Abstract
BACKGROUND AND PURPOSE: In the 1990s, the introduction of the Guglielmi detachable coil (GDC) system in clinical practice was followed by extensive clinical use of this endovascular device in the treatment of brain aneurysms. This technology is based on electrothrombosis and electrolytic detachment of platinum coils. Despite the extensive use of this treatment technique, the role of electrothrombosis has not been fully investigated and clarified. An in vitro electron microscopic study of human blood was performed to elucidate the role that electrothrombosis might play in triggering the biologic response of thrombosis of the aneurysmal sac.
METHODS: Human blood from five patients was used to fill plastic containers in which GDCs had been deposited. These five patients had subarachnoid hemorrhage and were similar in age and clinical presentation. Electron microscopic studies were performed on GDCs that had been electrically charged and on GDCs that had not.
RESULTS: All electron microscopic studies revealed that the electrically charged GDCs were covered by blood elements and fibrin adherent to the surface of the coil. Noncharged GDCs did not have deposits or adhesions of these blood constituents.
CONCLUSION: These findings demonstrated that passage of electric current through the GDC induces attraction of blood constituents. This attraction may trigger a thrombotic reaction on the surface of the coil. The greater the time of current application, the more pronounced the cellular reaction and the deposition of fibrin and blood cells on the GDC.
In the treatment of aneurysms with Guglielmi detachable coils (GDCs), the endovascularly placed platinum coils become positively charged for an average of about 2 min or less (1). This is the time usually required to electrolytically detach the coil from the delivery wire with a 1-mA electric current. Electrothrombosis occurs when an endovascular, positively charged electrode attracts the negatively charged red blood cells, white blood cells, platelets, and fibrinogen (2). Despite a growing number of aneurysms treated with GDCs, the role and amount of electrothrombosis has not been fully evaluated. Using human blood, an in vitro scanning electron microscopic (SEM) study was performed to elucidate the role of electrothrombosis in the treatment of aneurysms. This in vitro study was inspired by the histologic and SEM findings in a patient with subarachnoid hemorrhage who died 16 h after GDC treatment of a basilar tip aneurysm.
Methods
A 48-year-old woman was admitted with a grade 3 subarachnoid hemorrhage from a large, wide-necked basilar tip aneurysm. Subtotal occlusion of the aneurysm was obtained by using nine GDCs 24 h after the initial bleeding. Three hours after treatment, the patient became comatose and had severe systemic hypotension attributable to heart failure. A head CT scan showed no new bleeding, and the patient eventually died 16 h after treatment. Autopsy showed a diffuse subarachnoid hemorrhage over both cerebral hemispheres. The aneurysm was filled with coils that were surrounded and embedded in a thrombus. This thrombus adhered to the inner wall of the aneurysm and near the neck, without affecting the parent vessel. Formaldehyde fixation of the aneurysm was performed, and the GDC was rinsed with phosphate-buffered saline then washed with distilled water and alcohol. The specimen was then processed for ultra structural scanning by coating the surface with a 20-nm layer of gold and platinum to provide stability and conductivity under the electron beams. SEM, performed with a Jeol T300 apparatus (Akishima, Tokyo, Japan), revealed a layer of fibrin over and within the coils (Fig 1). A report of this case has been published (3).
fig 1.
