Abstract
BACKGROUND AND PURPOSE: Recent experience suggests that diffusion-weighted MR imaging may be decisive in the differential diagnosis of ring-enhancing cerebral lesions. Whether restricted diffusion within a ring-enhancing cerebral mass lesion is pathognomonic for abscess was studied.
METHODS: Seventeen patients with ring-enhancing cerebral lesions (three abscesses, six glioblastomas, eight metastases) on conventional contrast-enhanced T1-weighted images were examined with echo-planar diffusion-weighted MR imaging. Apparent diffusion coefficient (ADC) maps and the ADCs were calculated for all lesions. Lesions with signs of intralesional hemorrhage on unenhanced T1-weighted images were excluded.
RESULTS: The central portion of all six glioblastomas and seven of eight metastases showed unrestricted diffusion, whereas two of three abscesses showed restricted diffusion (low ADC values) in their cavity. However, restricted diffusion also was found in one metastasis, and one abscess within a postoperative cavity showed unrestricted diffusion within a larger nondependent portion.
CONCLUSION: In patients with ring-enhancing cerebral mass lesions, restricted diffusion might be characteristic but is not pathognomonic for abscess, as low ADC values also may be found in brain metastases.
With conventional MR imaging, the differential diagnosis of ring-enhancing cerebral lesions is difficult and often impossible. However, recent reports claim that brain abscesses can be differentiated from cystic or necrotic brain tumors with diffusion-weighted (DW) MR imaging (1–5), a method that provides unique information about water diffusion. While the centers of most primary and secondary necrotic or cystic brain tumors are hyperintense on apparent diffusion coefficient (ADC) maps corresponding to unrestricted diffusion, restricted diffusion with low ADC values within abscess cavities was reported by several authors. Yet, the reason for restricted diffusion in brain abscesses is poorly understood. Restricted diffusion might reflect high viscosity of proteinaceous fluid with high concentration of inflammatory cells, but these conditions are not confined to abscesses and might be present in various other brain diseases. Therefore, we used DW imaging in patients with ring-enhancing brain lesions to determine whether restricted diffusion within a ring-enhancing cerebral mass lesion was pathognomonic for abscess.
Methods
From October 1998 to September 1999, 17 consecutive patients with a cystic or necrotic cerebral mass lesion that showed ring enhancement on contrast-enhanced T1-weighted images were examined using DW imaging. Lesions with signs of intralesional hemorrhage on unenhanced T1-weighted images were excluded. Stereotactic aspiration biopsy or surgery was performed for all patients. The final diagnosis was pyogenic abscess for three patients, glioblastoma multiforme for six patients, and metastasis for eight patients.
All MR examinations were performed with a 1.5-T imager capable of echo-planar imaging (Marconi, Cleveland, Ohio). Conventional and DW MR imaging were performed during the same study for each patient. Conventional MR images were obtained with transverse proton density– and T2-weighted sequences (3000/20/2 [TR/TE/excitations]; matrix, 192 × 256; field of view, 23 × 23 cm2; section thickness, 6 mm; section gap, 1 mm), transverse T1-weighted sequences (650/25/ 2; matrix, 192 × 256; field of view, 23 × 23 cm2; section thickness, 6 mm; section gap, 1 mm), and transverse contrast-enhanced T1-weighted sequences (0.1 mmol/kg of gadopentetate dimeglumine [Magnevist; Schering, Berlin, Germany]). The contrast-enhanced images were obtained following DW imaging. Isotropic DW images were obtained in the transverse plane by using a single-shot echo-planar spin-echo pulse sequence (3000/120/1; matrix, 128 × 64; field of view, 31.5 × 17 cm; section thickness, 6 mm; section gap, 0.5 mm) with four b values (0, 333, 666, 1000 s/mm2). The total duration of the diffusion-sensitizing gradients was 80 m. To obtain isotropic diffusion weighting, a gradient scheme was used according to Heid and Weber (6). The maximum diffusion gradient was 26 mT/m. The ADC maps were calculated and the ADC value of the cystic or necrotic portion was measured in a region of interest of at least 1 cm2. On visual inspection, the signal intensities of the lesions on the DW images and on the ADC maps were interpreted relative to the contralateral brain parenchyma.
