Abstract
Melatonin (MT) is an indoleamine hormone that can counteract ischemia-induced organ injury through its antioxidant effects. The aim of the present study was to investigate the protective effects of exogenous MT against hemorrhagic shock (HS)-induced hepatic ischemic injury in rats, and the role of the nuclear factor (NF)-κB signaling pathway in this process. A rat model of HS-induced hepatic ischemic injury was established. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), glutamate dehydrogenase (GDH), tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6 and IL-1β were measured every 6 h, and the 24-h survival rate of the rats was analyzed. All surviving rats were sacrificed after 24 h. Pathological changes in the liver and the hepatocyte apoptosis rate were observed by hematoxylin and eosin staining and TUNEL assay, respectively, and the expression levels of NF-κB p65 and NF-κB inhibitor α (IκBα) were analyzed by reverse transcription-quantitative PCR analysis and western blotting. The results demonstrated that the serum levels of ALT, AST, LDH, GDH, TNF-α, IFN-γ, IL-6 and IL-1β gradually increased after HS compared with those in rats subjected to a sham procedure, but this increase was attenuated by MT. Furthermore, the survival rate of the MT group was significantly higher compared with that of the HS group. The degree of pathological hepatic injury, the hepatocyte apoptosis rate, and the hepatic levels of TNF-α, IFN-γ, IL-6 and IL-1β were significantly decreased in the MT group compared with the HS group. In addition, the mRNA expression of NF-κB p65 was significantly decreased and the mRNA expression of IκBα was significantly increased in the MT group compared with the sham group. Furthermore, the NF-κB p65 protein levels in the MT group were significantly increased in the cytosol but decreased in the nucleus, and the IκBα protein levels were increased while those of phosphorylated IκBα were decreased compared with those in the HS group. Therefore, it may be inferred that exogenous MT alleviates HS-induced hepatic ischemic injury in rats via the inhibition of NF-κB activation and IκBα phosphorylation.
Keywords: hemorrhagic shock, hepatic ischemia injury, melatonin, NF-κB/IκBα
Introduction
Hemorrhagic shock (HS) is a condition characterized by reduced tissue perfusion, which results in the delivery of oxygen and nutrients being inadequate for cellular function (1). As modern society has developed, the incidence of HS resulting from traffic accidents and natural disasters has increased, and HS has become one of the main causes of mortality worldwide (2). The induction of hepatic ischemic injury by HS is a common pathophysiological occurrence in the clinical setting, and is characterized by the release of inflammatory mediators and the activation and recruitment of neutrophils (3). The pathological mechanisms underlying hepatic ischemic injury remain unclear, but they may involve the apoptosis of hepatocytes, accumulation of reactive oxygen species, changes in mitochondrial permeability and endoplasmic reticulum stress (4). Current research also indicates that the main mechanism underlying hepatic ischemic injury is an excessive and uncontrolled inflammatory response in the liver (5).
Nuclear factor (NF)-κB is a nuclear transcription factor that participates in a number of signal transduction pathways in the process of inflammation, and NF-κB signaling plays an important role in the development of ischemic injury in organs (6). A recent study suggested that the suppression of activation of the NF-κB signaling pathway is the key to inhibiting the inflammatory response, and that NF-κB is a potential target for the alleviation of ischemic injury (7).
Melatonin (MT) is an indoleamine hormone synthesized by the pineal gland of vertebrates, which has a variety of strong direct and indirect antioxidant and anti-inflammatory properties (8). MT can counteract the ischemic injury of multiple organs through its antioxidant effects and strong free radical-scavenging capacity at the molecular level (9). Furthermore, MT has been demonstrated to prevent the development of ischemic injury by reducing oxidative stress-induced activation of the NF-κB signaling pathway (10). The aim of the present study was to investigate the protective effects of exogenous MT against HS-induced hepatic ischemic injury in rats and its association with the NF-κB pathway.
Materials and methods
Reagents
MT (cat. no. M5250) and TRIzol® were purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Alanine aminotransferase (ALT; cat. no. ml063179), aspartate aminotransferase (AST; cat. no. ml058577) lactate dehydrogenase (LDH; cat. no. ml037243) and glutamate dehydrogenase (GDH; cat. no. ml037964) assay kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. ELISA kits for the detection of tumor necrosis factor (TNF)-α (cat. no. ml037211), interferon (IFN)-γ (cat. no. ml063095), interleukin (IL)-6 (cat. no. ml002293) and IL-1β (cat. no. ml063132) were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. Hematoxylin and eosin (H&E) staining assay kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. DeadEnd™ Fluorometric TUNEL System kit was purchased from Promega Corporation. Nuclear and Cytoplasmic Protein Extraction kit (cat. no. P0028) was bought from Beyotime Institute of Biotechnology.