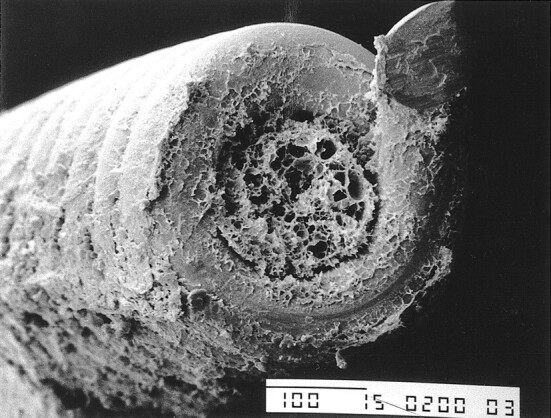
SEM photograph from a human case shows a thin layer of fibrin over, above, and within the coil (original magnification, × 200)
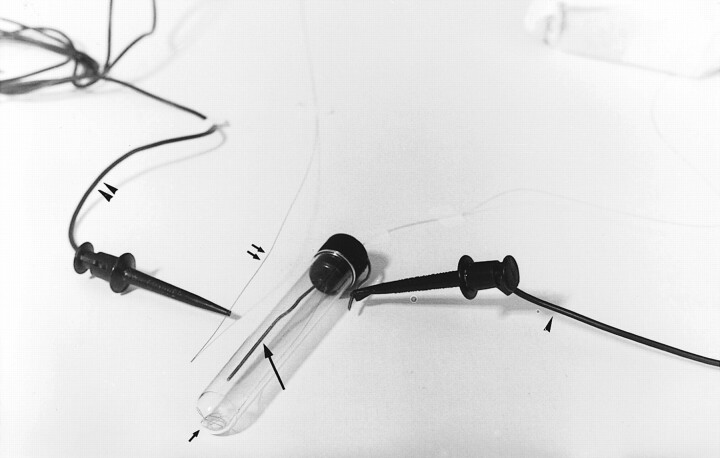
These findings prompted us to perform in vitro studies to assess the effect of the electric current on the platinum portion of GDCs. Five in vitro experiments were performed. A stainless steel (ground) electrode was positioned in the upper two thirds of each small, elongated plastic container (Fig 2). Blood from five patients similar in sex, age, and clinical characteristics and with acute subarachnoid hemorrhage was used; 24 mL of arterial blood was withdrawn from each patient during diagnostic angiography. For each experiment, three containers were filled with 8 mL of blood treated with heparin. A GDC was positioned in the blood, at the bottom of the container below the tip of the ground electrode (Fig 2). Three tests were performed for each of the five experiments. The first test involved leaving the GDC in the blood for 16 h, without applying electric current to the system. The second test involved applying a 1-mA current, for 30 s, to the GDC, the negative pole connected to the ground electrode and the positive pole connected to the proximal end of the delivery wire. The GDC was left in the blood for 16 h. The third test involved applying a 1-mA current, for 90 s, to the GDC, the negative pole connected to the ground electrode and the positive pole connected to the proximal end of the delivery wire. Again, each GDC was left in the blood for 16 h. After 16 h, the GDC was gently withdrawn from the blood and carefully examined. All experiments were performed at 37°C.
fig 2.
Photograph shows the experimental setup. The inner electrode (long arrow) is in the plastic container, as is a GDC (short arrow). The GDC delivery wire (double arrows), negative electrode (arrowhead), and positive electrode (double arrowheads) also are shown
For each experiment, the GDC was fixed with formalin, cleaned with an alcohol solution, and dried. The surface of the GDC was then covered with a 20-nm layer of gold and platinum to confer stability and conductivity during SEM. The portion of the coil with the most macroscopically visible deposits (eg, blood particles, fibrin) was carefully analyzed by using a Jeol T300 SEM apparatus (Akishima).
Results
In all experiments, the in vitro SEM studies demonstrated that charged GDCs had a different response than GDCs not exposed to an electric charge. A different response also was observed between GDCs charged for 30 s and GDCs charged for 90 s. In particular, noncharged GDCs did not have significant adhesion of blood components or fibrin on the platinum surface (Fig 3). The GDCs that were charged at 1 mA for 30 s had thin and irregular deposits of fibrin and blood components (eg, red blood cells, white blood cells, platelets) (Fig 4). The GDCs that were charged for 90 s had thick and regular deposits of the same materials (Fig 5). These SEM results were compared with those obtained in the patient with an acute basilar tip aneurysm who died 16 h after a GDC procedure. In this patient, the intraaneurysmal GDCs that had been charged for 30 s had similar thin and homogeneous deposits of fibrin and blood components in the in vitro setting.
fig 3.
