spermatocytes was also verified by immunohistochemistry in P20 testes (Figure 7E). Immunofluorescent staining for Emi2 in adult testes revealed Emi2 expression in spermatids (Figure 7F). Staining was observed in the acrosomal cap region of spermatids. Similar results were seen in human testicular sections (Figure 7H). No signal was detected in spermatids when staining was performed using immunoglobulin G (IgG) control or secondary antibody alone (data not shown). To determine whether Emi2 is also expressed in sperm, we performed immunofluorescent staining on mature sperm extracted from the cauda epididymis of wild-type mice and detected staining at the acrosomal cap for Emi2 (Figure 7G). We also performed staining for SP56, an acrosomal marker (Kim et al., 2001; Wassarman, 2009). To verify our results of Emi2 expression, we analyzed previously published RNaseq data on testicular elutriation fractions (Hammoud et al., 2014) and found the results to be similar to ours; Emi2 was expressed in spermatocytes, spermatids and sperm, with the highest expression in spermatocytes (data not shown), indicating that Emi2 is important not only in meiosis but potentially also in the post-meiotic development of spermatozoa.
DISCUSSION
Emi2 in Spermatogenesis
Over a decade after the identification of Emi2 as an APC/C inhibitor in Xenopus eggs (Schmidt et al., 2005), we uncovered its essential role in mammalian spermatogenesis. In the absence of Emi2 in testes, we observed the decreased activity of Cdk1. Based on prior reports that established an essential role for Emi2 in stabilizing cyclin B1 in eggs (Madgwick et al., 2006; Wu et al., 2007), it is likely that decreased Cdk1 activity is related to cyclin B1 levels in Emi2KO spermatocytes; however, this requires further investigation. Our experiments in Figure 7C, measuring steady-state levels of cyclin B1 in whole-testis lysates that contain germ cells and support cells, cannot adequately address cyclin B1 regulation in spermatocytes. In addition to association with cyclin B1, Cdk1 activity is also regulated by phosphorylation (Lew and Kornbluth, 1996). Interestingly, decreased activity of Cdk1 in the absence of Emi2 was associated with increased Tyr15 inhibitory phosphorylation. Based on prior models that postulate phosphorylation of Emi2 by Cdk1 (Tang et al., 2008; Tischer et al., 2012; Wu et al., 2007), it is intriguing that Cdk1 would undergo inhibitory phosphorylation in the absence of Emi2. Inhibitory phosphorylation of Cdk1 on Thr14 and Tyr15 residues by Wee1 and Myt1 kinases (Parker and Piwnica-Worms, 1992) (Kornbluth et al., 1994; Liu et al., 1997; Mueller et al., 1995) is antagonized by the Cdc25 family of phosphatases (Boutros et al., 2006); however, the role of Wee1/Myt1 and Cdc25 has not yet been studied extensively in mammalian meiosis.
In addition to Cdk1, we also observed the decreased activity of Cdk2 in Emi2KO testes (Figure S7A). The decrease in Cdk2 activity persisted upon Cdk1 activation in Emi2−/−Cdk1+/AF Cre testes (Figure S7B, lanes 1 and 2). Thus, in our model, the progression to metaphase I upon induction of Cdk1 activity in Emi2KO testes, although aberrant, did not require a concurrent increase in Cdk2 activity. Our Cdk1AF model offered the distinct benefit of being an in vivo system, and the rescue of prophase I arrest in Emi2KO spermatocytes using this model enforces its functionality. However, we were also limited due to the inherent lethality and damage-prone meiotic progression of Cdk1+/AF mice (Figures 4 and 6C). The aberrant and incomplete rescue may be related to these inherent defects and low Cdk2 activity.