Animal grouping and HS model preparation
A total of 45 healthy, clean-grade male Sprague Dawley rats, weighing 250–280 and aged 10–12 weeks, were provided and fed by the experimental animal center of Taizhou First People's Hospital. All animals underwent adaptive feeding for 7 days before the experiment, and were kept at a temperature between 20 and 25°C and humidity between 40 and 70%. The animals were allowed to eat and drink freely. All the animals, reagents and treatment methods used in the experiments were approved by the animal experiment ethics committee of Taizhou First People's Hospital, and all experimental procedures were performed in a manner that minimized suffering and reduced the number of animals used according to the Animal Research Reporting In Vivo Experiments (ARRIVE) Guidelines (11). The 45 Sprague Dawley rats were randomly divided into three groups: Sham group, HS model group and MT treatment group (n=15/group). Each rat was anesthetized with 5% pentobarbital sodium (30 mg/kg body weight) by intraperitoneal injection. The trachea was orally intubated, and spontaneous breathing was maintained. The right carotid artery and left jugular vein were separated, and polyethylene catheters were inserted into them for the withdrawal and transfusion of blood. Approximately 40% of the total blood volume was withdrawn in 30 min through a two-way automatic infusion pump to establish the HS model. After 30 min, fluid resuscitation was performed according to Advanced Trauma Life Support guidelines (12). All animals were injected with penicillin to avoid infection. The sham group was subjected to tracheal intubation without trauma modeling. In the HS and MT groups, a pressure-controlled traumatic HS model was established according to the standard protocol (13). Following traumatic HS for 1 h, rats in the MT group were injected intravenously with MT (10 mg/kg), whereas the sham and HS group rats received an equal volume of PBS. A 10 mg/kg dose of MT was used because 10 mg/kg MT showed a satisfactory anti-hepatic ischemia injury effect in the HS model in a preliminary study. Also, a 10 mg/kg dose is supported by previous studies (12–14). Following surgery, the animals were monitored by the laboratory group every 4 h. During the experiment, 12 Sprague Dawley rats (8 in the HS group and 4 in the MT group) died of shock. The 33 Sprague Dawley rats (15 in the sham group, 7 in the HS group and 11 in the MT group) that remained alive 24 h after surgery were euthanized by anesthesia with intraperitoneally injected pentobarbital (30 mg/kg) followed by cervical dislocation. Death was confirmed by lack of heartbeat, lack of respiration, lack of corneal reflex and the presence of rigor mortis. Livers were excised for pathological observation using a light microscope. The study established specific criteria, i.e., humane endpoints, to determine when animals should be euthanized according to ARRIVE Guidelines. The humane endpoints were a reduction of 4–6°C in body temperature, a weight loss of >10%, decreased activity (lethargy) and alertness, a rough coat and hunched posture, which are direct signs of illness, pain or distress.
Automatic biochemical analysis
Following traumatic HS for 6, 12, 18 and 24 h, blood samples were collected from the inferior vena cava of the rats and centrifuged at 4°C, 500 × g for 5 min. The upper layer of serum was collected and stored at −80°C until use. The levels of ALT, AST, LDH and GDH in the serum and hepatic tissue homogenate, were measured using the aforementioned kits in an automatic biochemical analyzer according to the manufacturer's instructions.
ELISA
Serum was prepared from blood samples as aforementioned. The hepatic tissues were removed from the rats when sacrificed, 24 h after surgery. After washing with normal saline, hepatic tissue homogenate was prepared using an automatic homogenizer, and centrifuged at 4°C and 500 × g for 5 min. A total of 1 ml cell suspension was obtained from each group and placed into 96-well ELISA plate, and the absorbance of each well at a wavelength of 490 nm was detected using an ELISA reader. The concentrations of TNF-α, IFN-γ, IL-6 and IL-1β in the serum and tissue homogenate were measured and expressed as pg/ml in each sample.
H&E staining
Hepatic tissue samples were fixed in 4% paraformaldehyde at 37°C for 30 min and then dehydrated, transparentized, embedded in paraffin and cut into 4-µm sections. The sections were then stained with H&E for 4 h at room temperature. The stained tissue was placed in 1% hydrochloric acid ethanol (Merck KGaA) for differentiation at room temperature for 10 sec and sealed using neutral gum. The degree of fatty degeneration, inflammation and necrosis of the H&E stained liver samples were then evaluated under a light microscope. Hepatic pathology was scored by Suzuki's criteria (14).
TUNEL assay
Hepatic tissue samples were fixed in 4% paraformaldehyde for 30 min at room temperature and then dehydrated, transparentized, embedded in paraffin and cut into 4-µm sections. Subsequently, the sections were treated with 100 µl TUNEL reaction mixture (cat. no. C1086; Beyotime Institute of Biotechnology) at 37°C for 1 h, followed by incubation with 100 µl DNase at room temperature for 5 min. Following washing with PBS, the sections were treated with 100 µl DAB for at room temperature 10 min in the dark. Apoptotic cells were observed using a fluorescence microscope (Olympus Corporation) at ×400 magnification.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA of hepatic tissue was extracted from using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. cDNA was synthesized using a PrimeScript™ One Step RT-PCR kit (cat. no. AM1558; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The primer sequences for NF-κB p65 and NF-κB inhibitor α (IκBα) were designed using Primer Premier 5 (premierbiosoft.com). The sequences are as follows: NF-κB p65, forward, 5′-AGGCAAGGAATAATGCTGTCCTG-3′ and reverse, 5′-ATCATTCTCTAGTGTCTGGTTGG-3′; IκBα, forward, 5′-CACTCCATCCTGAAGGCTACCAA-3′ and reverse, 5′-AAGGGCAGTCCGGCCATTA-3′; GAPDH, forward 5′-TGAGAGGGAAATCGTGCGTG-3′, and reverse, 5′-TGCTTGCTGATCCACATCTGC-3′. The thermocycling conditions were as follows: 95°C for 15 min, followed by 40 cycles of 94°C for 15 sec, at 55°C for 30 sec and 70°C for 30 sec for a total of 40 cycles. After qPCR, the relative expression levels were calculated using the 2−Δ∆Cq method (15).