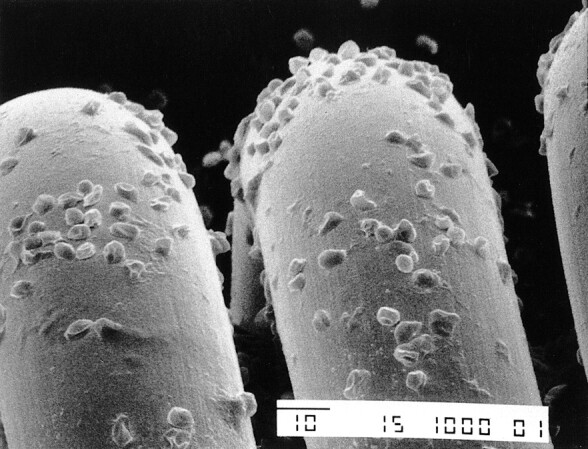
SEM photograph of the loops of a GDC obtained without passage of electric current shows deposition of scattered blood components (original magnification, ×1000)
fig 4.
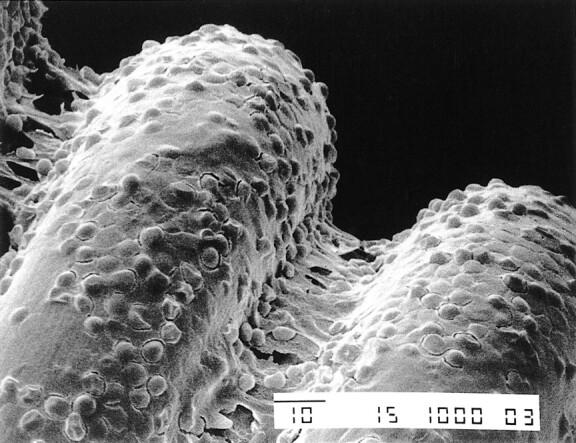
SEM photograph of the loops of a GDC obtained after passage of a 1-mA electric current for 30 s shows a thin layer of fibrin and blood components (original magnification, × 200)
fig 5.
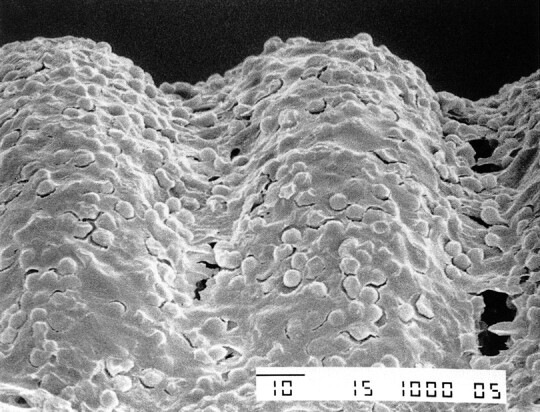
SEM photograph of the loops of a GDC obtained after passage of a 1-mA electric current for 90 s shows a thick layer of fibrin and blood components (original magnification, × 200)
Discussion
In 1953, Sawyer et al (2) reported that red blood cells, white blood cells, platelets, and fibrinogen are negatively charged. They performed in vitro experiments using a positively charged electrode dipped into blood; because of the opposite electric charge, the aforementioned blood components were attracted by the positive electrode, forming a thrombus (electrothrombosis). The GDC system is based on two electrochemical principles: electrothrombosis and electrolysis (4). The endovascular GDCs become positively charged in the vascular lumen for an average of 2 min or less; this is the time required in electrolysis to detach the platinum portion from the stainless steel delivery wire with a 1-mA direct electric current (1). Whereas it is clear that electrolysis detaches the coil in the vascular lumen, the role of electrothrombosis has not been fully clarified.
Several reports about experimental aneurysms treated with GDCs exist in the literature (4–13). These studies were performed to evaluate the acute and chronic histopathologic response and to assess the short- and long-term efficacy of this treatment technique. Some authors report a low trombogenicity of GDCs because of the limited efficacy of intraaneurysmal electrothrombosis (5, 6, 8, 9). In particular, it was speculated that the GDC occlusion was comparable with that obtained by using mechanically detachable coils and that it was due only to the “mechanical space-occupying effect.” Human cases of GDC-treated aneurysms that subsequently underwent surgical clipping have been reported; no acute endosaccular thrombosis was observed (14). In contrast, many reports of the endosaccular presence of a fresh thrombus in experimental aneurysms treated with platinum coils exist (10–13, 15). In particular, using experimental aneurysms in rabbits and Japanese monkeys, Reul et al (12) and Tenjin et al (13) demonstrated the presence of an endosaccular fresh thrombus and fibrinlike proteins 24–48 h after coil treatment. In the long term, they also observed complete occlusion of the aneurysmal sac by connective tissue and the presence of a thick membrane covering the aneurysmal neck.