Testicular meiotic arrest in the absence of Emi2 was associated with decreased expression of cyclin A1 (Figure 7C). Interestingly, cyclin A1 expression followed a similar pattern as Emi2 and increased with age, with Emi2KO testes displaying delayed upregulation. Similar to Emi2KO male mice, cyclin A1KO male mice are sterile, with spermatocytes arresting in diplotene (Liu et al., 1998; Nickerson et al., 2007) associated with impaired Cdk1 activation (Liu et al., 2000). The involvement of Emi2 and cyclin A1 at similar stages is suggestive of co-operation between the two in spermatogenic regulation. While awaiting further research into these aspects of spermatogenesis, it may be worthwhile for now to draw parallels from mitosis, where Emi1-mediated inhibition of APC/C results in the stabilization of cyclin A2, allowing for further cyclin A2-mediated APC/C inhibition and mitotic progression (Lukas et al., 1999; Sørensen et al., 2001). Cooperative APC/C inhibition leading to cyclin A1 and cyclin B1 stabilization may be similarly essential for meiotic progression in spermatogenesis. A potential role for Cdk2, a binding partner of cyclin A1 in spermatogenesis (Sweeney et al., 1996) that was discussed earlier, provides further reason for evaluating these links. While a role for APC/C in female meiosis is well established (Homer, 2013; Jones, 2011), evidence for its involvement in spermatogenesis is limited (Holt et al., 2014; Zhao et al., 2013). The identification of FZR1 as an essential APC/C coactivator for the completion of prophase I in spermatocytes (Holt et al., 2014) implores further investigation into the substrates and regulatory pathways directed by APC/C in male meiosis.
Emi2 in Mitosis
The general acceptance of Emi2 as a specialized meiotic CSF component in eggs was challenged when T.U. Mayer’s group reported its requirement for mitotic embryonic divisions in Xenopus (Tischer et al., 2012). In contrast, our study indicates that Emi2 is dispensable for mitotic divisions in mice, since germline Emi2KO mice are viable. Even in mouse spermatogenesis, where we report that Emi2 is essential, no requirement of Emi2 was observed in mitotic spermatogonial divisions (Emi2KO testes are normal at P15; Figure 3B). The contrasting mitotic requirements of Emi2 across species may reflect differences in Emi2 expression patterns. While the premise for investigating Emi2 functions in Xenopus embryos was the reaccumulation of Emi2 in early embryos following fertilization (Tischer et al., 2012), Emi2 levels have been reported to decline following fertilization in mouse embryos (Shoji et al., 2006). Additional investigations into Emi2 expression across species, and into stage-specific deletions of Emi2 during development, may yield fresh insights into roles of Emi2 beyond meiosis.
Emi2 in Spermiogenesis and Fertility
The expression of Emi2 in spermatocytes, spermatids, and sperm alludes to potential roles during differentiation and spermiogenesis. In particular, its expression in sperm may be suggestive of functions during fertilization. Although immunostaining revealed expression of Emi2 at the acrosomal cap (Figures 7F–7H), preliminary western blot experiments revealed Emi2 expression in both acrosomal and non-acrosomal fractions of cauda sperm (data not shown). Since the acrosomal region in sperm is prone to non-specific staining by antibodies, additional experiments are necessary to validate this finding. We attempted to use our CreERT2 models to delete Emi2 in sperm by administering tamoxifen to adult mice (Figure S2). However, post-meiotic expression of CreERT2 was very weak, and we were unable to effectively use this system for deletion of Emi2 in spermatids or sperm. Use of post-meiotic Cre strains such as protamine-cre (O’Gorman et al., 1997) and evaluating the behavior of Emi2-depleted sperm could help ascertain why Emi2 is expressed post-meiotically. Identification of interacting partners in spermatid fractions or sperm will aid in fitting Emi2 into the vast post-meiotic regulatory network of testis-specific transcription factors, transition proteins, and morphogenetic factors.