Western blotting
Total protein was extracted from the tissues using RIPA protein lysis buffer (Beyotime Institute of Biotechnology). Nuclear and cytoplasmic proteins were separated using the Nuclear and Cytoplasmic Protein Extraction Kit. The protein concentration was determined using a BCA protein concentration kit (Beyotime Institute of Biotechnology) Total protein (50 µg/lane) was separated by SDS-PAGE on 10% gels and then transferred onto PVDF membranes. After blocking with 5% non-fat milk for 2 h at room temperature, the membranes were incubated overnight at 4°C with the following primary antibodies: NF-κB p65 (1:1,000; cat. no. ab16502; Abcam), NF-κB inhibitor α (IκBα; 1:1,000; cat. no. ab32518; Abcam), phosphorylated (p)-IκBα (1:1,000; cat. no. ab133462; Abcam) and β-actin (1:5,000; cat. no. ab8226; Abcam) at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) secondary antibody (1:5,000; cat. no. AS014; ABclonal Biotech Co., Ltd.) at room temperature for 1 h. Lamin B1 antibody (cat. no. ab16048; Abcam) was used to detect lamin B1 as a housekeeping protein in the nuclear fraction. Protein bands were visualized using an enhanced chemiluminescence detection kit (ECL Plus; EMD Millipore) and measured using Image J 1.47 software (National Institutes of Health).
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc.) was used to carry out the statistical analysis. Each experiment was performed three times, and the data are expressed as the mean ± SD. Comparisons among groups were analyzed by one-way analysis of variance followed by Tukey's multiple comparison tests. Survival rates were analyzed by the Kaplan-Meier method and log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Serum levels of ALT, AST, LDH and GDH
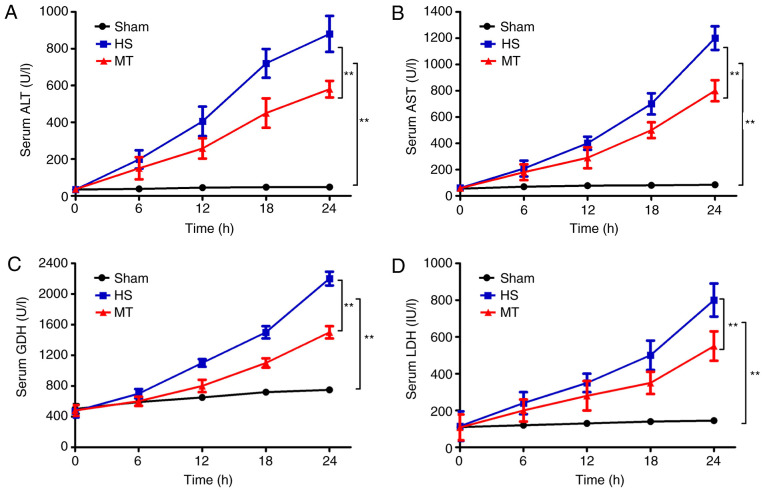
An automatic biochemical analyzer was used to measure the serum levels of ALT, AST, LDH and GDH every 6 h. The results revealed that, compared with the sham group, the serum levels of ALT, AST, LDH and GDH in the HS group gradually increased over time (P<0.01; Fig. 1). In addition, although the serum levels of ALT, AST, LDH and GDH also increased in the MT group, the increase was significantly reduced compared with that in the HS group (P<0.01; Fig. 1).
Figure 1.
Serum levels of ALT, AST, LDH and GDH in each group at 6-h intervals as measured using an automatic biochemical analyzer. Serum (A) ALT, (B) AST, (C) GDH and (D) LDH levels. **P<0.01 as indicated. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; GDH, glutamate dehydrogenase; HS, hemorrhagic shock; MT, melatonin.
Serum levels of TNF-α, IFN-γ, IL-6 and IL-1β
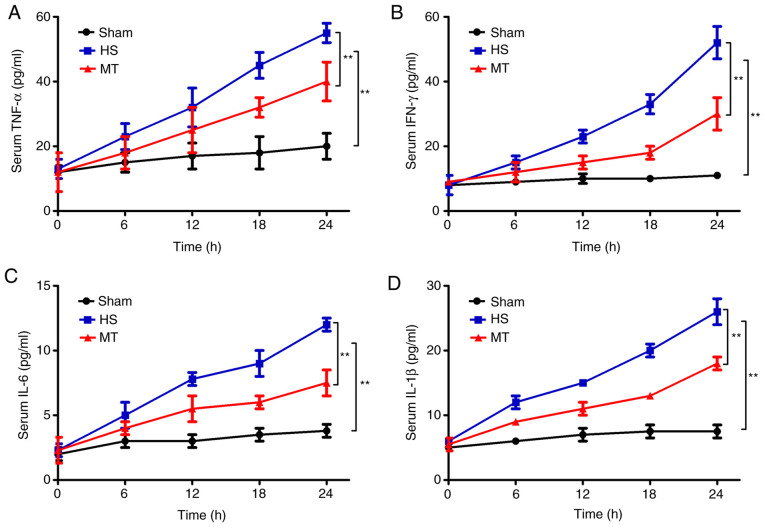
ELISAs were used to detect the serum levels of TNF-α, IFN-γ, IL-6 and IL-1β every 6 h. The results revealed that the serum levels of TNF-α, IFN-γ, IL-6 and IL-1β in the HS group were significantly increased compared with those in the sham group (P<0.01; Fig. 2). In addition, the serum levels of TNF-α, IFN-γ, IL-6 and IL-1β were also increased in the MT group, but the increase was significantly reduced compared with that in the HS group (P<0.01; Fig. 2).