It is, however, improper to extrapolate the results of experimental studies to human aneurysms after GDC treatment; the technique of creating experimental aneurysms (venous or arterial and side-wall or terminal) and the differences in coagulation systems between species constitute the main limitations (8, 9, 13, 16–18). Such differences make comparing the process of coil-induced thrombosis in animals and humans difficult.
Despite the growing number of patients who are treated with the GDC system, only a few reports about the histologic examination of aneurysms exist; particularly few are those about the acute phase after treatment (3, 16–28). Some of these reports describe that, in the acute phase, a fresh thrombus comprising fibrin and blood cells occupies the aneurysm sac. Subsequently, fibroblastic and endothelial cellular proliferation occurs within the sac and at the aneurysmal ostium.
Our in vitro study shows that the passage of electric current through GDCs induces the attraction of blood components. The longer the duration of current application, the more pronounced this cellular reaction and deposition of fibrin on the coil surface. It is possible to speculate that this might 1) trigger an early and enhanced thrombotic reaction that protects the aneurysm from an early rebleeding (17, 29) and 2) encourage the formation of an intraaneurysmal mature scar, with subsequent neoendothelialization of the aneurysmal neck (21, 24, 27, 28). The role of electrothrombosis, however, might have a negative effect, in that it could promote unwanted thrombus formation, with possible consequent downstream thromboembolic complications.
This study was performed with stagnant blood; therefore, we can only speculate that the same events would occur in vivo or in clinical cases where flow was present. It can be pointed out, however, that deposition of GDCs rather rapidly causes a reduction of intraaneurysmal flow so that, in some parts of the aneurysm, areas where flow is very slow or even stagnant are likely.
Unquestionably, total exclusion of the aneurysm from the blood circulation will always depend on the degree of aneurysmal packing, the size of the aneurysmal neck, and the geometry of the aneurysm–parent-artery complex. However, precocious formation of a fresh, electrically enhanced thrombus may induce a rapid and progressive aneurysmal occlusion.
Footnotes
Address reprint requests to Riccardo Padolecchia, MD, Section of Neuroradiology, S. Chiara Hospital, Via Roma 67, 56100 Pisa, Italy.
References
- 1.Byrne JV, Guglielmi G. Endovascular Treatment of Intracranial Aneurysms. Berlin, Germany: Springer; 1997; 146–148
- 2.Sawyer PN, Pate JW, Weldon CS. Relationship of abnormal and injury electric potential differences to intravascular thrombosis. Am J Physiol 1953;175:108-112 [DOI] [PubMed] [Google Scholar]
- 3.Padolecchia R, Puglioli M, Collavoli PL, et al. Acute histologic and ultrastructural study in one case of human basilar tip aneurysm embolised with Guglielmi detachable coils. Intervent Neuroradiol 1999;5:257-260 [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach, I: electrochemical basis, technique and experimental results. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 5.Ahuja A, Hergenrother RW, Strother CM, Rappe AA, Cooper SL, Graves WB. Platinum coil coating to increase thrombogenicity: a preliminary study in rabbits. AJNR Am Neuroradiol 1993;14:794-798 [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JV, Hope JK, Hubbard N, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. AJNR Am J Neuroradiol 1997;18:29-33 [PMC free article] [PubMed] [Google Scholar]
- 7.Mawad M, Mawad J, Cartwright J Jr, Gokaslan Z. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7-13 [PMC free article] [PubMed] [Google Scholar]
- 8.Reul J, Weis J, Spetzger U. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol 1997;18:35-42 [PMC free article] [PubMed] [Google Scholar]