Results
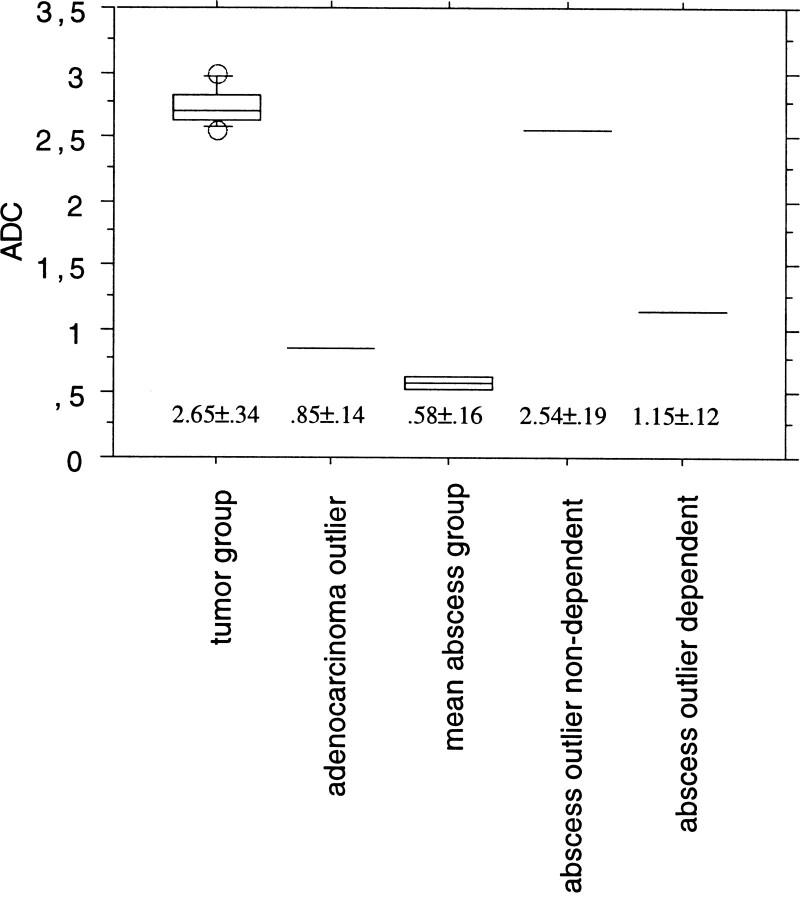
Differentiation between abscess, metastasis, and glioblastoma was not possible by conventional MR imaging alone. The necrotic or cystic portion of six metastases and of all glioblastomas (mean tumor group) was markedly hypointense on DW images, with corresponding marked hyperintensity on the ADC maps. The mean ADC value of the central portion was 2.65 ± 0.34 × 10−3 mm2/s. However, in one metastasis (adenocarcinoma), the central portion was markedly hyperintense on DW images relative to normal brain parenchyma. On the ADC maps, there was a corresponding low signal intensity (Fig 1) and the ADC value was 0.85 ± 0.14 × 10−3 mm2/s. Two abscesses showed markedly hyperintense signal on DW images and were markedly hypointense on the ADC maps (Figs 2 and 3). One abscess showed extraparenchymal extension corresponding to a parafalcial subdural empyema that had low ADC values as well (Fig 3). On quantitative analysis, the mean ADC value of the central portion of both abscesses was 0.58 ± 0.16 × 10−3 mm2/s. The central portion of the third abscess in a patient with resection of a right temporal metastasis 2 months earlier showed a fluid-fluid level within the cavity. The DW imaging intensity of the gravity-dependent portion was markedly hyperintense and showed markedly hypointense signal on the ADC map, indicating restricted diffusion. The DW imaging intensity of the nondependent portion was hypointense with corresponding marked hyperintensity on the ADC map. The ADC value of the gravity-dependent area was 1.15 ± 0.12 × 10−3 mm2/s; for the nondependent portion, 2.54 ± 0.19 × 10−3 mm2/s (Fig 4).
fig 1.