Figure 2.
Serum levels of TNF-α, IFN-γ, IL-6 and IL-1β in each group at 6-h intervals as measured by ELISA assay. Serum (A) TNF-α, (B) IFN-γ, (C) IL-6 and (D) IL-1β levels. **P<0.01 as indicated. TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; HS, hemorrhagic shock; MT, melatonin.
Survival rate
The survival rate of the rats in each group was analyzed by the Kaplan-Meier method and log-rank test. A total of 12 rats (8 in the HS group and 4 in the MT group) died of shock during the experiment, and the remaining 33 rats (15 in sham group, 7 in HS group and 11 in MT group) survived for 24 h. The results show that the 24-h survival rate of the sham group was 100%, whereas the 24-h survival rate of the MT group was significantly higher compared with that of the HS group [73.33% (11/15) vs. 46.67% (7/15), respectively; P=0.003; Fig. 3].
Figure 3.
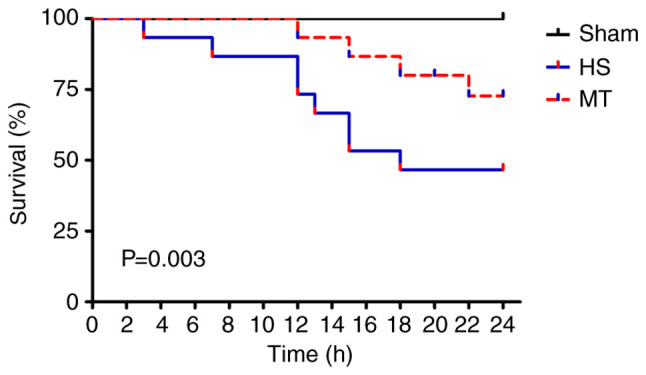
Survival rate of the rats in each group. HS, hemorrhagic shock; MT, melatonin.
Pathological changes
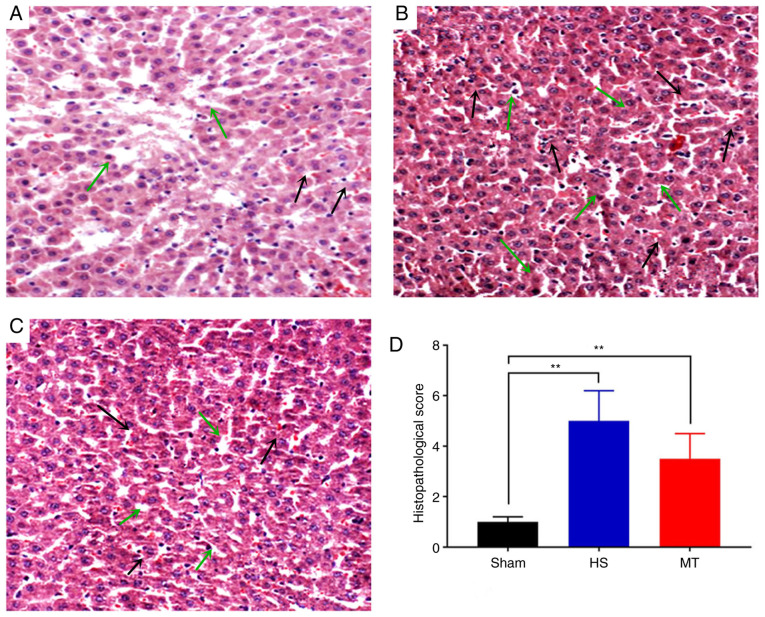
Following sacrifice of the rats 24 h after surgery, hepatic tissues were removed and stained with H&E. The results revealed that the hepatic lobule structure of the sham group was complete, the hepatocytes were arranged linearly and no cell denaturation or inflammatory cell infiltration was present (Fig. 4A). In the HS group, moderate edema of the hepatocytes was observed, with fatty degeneration, marked sinusoidal expansion, central venous congestion, expansion of the portal area interlobular veins and notable inflammatory cell infiltration (Fig. 4B). In the MT group, the hepatic lobule structure was well-defined, with only a scattered infiltration of fat droplets. Furthermore, the hepatocytes exhibited only diffuse mild edema, with a small number of lymphocytes visible in the hepatic cords and the portal area (Fig. 4C). The HS group displayed significant exacerbation of hepatic pathological injury compared with the sham group (Suzuki score, 5.12±1.23 vs. 1.05±0.45, respectively; P<0.01; Fig. 4D) and the MT group (Suzuki score, 5.12±1.23 vs. 3.53±1.12, respectively; P<0.01; Fig. 4D).
Figure 4.
Morphological changes of hepatic tissue were detected by H&E staining. Representative images of H&E staining in the (A) sham, (B) HS and (C) MT groups. (D) Suzuki score in each group. Magnification, ×200. **P<0.01 as indicated. Black arrows indicate inflammatory infiltration and green arrows indicate edema. H&E, hematoxylin and eosin; HS, hemorrhagic shock; MT, melatonin.