Emi2 in Oocytes
Decades of quest into the identity of CSF components in eggs established pathways of APC/C inhibition by Emi2 and cMos/MAPK (mitogen-activated protein kinase) leading to MPF (Cdk1/cyclin B1) stabilization (Masui, 2000). Unlike the c-Mos/ MEK/MAPK/p90rsk pathway, which is not fully conserved between frog and mouse eggs (Dumont et al., 2005), the role of Emi2 to maintain MII arrest in eggs appears to be well conserved in vertebrates (see Figure 2; Wu and Kornbluth, 2008). Since we chose to focus on the spermatogenesis phenotype and did not examine oocytes from Emi2KO mice in much detail, specifics of meiosis I progression, as well as the exact kinetics of MII entry/exit (earlier studies demonstrated that Emi2 is required for both establishment and maintenance of metaphase II arrest; Liu et al., 2007), are not clear from our study. We were also keenly interested in using our in vivo model to investigate any link between the parthenogenetic phenotype in Emi2KO oocytes and ovarian cancer. In the absence of Emi2, we did not detect any attenuation of lifespan or ovarian cancer. Since the number of Emi2KO female mice that exhibited oocyte parthenogenesis were few (2 out of 11), it is possible that ovarian cancer in the absence of Emi2 is a rare occurrence and will require the analysis of several more mice for conclusive findings. Despite this, the in vivo nature of our study design and the unambiguous sterility in Emi2KO females clearly establish an essential physiological role for Emi2 in female mammalian meiosis.
EXPERIMENTAL PROCEDURES
All animal work was done in a humane way and was approved by the Biological Resource Center (BRC) of Biopolis (IACUC #140927).
Testis Immunohistochemistry
Freshly excised mouse testes were fixed in Bouin’s fixative or modified Davidson’s fluid (30% formalin, 15% ethanol, 5% glacial acetic acid) for 16 hr. Paraffin-embedded tissues were sectioned and processed for Periodic Acid Schiff staining, H&E staining, or anti-Emi2 antibody immunostaining.
Immunofluorescence Analysis of Testis
Please refer to the Supplemental Information for detailed protocols. Briefly, slides with chromosome spreads were incubated overnight with primary antibodies at 4°C, followed by incubation with appropriate Alexa Fluor-conjugated secondary antibodies for 1 hr at room temperature. Slides were counterstained with DAPI before epifluorescence images were taken.
Testicular Cell Elutriation
Centrifugal elutriation of testicular germ cell fractions was performed as described previously (Barchi et al., 2009). Briefly, a testicular cell suspension was prepared from testes of three 8-week-old wild-type mice using collagenase and trypsin digestion. The pump flow rates and velocity were adjusted to obtain five fractions from the cell suspension. Approximate proportions of cell fractions are as described here. Fractions 1 and 2, 65% residual bodies + 34% elongated spermatids, Fraction 3, 72% round spermatids; Fraction 4: 50% round spermatids + 30% primary spermatocytes + 12% secondary spermatocytes; and Fraction 5: 80% primary spermatocytes. Content and purity of fractions were observed using Giemsa staining.
Western Blot Analysis
Freshly excised or frozen testes were lysed in EBN buffer (80 mM β-glycerophosphate [pH 7.3], 20 mM EGTA, 15 mM MgCl2, 150 mM NaCl, 0.5% NP-40, 1 mM DTT, and protease inhibitors [20 μg/mL each of leupeptin, chymostatin, and pepstatin; Chemicon, EI8, EI6, and EI10, respectively]) for 20 min on ice and clarified by centrifugation. Protein extracts were resolved by SDS-PAGE, followed by western blot analysis.
Real-Time PCR
Quantification of mRNA levels from mouse testes and testicular elutriation fractions was performed by real-time PCR using a Rotor-Gene 6000 instrument (Corbett Life Science) and the Maxima SYBR Green Kit (Fermentas). Sequences of primers used are listed in Tables S1 and S2.
Tamoxifen Treatment and Superovulation of Mice
Emi2flox ROSA26-CreERT2 or Emi2flox Mvh-CreERT2 male mice, aged 6–8 weeks, were injected intraperitoneally with 1.5 mg tamoxifen dissolved in corn oil. Mice received a total of three injections, one injection per day on 3 consecutive days. The next day, each tamoxifen-treated mouse was moved to a cage with two wild-type females (5–6 weeks old). After 2 months, the breeding females were replaced with two new females, and breeding with new females was continued for 1–2 more months. Separated females were monitored for 3 more weeks to check for pregnancy/litters. All breeding cages were regularly monitored for litter number and size.
For superovulation, female mice aged 3 weeks or older were intraperitoneally injected with 5 IU PMSG (pregnant mare serum gonadotropin; Calbiochem, catalog #367222). 48 hr later, mice were intraperitoneally injected with 5 IU human chorionic gonadotropin (HCG; Calbiochem, catalog #230734). Eggs were collected from the oviducts of sacrificed mice 12–16 hr after HCG administration.