- 9.Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach JM. Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization. J Neurosurg 1996;85:488-495 [DOI] [PubMed] [Google Scholar]
- 10.Macdonald RL, Mojtahedi S, Johns L, Kowalczuk A. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke 1998;29:478-486 [DOI] [PubMed] [Google Scholar]
- 11.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667-672 [PMC free article] [PubMed] [Google Scholar]
- 12.Reul J, Spetzger U, Weis J, Von Buelow S, Ince A, Thron A. The nature of early intraluminal thrombosis in terminal aneurysms occluded with Guglielmi detachable coils. Intervent Neuroradiol 1998;4:39-48 [DOI] [PubMed] [Google Scholar]
- 13.Tenjin H, Fushiki S, Nakahara Y, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke 1995;26:2075-2080 [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Samson D, Purdy P. Does electrothrombosis occur immediately after embolization of an aneurysm with Guglielmi detachable coils? AJNR Am J Neuroradiol 1997;18:510-513 [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson RC III, Krisht AF, Barrow DL, Joseph GJ, Shengelaia GG, Bonner G. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133-140 [DOI] [PubMed] [Google Scholar]
- 16.Stiver SI, Porter PJ, Willinsky RA, Wallace CW. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of the literature. Neurosurgery 1998;43:1203-1208 [DOI] [PubMed] [Google Scholar]
- 17.Manabe H, Fujita S, Hatayama T, Ohkuma H, Suzuki S, Yagihashi S. Embolisation of ruptured cerebral aneurysms with interlocking detachable coils in acute stage. Intervent Neuroradiol 1997;3:49-63 [DOI] [PubMed] [Google Scholar]
- 18.Manabe H, Fujita S, Hatayama T, Suzuki S, Yagihashi S. Rerupture of coil-embolized aneurysm during long-term observation. J Neurosurg 1998;88:1096-1098 [DOI] [PubMed] [Google Scholar]
- 19.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils. J Neurosurg 1995;83:129-132 [DOI] [PubMed] [Google Scholar]
- 20.Mizoi K, Yoshimoto T, Takahashi A, Nagamine Y. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation. Neurosurgery 1996;39:165-168 [DOI] [PubMed] [Google Scholar]
- 21.Horowitz M, Purdy P, Burns D, Bellotto D. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:688-690 [PMC free article] [PubMed] [Google Scholar]
- 22.Gaebel C, Reul J, Reusche E, et al. Pathology of human intracranial aneurysms after GDC-embolization and correlation with animal model data. Clin Neuropathol 1996;15:270 [Google Scholar]
- 23.Ozawa T, Koike T, Takeuchi S, Tanaka R. Scanning electron microscopic study of the migrated platinum coil after endovascular embolization of a giant cerebral aneurysm. AJNR Am J Neuroradiol 1998;19:594-595 [PMC free article] [PubMed] [Google Scholar]
- 24.Koizumi T, Kawano T, Kazekawa K, et al. Histological findings in aneurysm treated with IDC: scanning electron microscopical study. No Shinkei Geka 1997;25:1027-1031 [PubMed] [Google Scholar]
- 25.Shimizu S, Kurata A, Takano M, et al. Tissue response of a small saccular aneurysm after incomplete occlusion with a Guglielmi detachable coil. AJNR Am J Neuroradiol 1999;20:546-548 [PMC free article] [PubMed] [Google Scholar]
- 26.Romeike BF, Niedermayer I, Feiden W. Histopathologic findings in cerebral artery aneurysms after embolization with Guglielmi detachable platinum coils (GDC): report of two cases. Radiologe 1999;39:900-903 [DOI] [PubMed] [Google Scholar]
- 27.Castro E, Fortea F, Villoria F, Lacruz C, Ferreras B, Carrillo R. Long-term histopathologic findings in two cerebral aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:549-552 [PMC free article] [PubMed] [Google Scholar]
- 28.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284-293 [DOI] [PubMed] [Google Scholar]
- 29.Graves VB, Strother CM, Duff TA, Perl J II. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640-647 [DOI] [PubMed] [Google Scholar]