Hepatocyte apoptosis rate
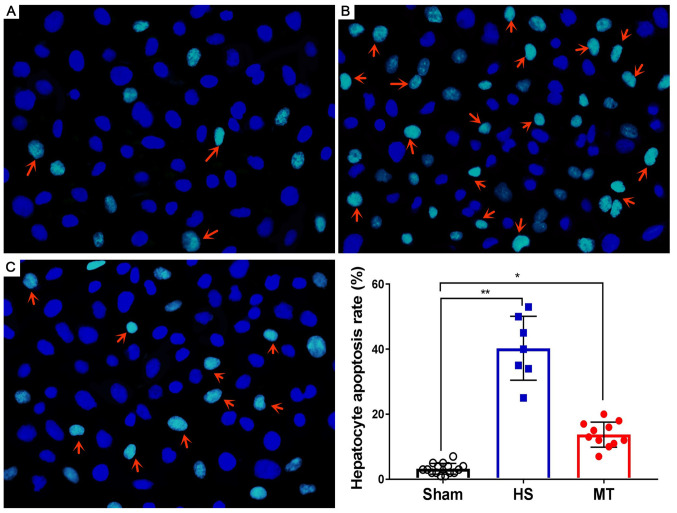
After the rats were sacrificed, hepatic tissues were removed and subjected to analysis of the hepatocyte apoptosis rate using the DeadEnd™ Fluorometric TUNEL System. The results demonstrated that the hepatocyte apoptosis rate was very low in the sham group (Fig. 5A) and significantly higher in the HS group (P<0.01; Fig. 5B); however, the hepatocyte apoptosis rate was significantly decreased following treatment with MT (Fig. 5C), and the difference between the HS and MT groups was statistically significant (P<0.05; Fig. 5D).
Figure 5.
Hepatocyte apoptosis rate as detected by TUNEL assay. Representative images of TUNEL staining in the (A) sham, (B) HS and (C) MT groups. Hepatocyte apoptosis rate. Arrows indicate apoptotic cells. Magnification, ×400. *P<0.05, **P<0.01 as indicated. HS, hemorrhagic shock; MT, melatonin.
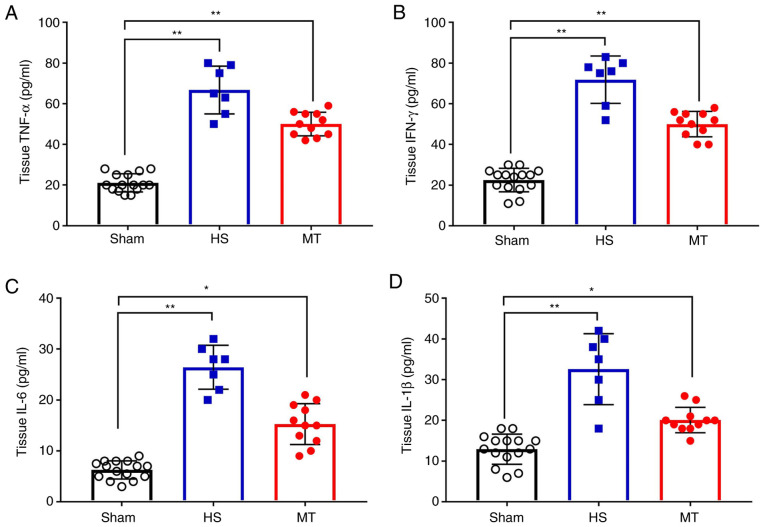
Hepatic tissue levels of TNF-α, IFN-γ, IL-6 and IL-1β
When the rats were sacrificed 24 h after surgery, hepatic tissues were removed and a hepatic tissue homogenate was prepared. Analysis of the homogenate revealed that, the levels of TNF-α, IFN-γ, IL-6 and IL-1β in the hepatic tissue homogenate were significantly increased in the HS group compared with those in the sham group; however, these levels were significantly decreased following treatment with MT compared with those in the HS group (P<0.05; Fig. 6).
Figure 6.
Hepatic tissue levels of TNF-α, IFN-γ, IL-6 and IL-1β in each group after sacrifice were measured by ELISA. Hepatic tissue levels of (A) TNF-α, (B) IFN-γ, (C) IL-6 and (D) IL-1β. *P<0.05, **P<0.01 as indicated. TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; HS, hemorrhagic shock; MT, melatonin.
