Abstract
Summary: Thrombotic occlusion of the anterior communicating and right anterior cerebral arteries occurred during embolization of an acutely ruptured aneurysm of the anterior communicating artery. Traditional management, including superselective infusion of a fibrinolytic agent, was unsuccessful in reestablishing normal vessel patency. Therefore, an intravenous dose of abciximab was administered. Serial angiography showed that normal vessel patency was reestablished within 10 min. There were no adverse events related to abciximab administration, and the patient recovered from the procedure without neurologic deficit.
Guglielmi detachable coil (GDC) embolization of intracranial aneurysms has become a valid therapeutic alternative to open neurosurgery in many cases. Potential complications include intraprocedural aneurysm rupture and thromboembolism of the parent artery. We report the successful use of abciximab (ReoPro; Centocor, Malvern, PA) for treatment of thrombus in a parent artery during GDC treatment of an acutely ruptured aneurysm.
Case Report
A 44-year-old woman with a history of diabetes mellitus, clinical hypothyroidism, and asthma presented with a Hunt and Hess grade 1 subarachnoid hemorrhage (1). A CT study showed diffuse subarachnoid blood in the sylvian fissures and basal cisterns. Cerebral angiography revealed an 8-mm aneurysm in the right A1-A2 junction of the anterior communicating artery (Fig 1A). The right A1 segment was absent. The patient agreed to participate in the International Subarachnoid Hemorrhage Trial and was randomly assigned to have endovascular therapy.
fig 1.
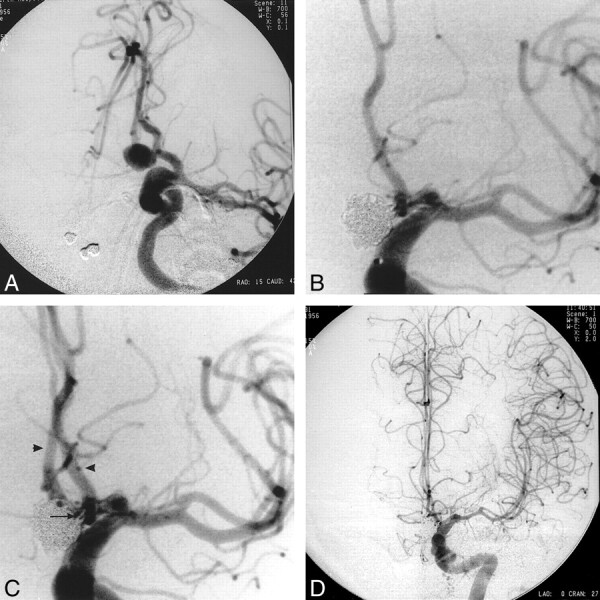
44-year-old woman with a history of diabetes mellitus, clinical hypothyroidism, and asthma who presented with a Hunt and Hess grade 1 subarachnoid hemorrhage.
A, Diagnostic angiogram shows a unilocular aneurysm of the anterior communicating artery arising from the right A1-A2 junction with its fundus directed inferiorly.
B, Angiogram obtained after detachment of the second coil shows nonfilling of the right anterior cerebral artery. The aneurysm is completely occluded. Note incidental intracavernous aneurysm.
C, Angiogram obtained immediately after abciximab administration shows complete resolution of thrombus and normal filling of both anterior cerebral arteries. Note vasospasm involving the M1 segment.
D, Angiogram obtained 15 hours after the procedure shows normal filling of both anterior cerebral arteries. Mild vasospasm involving the M1 segment is again evident.
Under general anesthesia and systemic heparinization to achieve an activated clotting time (ACT) of 2.5 × baseline, the aneurysm was superselectively catheterized with a Tracker Excel-14 microcatheter (Target Therapeutics, Fremont, CA). A 2D GDC-18 8-mm × 30-cm coil (Target Therapeutics) was positioned satisfactorily within the aneurysm and detached. A GDC-18 7-mm × 30-cm coil (Target Therapeutics) was then positioned within the aneurysm. A small single-coil loop was seen prolapsing through the neck for a distance of 2 mm. However, as there was now complete aneurysmal thrombosis with normal parent artery flow, this coil was not repositioned. An ACT obtained at that time was therapeutic, and repeat angiography over a 10-min interval showed continued normal filling of both anterior cerebral arteries. Nonfilling of the right A2 segment was noted immediately after coil detachment (Fig 1B).