Supplementary Material
Highlights.
Deletion of Emi2 leads to sterility in male and female mice
In the absence of Emi2, oocytes exhibit defective entry into meiosis II
Emi2KO spermatocytes are unable to complete meiotic divisions
Elevated Cdk1 activity partially rescues this spermatogenic arrest
ACKNOWLEDGMENTS
We thank Zakiah Talib, June Wang, Vithya Anantaraja, and Chloe Sim for animal care; Stefan Lim for generating the targeting vector; Chee Leng Lee for help with centrifugal elutriation; Julius Muller for initial analysis of the gene expression data; Daniel Messerschmidt for help with oocyte studies; Shuhui Lim for help with mouse injections; Shawn Lu Wen Tan for help with testes isolations and chromosome spreads; Oliver Jones for help with gene expression analysis; Joanna Niska for proofreading; Keith Jones for discussions; and all past and current members of the P.K. lab for support and discussions. We are grateful to Eileen Southon and Susan Reid for help in generating the Emi2flox and Cdk1AF mice. We thank Jos Jonkers and Anton Berns for providing the Rosa26-CreERT2 mice, Mark Lewandoski for the β-actin–Cre/Flpe mice, and David Page for the Stra8-Cre mice. We acknowledge the tech-nical expertise provided by the Advanced Molecular Pathology Laboratory at IMCB. This work was supported by the Biomedical Research Council of A*STAR (Agency for Science, Technology and Research), Singapore.
Footnotes
ACCESSION NUMBERS
The accession number for the microarray data reported in this paper is GEO: GSE77309.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.06.033.
REFERENCES
- Adhikari D, Busayavalasa K, Zhang J, Hu M, Risal S, Bayazit MB, Singh M, Diril MK, Kaldis P, and Liu K (2016). Inhibitory phosphorylation of Cdk1 mediates prolonged prophase I arrest in female germ cells and is essential for female reproductive lifespan. Cell Res. 26, 1212–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaric I, Miller G, and Dear TN (2007). Appearances can be deceiving: phenotypes of knockout mice. Brief. Funct. Genomics Proteomics 6, 91–103. [DOI] [PubMed] [Google Scholar]
- Barchi M, Geremia R, Magliozzi R, and Bianchi E (2009). Isolation and analyses of enriched populations of male mouse germ cells by sedimentation velocity: the centrifugal elutriation. Methods Mol. Biol 558, 299–321. [DOI] [PubMed] [Google Scholar]
- Bhatt RR, and Ferrell JE Jr. (1999). The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286, 1362–1365. [DOI] [PubMed] [Google Scholar]
- Bisig CG, Guiraldelli MF, Kouznetsova A, Scherthan H, Höög C, Dawson DS, and Pezza RJ (2012). Synaptonemal complex components persist at centromeres and are required for homologous centromere pairing in mouse spermatocytes. PLoS Genet. 8, e1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros R, Dozier C, and Ducommun B (2006). The when and wheres of CDC25 phosphatases. Curr. Opin. Cell Biol 18, 185–191. [DOI] [PubMed] [Google Scholar]
- Cobb J, Cargile B, and Handel MA (1999). Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev. Biol 205, 49–64. [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, and Evans MJ (1994). Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370, 65–68. [DOI] [PubMed] [Google Scholar]
- de Vries FA, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu JG, van Zeeland AA, Heyting C, and Pastink A (2005). Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev. 19, 1376–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, and Moens PB (1994). Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J. Cell Sci 107, 2749–2760. [DOI] [PubMed] [Google Scholar]
- Dumont J, Umbhauer M, Rassinier P, Hanauer A, and Verlhac MH (2005). p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J. Cell Biol 169, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Varnum DS, and Nadeau JH (1996). Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer Res. 56, 5047–5054. [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, and Castrillon DH (2007). Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M, Damestoy A, Mouradian S, Rudkin BB, and Durand P (2004). Key role for cyclin-dependent kinases in the first and second meiotic divisions of rat spermatocytes. Biol. Reprod 70, 1147–1152. [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Lewellyn AL, and Maller JL (1999). Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science 286, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Haccard O, Sarcevic B, Lewellyn A, Hartley R, Roy L, Izumi T, Erikson E, and Maller JL (1993). Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science 262, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Hammoud SS, Low DH, Yi C, Carrell DT, Guccione E, and Cairns BR (2014). Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell 15, 239–253. [DOI] [PubMed] [Google Scholar]