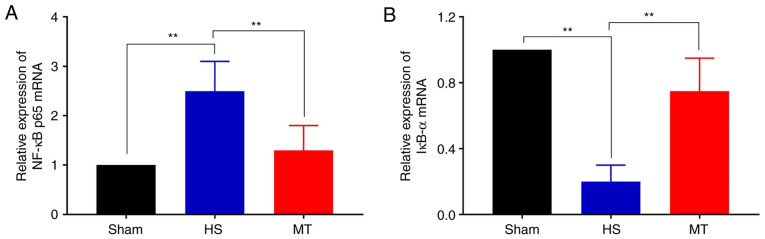
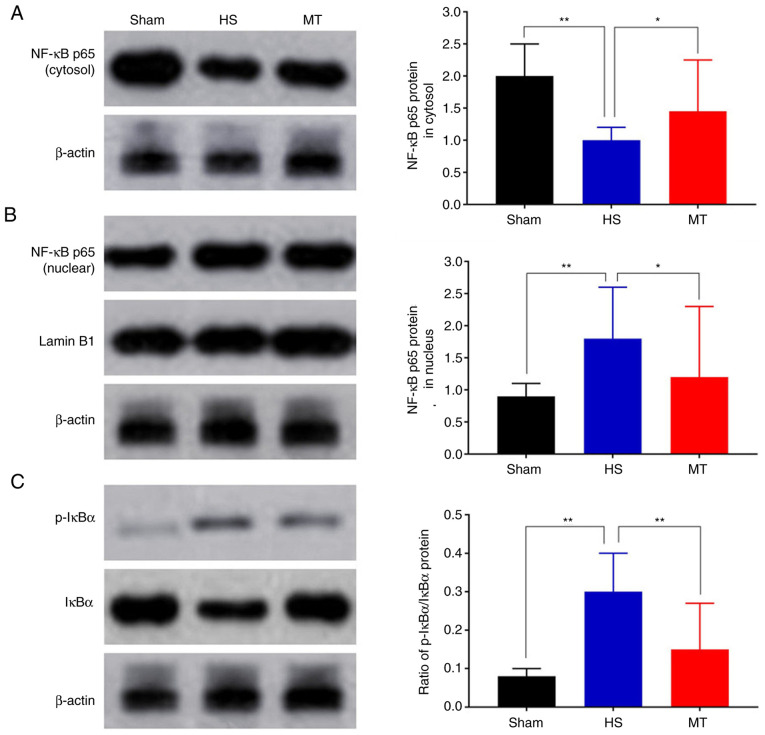
Activity of the NF-κB/IκBα pathway
The expression levels of NF-κB p65 and IκBα in the hepatic tissues of the rats were analyzed by RT-qPCR and western blotting. The RT-qPCR results demonstrated that the mRNA expression of NF-κB p65 was significantly decreased whereas that of IκBα was increased in the MT group compared with the HS group (P<0.01; Fig. 7). Furthermore, the western blotting results revealed that the protein level of NF-κB p65 in the HS group was significantly decreased in the cytosol but increased in the nucleus compared with that in the control group, but these HS-induced changes were significantly attenuated following treatment with MT; also, the p-IκBα/IκBα ratio was significantly increased in HS group compared with the control, but the increase was significantly attenuated following treatment with MT (P<0.05; Fig. 8).
Figure 7.
Transcriptional activity of the NF-κB/IκBα pathway. Relative expression of (A) NF-κB p56 and (B) IκBα mRNA in hepatic tissues. **P<0.01 as indicated. HS, hemorrhagic shock; MT, melatonin; NF, nuclear factor; IκBα, NF-κB inhibitor α.
Figure 8.
Hepatic levels of NF-κB p65, IκBα and p-IκBα proteins. NF-κB p56 protein in the (A) cytosol and (B) nucleus. (C) Relative ratio of p-IκBα/IκBα proteins. *P<0.05, **P<0.01 as indicated. HS, hemorrhagic shock; MT, melatonin; p, phosphorylated; NF, nuclear factor; IκBα, NF-κB inhibitor.
Discussion
Trauma accounts for 10% of fatalities and 16% of disabilities worldwide (16). HS is the most common preventable cause of mortality subsequent to trauma. HS can stimulate the body to release excessive amounts of inflammatory mediators and produce a systemic inflammatory response, which may eventually lead to multiple organ dysfunction syndrome (17). HS-induced hepatic ischemic injury is the most common pathophysiological process in the clinical setting. Recent studies (18,19) report that an excessive and uncontrolled inflammatory response is the key mechanism by which hepatic ischemic injury is mediated during HS, with TNF-α, IFN-γ, IL-6 and IL-1β being the most important inflammatory cytokines in this process.
MT is an indoleamine hormone synthesized by the pineal gland of vertebrates, which has a variety of strong direct and indirect antioxidant and anti-inflammatory effects (20). Recent studies (21,22) demonstrated that the early administration of MT to rats is able to attenuate oxidative and inflammatory ischemic organ injury. In the present study, a rat HS model was successfully established and MT treatment was administered. It was observed that the hepatic function of the rats gradually worsened as time progressed, and the levels of inflammatory factors also gradually increased, but these effects were reversed by MT treatment. In addition, the survival rate of the rats was significantly increased, and the hepatic pathological injury score and hepatocyte apoptosis rate were significantly decreased in the rats treated with MT compared with those in the untreated HS group. To the best of our knowledge, the present study is the first to report that exogenous MT is able to alleviate HS-induced hepatic ischemic injury in rats.
NF-κB is a transcription factor that is involved in the transcription and modulation of several genes that serve as inflammatory mediators, including TNF-α, IFN-γ, IL-6 and IL-1β, and plays an important role in the inflammatory immune response, oxidation and cell apoptosis (23). NF-κB consists of 5 subunits (24): Rel (cRel), p65 (RelA, NF-κB3), RelB, p50 (NF-κB1) and p52 (NF-κB2), of which NF-κB p65 (25) is the most important subunit in the NF-κB signaling pathway. IκBα is the main inhibitory regulatory protein of NF-κB, and the phosphorylation of IκBα is an important pathway mediating NF-κB activation (26,27). In unstimulated cells, IκBα inhibits NF-κB signaling by sequestering NF-κB in an inactive state in the cytoplasm and reducing its nuclear localization (28). However, various extracellular stimuli may cause the phosphorylation and degradation of IκBα. The detachment of IκBα from the NF-κB complex leads to the activation of NF-κB and its entry into the nucleus, thereby increasing the transcriptional activity of inflammatory mediator genes (29). The balance of NF-κB p65 and IκBα expression is key to activation of the NF-κB signaling pathway (30).