A 2000-U bolus of intravenous heparin was administered, followed by 2 mg of nimodipine superselectively infused via the microcatheter to treat any contributing vasospasm. Nonfilling of the right A2 segment persisted. Therefore, 20 mg of ketorolac was administered intravenously followed by superselective infusion of 300,000 U of urokinase via the microcatheter, its tip initially in the aneurysm but later withdrawn into the anterior communicating artery.
Partial unsatisfactory flow was reestablished within the right anterior cerebral artery. Abciximab 20 mg was then administered intravenously over a 10-min interval, resulting in restoration of normal right anterior cerebral artery flow without any branch occlusions (Fig 1C). The aneurysm remained completely occluded.
The patient was transferred to the intensive care unit, where oral clopidogrel (75 mg daily) and aspirin (300 mg daily) were begun and continued for 6 wk. Full systemic heparinization was continued for 48 h. The patient recovered from anesthesia without neurologic deficit. Angiography performed the next day showed both anterior cerebral arteries to be normal in appearance (Fig 1D). The patient was discharged home 11 d after the procedure and remained neurologically intact at the 3-mo clinical follow-up.
Discussion
GDC embolization is a safe and effective treatment for intracranial aneurysms. Progressive refinements in technique and improvements in catheter, coil, and guidewire technology have reduced the risk of thromboembolic complications to approximately 3% of cases (2, 3). Permanent neurologic deficits occur in 1.7% to 5% of cases (4). Treatment of parent artery thrombosis or branch occlusion has until recently been limited to intravascular volume expansion, local infusion of fibrinolytics, maintenance of a therapeutic ACT, and pharmacologic elevation of blood pressure to optimize collateral flow. Reports of superselective infusion of urokinase have documented moderate success, with partial recanalization achieved in 47% of patients (5).
Abciximab is a potent antiplatelet agent that binds to the IIb-IIIa surface-membrane glycoprotein platelet receptor, preventing platelet cross-linking and aggregation (6). Its primary role has been in the prevention and treatment of thromboembolic and ischemic complications in coronary interventions. Potential complications include intracranial, genitourinary, gastrointestinal, retroperitoneal, and vascular access site hemorrhage as well as thrombocytopenia and arrhythmias (7).
Reports of the use of abciximab for neurovascular applications include its use as an adjunct to vertebrobasilar (Samuels OB, Mohammad Y, Stern BJ, et al, paper presented at the American Society of Interventional and Therapeutic Neuroradiology meeting, January 1998) and carotid (8) angioplasty, prevention of basilar artery rethrombosis after transluminal angioplasty (9), and rescue in acute carotid stent thrombosis (10). To our knowledge, the only report documenting abciximab use during an intracranial GDC embolization procedure described successful rescue of localized parent artery thrombus formation during endovascular therapy for a basilar tip aneurysm 1 month after rupture (11).
Acute thrombus is platelet rich (white thrombus), and abciximab is therefore better suited to dissolution than are fibrinolytic agents, such as urokinase (12). However, the possibility that dissolution of intraaneurysmal thrombus would result in subarachnoid hemorrhage dissuaded us from using abciximab as a first-line rescue therapy. Initially, we opted to treat any compounding effects of vasospasm with locally infused nimodipine and use the less potent antiplatelet agent ketorolac. As with other nonsteroidal antiinflammatory drugs, ketorolac derives its antiplatelet effects through inhibition of prostaglandin-synthetase activity, resulting in platelet dysfunction for over 24 h (13). We used ketorolac, because it is administered intravenously, and our patient did not have a nasogastric tube in situ. When superselective infusion of urokinase failed to satisfactorily reestablish flow, we chose to administer 20 mg of abciximab intravenously while carefully observing the patient's vital signs. The recommended bolus dose is 0.25 mg/kg; however, we opted for a smaller dose in our 103-kg patient in view of the acute nature of the aneurysm. Complete clot dissolution with reestablishment of normal flow within the parent artery and its distal territory was seen immediately after the infusion, and the aneurysm remained completely occluded.