- Handel MA, Caldwell KA, and Wiltshire T (1995). Culture of pachytene spermatocytes for analysis of meiosis. Dev. Genet. 16, 128–139. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. (1994). Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature 370, 68–71. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, and Allis CD (1997). Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360. [DOI] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, and Smith CE (2010). Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech 73, 241–278. [DOI] [PubMed] [Google Scholar]
- Heyting C, Dietrich AJ, Redeker EJ, and Vink AC (1985). Structure and composition of synaptonemal complexes, isolated from rat spermatocytes. Eur. J. Cell Biol 36, 307–314. [PubMed] [Google Scholar]
- Heyting C, Moens PB, van Raamsdonk W, Dietrich AJ, Vink AC, and Redeker EJ (1987). Identification of two major components of the lateral elements of synaptonemal complexes of the rat. Eur. J. Cell Biol 43, 148–154. [PubMed] [Google Scholar]
- Holt JE, Pye V, Boon E, Stewart JL, García-Higuera I, Moreno S, Rodríguez R, Jones KT, and McLaughlin EA (2014). The APC/C activator FZR1 is essential for meiotic prophase I in mice. Development 141, 1354–1365. [DOI] [PubMed] [Google Scholar]
- Homer H (2013). The APC/C in female mammalian meiosis I. Reproduction 146, R61–R71. [DOI] [PubMed] [Google Scholar]
- Jones KT (2011). Anaphase-promoting complex control in female mouse meiosis. Results Probl. Cell Differ. 53, 343–363. [DOI] [PubMed] [Google Scholar]
- Kim KS, Cha MC, and Gerton GL (2001). Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol. Reprod 64, 36–43. [DOI] [PubMed] [Google Scholar]
- Kornbluth S, Sebastian B, Hunter T, and Newport J (1994). Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol. Biol. Cell 5, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, and Kornbluth S (1996). Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol. 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, and Martin GR (1997). Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb. Symp. Quant. Biol 62, 159–168. [PubMed] [Google Scholar]
- Liu F, Stanton JJ, Wu Z, and Piwnica-Worms H (1997). The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol. Cell. Biol 17, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, and Wolgemuth DJ (1998). Cyclin A1 is required for meiosis in the male mouse. Nat. Genet 20, 377–380. [DOI] [PubMed] [Google Scholar]
- Liu D, Liao C, and Wolgemuth DJ (2000). A role for cyclin A1 in the activation of MPF and G2-M transition during meiosis of male germ cells in mice. Dev. Biol 224, 388–400. [DOI] [PubMed] [Google Scholar]
- Liu J, Grimison B, and Maller JL (2007). New insight into metaphase arrest by cytostatic factor: from establishment to release. Oncogene 26, 1286–1289. [DOI] [PubMed] [Google Scholar]
- Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J, and Lukas J (1999). Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401, 815–818. [DOI] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, and Jones KT (2006). Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J. Cell Biol 174, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodríguez J, Jasin M, Keeney S, Bonner WM, and Burgoyne PS (2001). Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet 27, 271–276. [DOI] [PubMed] [Google Scholar]
- Masui Y (2000). The elusive cytostatic factor in the animal egg. Nat. Rev. Mol. Cell Biol 1, 228–232. [DOI] [PubMed] [Google Scholar]
- Masui Y, and Markert CL (1971). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool 177, 129–145. [DOI] [PubMed] [Google Scholar]
- Meuwissen RL, Offenberg HH, Dietrich AJ, Riesewijk A, van lersel M, and Heyting C (1992). A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 11, 5091–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB (1995). Histones H1 and H4 of surface-spread meiotic chromosomes. Chromosoma 104, 169–174. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, and Dunphy WG (1995). Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90. [DOI] [PubMed] [Google Scholar]
- Nickerson HD, Joshi A, and Wolgemuth DJ (2007). Cyclin A1-deficient mice lack histone H3 serine 10 phosphorylation and exhibit altered aurora B dynamics in late prophase of male meiosis. Dev. Biol 306, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman S, Dagenais NA, Qian M, and Marchuk Y (1997). Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA 94, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LL, and Piwnica-Worms H (1992). Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257, 1955–1957. [DOI] [PubMed] [Google Scholar]