Recent studies have demonstrated that MT plays a regulatory role in a variety of diseases via the inhibition of NF-κB pathways. Gu et al (31) reported that MT inhibits the migration and invasion of TE-1 esophageal cancer cells by suppressing the NF-κB signaling pathway and decreasing the expression of matrix metalloproteinase-9. Chen et al (32) observed that MT alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLR pyrin domain containing 3 inflammasome positive feedback loop. Furthermore, Tiong et al (33) demonstrated that MT prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high-glucose-treated Schwann cells via the upregulation of Bcl2, NF-κB, mammalian target of rapamycin and Wnt signaling pathways. In the present study, RT-qPCR and western blotting assays were employed to assess the activity of the NF-κB signaling pathway. The results demonstrated that MT exerted a marked inhibitory effect on the transcriptional activity of NF-κB p65, whereas it enhanced IκBα activity. Furthermore, MT inhibited the transfer of NF-κB p65 into the nucleus and the phosphorylation of IκBα.
There were several limitations to the present study. One was that only treatment with 10 mg/kg MT was conducted; the effects of MT may be dose-dependent and various treatment groups using different doses of MT should be investigated in the future. Also, the specific location of NF-κB p65 in the nucleus and cytoplasm has not been explored in depth and merits further study.
In summary, the present study was the first to determine that exogenous MT alleviates HS-induced hepatic ischemic injury in rats. The underlying mechanism may involve the inhibition of NF-κB activation and IκBα phosphorylation, which could reduce inflammation. Although the exact mechanism requires further elucidation, to the best of our knowledge, the present study was the first to provide evidence on the efficacy of MT against HS-induced hepatic ischemic injury.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Public Welfare Basic Research Project of Zhejiang Province (grant no. LGF19H150001).
Funding
The present study was supported by the Public Welfare Basic Research Project of Zhejiang Province (grant no. LGF19H150001).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XLW was responsible for overall planning of the research. HWL and PY were responsible for most of the experiments and manuscript preparation. HWL and QQC were involved in data analysis and manuscript preparation. ZHY participated in acquisition, analysis and interpretation of data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Taizhou First People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378:370–379. doi: 10.1056/NEJMra1705649. [DOI] [PubMed] [Google Scholar]
- 2.Kezelman C. Trauma-informed care and practice in nursing. Aust Nurs Midwifery J. 2016;24:28. [PubMed] [Google Scholar]
- 3.Slim C, Zaouali MA, Nassrallah H, Ammar HH, Majdoub H, Bouraoui A, Abdennebi HB. Protective potential effects of fucoidan in hepatic cold ischemia-reperfusion injury in rats. Int J Biol Macromol. 2020;155:498–507. doi: 10.1016/j.ijbiomac.2020.03.245. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Gao M, Xu LN, Yin LH, Qi Y, Peng JY. MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury via targeting of myristoylated alanine-rich C-kinase substrate. Pharmacol Res. 2020;156:104783. doi: 10.1016/j.phrs.2020.104783. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim SG, El-Emam SZ, Mohamed EA, Abd Ellah MF. Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int Immunopharmacol. 2020;80:106131. doi: 10.1016/j.intimp.2019.106131. [DOI] [PubMed] [Google Scholar]
- 6.Fliegauf M, Grimbacher B. Nuclear factor κB mutations in human subjects: The devil is in the details. J Allergy Clin Immunol. 2018;142:1062–1065. doi: 10.1016/j.jaci.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Huang J, Xu J, Zeng L, Tian J, Lou Y, Liu Y, Hu B, Tong F, Shen R. Targeted delivery of puerarin/glycyrrhetinic acid-PEG-PBLA complex attenuated liver ischemia/reperfusion injury via modulating Toll-like receptor 4/nuclear factor-κB pathway. Ther Deliv. 2018;9:245–255. doi: 10.4155/tde-2017-0106. [DOI] [PubMed] [Google Scholar]
- 8.Amaral FGD, Cipolla-Neto J. A brief review about melatonin, a pineal hormone. Arch Endocrinol Metab. 2018;62:472–479. doi: 10.20945/2359-3997000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrascal L, Nunez-Abades P, Ayala A, Cano M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr Pharm Des. 2018;24:1563–1588. doi: 10.2174/1381612824666180426112832. [DOI] [PubMed] [Google Scholar]
- 10.Tang YL, Sun X, Huang LB, Liu XJ, Qin G, Wang LN, Zhang XL, Ke ZY, Luo JS, Liang C, et al. Melatonin inhibits MLL-rearranged leukemia via RBFOX3/hTERT and NF-κB/COX-2 signaling pathways. Cancer Lett. 2019;443:167–178. doi: 10.1016/j.canlet.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 11.https://www.nc3rs.org.uk/arrive-guidelines