We think that a high degree of occlusion by the coil ball mass, as in this case, is a prerequisite for the safe use of abciximab during treatment of acutely ruptured aneurysms. We opted not to continue a maintenance abciximab infusion (recommended 10 μg/min) to reduce the risk of aneurysm rebleeding, and instead used oral clopidogrel, 75 mg per day, and aspirin, 300 mg per day.
Conclusion
This case illustrates that glycoprotein IIb-IIIa inhibitors can be used successfully for rescue of thrombotic parent artery occlusion during coil embolization of acutely ruptured aneurysms. Importantly, the aneurysm was completely occluded at the time of abciximab administration. Further data are required to better define the indications, optimal dosage, method of administration (bolus vs maintenance infusion), and safety profile of abciximab in neurovascular interventions.
Footnotes
Address reprint requests to A/Prof. Makhan Khangure, Department of Diagnostic and Interventional Radiology, Royal Perth Hospital, P.O. Box X2213 GPO, Perth, Western Australia WA 6847.
References
- 1.Hunt WE, Hess RM. Surgical risks as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14-20 [DOI] [PubMed] [Google Scholar]
- 2.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 3.McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393-399 [DOI] [PubMed] [Google Scholar]
- 4.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multi-center clinical trial. J Neurosurg 1998;89:81-86 [DOI] [PubMed] [Google Scholar]
- 5.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracranial aneurysms: a comparison of anatomic results and clinical outcome [see comments]. AJNR Am J Neuroradiol 1998;19:157-165 [PMC free article] [PubMed] [Google Scholar]
- 6.Topol EEJ, Califf RM, Lincoff AM, et al. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization: the EPILOG investigators [see comments]. N Engl J Med 1997;336:1689-1696 [DOI] [PubMed] [Google Scholar]
- 7.Wojak JC. Pharmacology in interventional neuroradiology. In: Connors JJ III, Wojak JC, eds. Interventional Neuroradiology Strategies and Practical Techniques. Philadelphia: Saunders; 1999:59–76
- 8.Qureshi AI, Suri MF, Khan J, Fessler RD, Guterman LR, Hopkins LN. Abciximab as an adjunct to high-risk carotid or vertebrobasilar angioplasty: preliminary experience. Neurosurgery 2000;46:1316-1324 [DOI] [PubMed] [Google Scholar]
- 9.Wallace RC, Furlan AJ, Moliterno DJ, Stevens GH, Masaryk TJ, Perl J III. Basilar artery rethrombosis: successful treatment with platelet glycoprotein IIb/IIIa receptor inhibitor. AJNR Am J Neuroradiol 1997;18:1257-1260 [PMC free article] [PubMed] [Google Scholar]
- 10.Tong FC, Cloft HJ, Joseph GJ, Samuels OB, Dion JE. Abciximab rescue in acute carotid stent thrombosis. AJNR Am J Neuroradiol 2000;21:1750-1752 [PMC free article] [PubMed] [Google Scholar]
- 11.Lempert TE, Malek AM, Halbach VV, Phatouros CC, Dowd CF, Higashida RT. Rescue treatment of acute parent vessel thrombosis with glycoprotein IIb/IIIa inhibitor during GDC coil embolization. Stroke 1999;30:693-695 [PubMed] [Google Scholar]
- 12.Nguyen-Ho P, Lakkis NM. Platelet glycoprotein IIb/IIIa antagonists and coronary artery disease. Curr Atheroscler Rep 2001;3:139-148 [DOI] [PubMed] [Google Scholar]
- 13.Niemi TT, Backman JT, Syrjala MT, Viinikka LU, Rosenberg PH. Platelet dysfunction after ketorolac or propacetamol. Acta Anaesthesiol Scand 2000;44:69-74 [DOI] [PubMed] [Google Scholar]