- Parra MT, Viera A, Gómez R, Page J, Benavente R, Santos JL, Rufas JS, and Suja JA (2004). Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J. Cell Sci 117, 1221–1234. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, and Bonner WM (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol 10, 886–895. [DOI] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, and Jackson PK (2001). Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645–655. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, and Stainier DY (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233. [DOI] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Payne CJ, Dearth AT, and Braun RE (2008). Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 46, 738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, and Ikawa Y (1989). The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342, 512–518. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, and Mayer TU (2005). Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 19, 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Rauh NR, Nigg EA, and Mayer TU (2006). Cytostatic factor: an activity that puts the cell cycle on hold. J. Cell Sci 119, 1213–1218. [DOI] [PubMed] [Google Scholar]
- Shibuya EK, and Masui Y (1988). Stabilization and enhancement of primary cytostatic factor (CSF) by ATP and NaF in amphibian egg cytosols. Dev. Biol 129, 253–264. [DOI] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, and Perry AC (2006). Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 25, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, and Lukas J (2001). A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol. Cell. Biol 21, 3692–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, and Handel MA (2008). Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes. Chromosoma 117, 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Suzuki E, Yoshida N, Kubo A, Li H, Okuda E, Amanai M, and Perry AC (2010). Mouse Emi2 as a distinctive regulatory hub in second meiotic metaphase. Development 137, 3281–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, Wolgemuth DJ, and Carrington M (1996). A distinct cyclin A is expressed in germ cells in the mouse. Development 122, 53–64. [DOI] [PubMed] [Google Scholar]
- Tang W, Wu JQ, Guo Y, Hansen DV, Perry JA, Freel CD, Nutt L, Jackson PK, and Kornbluth S (2008). Cdc2 and Mos regulate Emi2 stability to promote the meiosis I-meiosis II transition. Mol. Biol. Cell 19, 3536–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer T, Hörmanseder E, and Mayer TU (2012). The APC/C inhibitor XErp1/Emi2 is essential for Xenopus early embryonic divisions. Science 338, 520–524. [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR 3rd, and Jackson PK (2005). A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA 102, 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Schwab MS, Chen LG, and Maller JL (2002). The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr. Biol 12, 1027–1033. [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Eyers PA, Chen LG, Lewellyn AL, and Maller JL (2003). Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J. Cell Biol 163, 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC, Shaw TJ, and Ethier JF (2003). Animal models of ovarian cancer. Reprod. Biol. Endocrinol 1, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM (2009). Mammalian fertilization: the strange case of sperm protein 56. BioEssays 31, 153–158. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Park C, Caldwell KA, and Handel MA (1995). Induced premature G2/M-phase transition in pachytene spermatocytes includes events unique to meiosis. Dev. Biol 169, 557–567. [DOI] [PubMed] [Google Scholar]
- Wu JQ, and Kornbluth S (2008). Across the meiotic divide – CSF activity in the post-Emi2/XErp1 era. J. Cell Sci 121, 3509–3514. [DOI] [PubMed] [Google Scholar]
- Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, et al. (2007). A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol 17, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Amanai M, Fukui T, Kajikawa E, Brahmajosyula M, Iwahori A, Nakano Y, Shoji S, Diebold J, Hessel H, et al. (2007). Broad, ectopic expression of the sperm protein PLCZ1 induces parthenogenesis and ovarian tumours in mice. Development 134, 3941–3952. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gou LT, Zhang M, Zu LD, Hua MM, Hua Y, Shi HJ, Li Y, Li J, Li D, et al. (2013). piRNA-triggered MIWI ubiquitination and removal by APC/C in late spermatogenesis. Dev. Cell 24, 13–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.