- 12.ATLS Subcommittee: American College of Surgeons' Committee on Trauma; International ATLS working group, corp-author. Advanced trauma life support (ATLS(R)): The ninth edition. J Trauma Acute Care Surg. 2013;74:1363–1366. doi: 10.1097/01586154-201305000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Jiang H, Liu J, Xu Z, Zheng C. Efficacy of different fluid resuscitation methods on coagulation function of rats with traumatic hemorrhagic shock. J Surg Res. 2020;260:259–266. doi: 10.1016/j.jss.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, Fujise Y. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation. 1991;52:979–983. doi: 10.1097/00007890-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg. 2010;14:528–535. doi: 10.1007/s11605-009-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer JL, El-Gabalawy R, Taillieu T, Afifi TO, Carleton RN. Associations between trauma exposure and physical conditions among public safety personnel. Can J Psychiatry. 2020;12:548–558. doi: 10.1177/0706743720919278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Türedi S, Şahin A, Akça M, Demir S, Köse GDR, Çekiç AB, Yıldırım M, Yuluğ E, Menteşe A, Türkmen S, Acar S. Ischemia-modified albumin and the IMA/albumin ratio in the diagnosis and staging of hemorrhagic shock: A randomized controlled experimental study. Ulus Travma Acil Cerrahi Derg. 2020;26:153–162. doi: 10.14744/tjtes.2019.32754. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakopoulos G, Tsaroucha AK, Valsami G, Lambropoulou M, Kostomitsopoulos N, Christodoulou E, Kakazanis Z, Anagnostopoulos C, Tsalikidis C, Simopoulos CE. Silibinin improves TNF-α and M30 expression and histological parameters in rat kidneys after hepatic ischemia/reperfusion. J Invest Surg. 2018;31:201–209. doi: 10.1080/08941939.2017.1308044. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Wang W, Zhang W, Peng Y, Liu Y, Yu S, Chen Q, Geng L, Zhou L, Xie H, et al. Galectin-1 attenuates hepatic ischemia reperfusion injury in mice. Int Immunopharmacol. 2019;77:105997. doi: 10.1016/j.intimp.2019.105997. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Yan X, Tian Y, Li W, Wang H, Li Q, Li Y, Li Z, Wu T. Synthesis of a new water-soluble melatonin derivative with low toxicity and a strong effect on sleep aid. ACS Omega. 2020;5:6494–6499. doi: 10.1021/acsomega.9b04120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pranil T, Moongngarm A, Loypimai P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon. 2020;6:e03648. doi: 10.1016/j.heliyon.2020.e03648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ullah U, Badshah H, Malik Z, Uddin Z, Alam M, Sarwar S, Aman A, Khan AU, Shah FA. Hepatoprotective effects of melatonin and celecoxib against ethanol-induced hepatotoxicity in rats. Immunopharmacol Immunotoxicol. 2020;42:255–263. doi: 10.1080/08923973.2020.1746802. [DOI] [PubMed] [Google Scholar]
- 24.Yu HY, Meng LF, Lu XH, Liu LH, Ci X, Zhuo Z. Protective effect of miR-146 against kidney injury in diabetic nephropathy rats through mediating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:3215–3222. doi: 10.26355/eurrev_202003_20688. [DOI] [PubMed] [Google Scholar]
- 25.Jia Y, He W, Zhang H, He L, Wang Y, Zhang T, Peng J, Sun P, Qian Y. Morusin ameliorates IL-1β-induced chondrocyte inflammation and osteoarthritis via NF-κB signal pathway. Drug Des Devel Ther. 2020;14:1227–1240. doi: 10.2147/DDDT.S244462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, Duan T, Du Y, Jin S, Wang M, Cui J, Wang RF. LRRC25 functions as an inhibitor of NF-κB signaling pathway by promoting p65/RelA for autophagic degradation. Sci Rep. 2017;7:13448. doi: 10.1038/s41598-017-12573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boisson B, Puel A, Picard C, Casanova JL. Human IκBα gain of function: A severe and syndromic immunodeficiency. J Clin Immunol. 2017;37:397–412. doi: 10.1007/s10875-017-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimova N, Babazada H, Higuchi Y, Yamashita F, Hashida M. Development of mKO2 fusion proteins for real-time imaging and mechanistic investigation of the degradation kinetics of human IκBα in living cells. Biochim Biophys Acta Mol Cell Res. 2019;1866:190–198. doi: 10.1016/j.bbamcr.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Kanan T, Kanan D, Erol I, Yazdi S, Stein M, Durdagi S. Targeting the NF-κB/IκBα complex via fragment-based E-Pharmacophore virtual screening and binary QSAR models. J Mol Graph Model. 2019;86:264–277. doi: 10.1016/j.jmgm.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Shi CX, Qi QH, Xu J, Zhao WW. Protective effect of magnesium sulfate on cranial nerves in preeclampsia rats through NF-κB/ICAM-1 pathway. Eur Rev Med Pharmacol Sci. 2020;24:2785–2794. doi: 10.26355/eurrev_202003_20639. [DOI] [PubMed] [Google Scholar]
- 31.Gu H, Shen Q, Mei D, Yang Y, Wei R, Ni M. Melatonin inhibits TE-1 esophageal cancer cells metastasis by suppressing the NF-κB signaling pathway and decreasing MMP-9. Ann Clin Lab Sci. 2020;50:65–72. [PubMed] [Google Scholar]
- 32.Chen F, Jiang G, Liu H, Li Z, Pei Y, Wang H, Pan H, Cui H, Long J, Wang J, Zheng Z. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8:10. doi: 10.1038/s41413-020-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated schwann cells via upregulation of Bcl2, NF-κB, mTOR, wnt signalling pathways. Antioxidants (Basel) 2019;8:198. doi: 10.3390/antiox8070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.