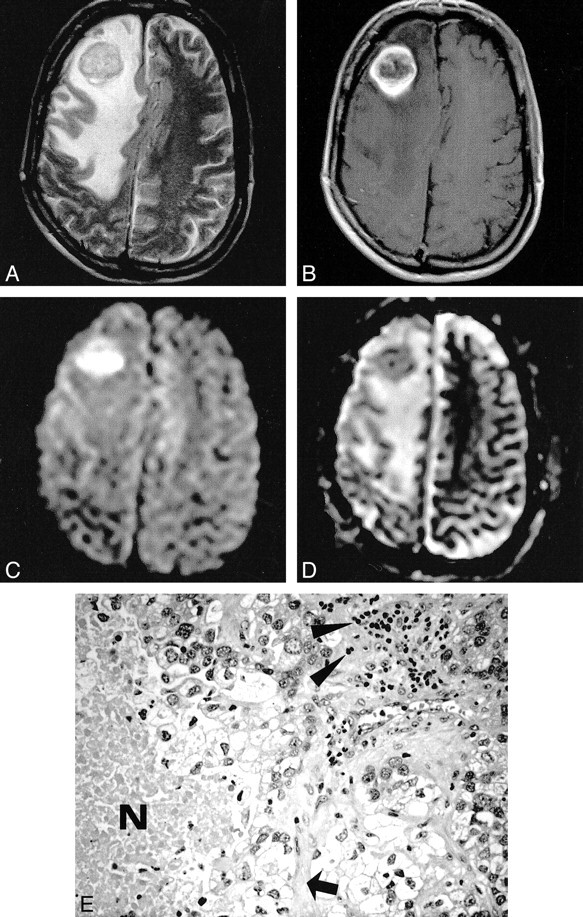
Metastatic adenocarcinoma. The T2-weighted image (A) shows a hypointense mass lesion surrounded by massive edema. On the contrast-enhanced T1-weighted image (B), there is ring enhancement presumably due to central necrosis. With diffusion weighting (C), the central part of the tumor becomes markedly hyperintense, while the ADC map (D) reveals low values, indicating restricted diffusion. Histopathologic section (E): Note that besides tumor cells, additional geographic necrosis (N), lymphocytes (arrowheads), and connective tissue (arrow) are present (hematoxylin and eosin stain; original magnification, × 200)
fig 2.
Pyogenic brain abscess. On the T2-weighted image (A), the lesion has a thick hypointense wall with a hyperintense center, consistent with necrosis. Dense ring enhancement is noted on the contrast- enhanced T1-weighted image (B). The DW image (C) and the ADC map (D) reveal restricted diffusion within the center
fig 3.
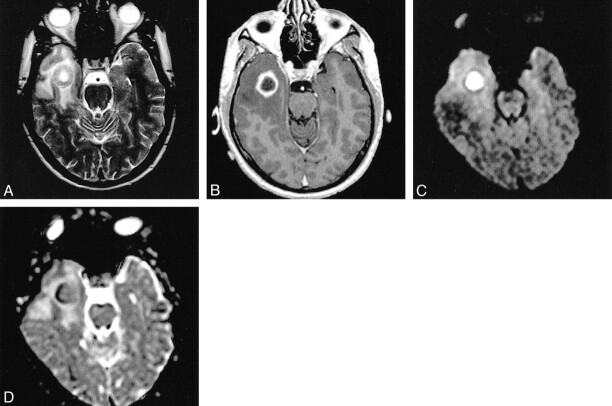
Pyogenic brain abscess with parafalcial subdural empyema. On T2-weighted image (A) the lesion has a thick hypointense wall. The contrast-enhanced T1-weighted image (B) shows ring enhancement. Evidence for restricted diffusion within the abscess cavity and the parafalcial empyema is provided by the DW image (C) and the ADC map (D)
fig 4.
Mean ADC values ± SD (×10−3 mm2/s) of the mean tumor group (n = 13), adenocarcinoma outlier (n = 1), mean abscess group (n = 2), and nondependent and dependent portions of the abscess outlier (n = 1)
Discussion
In most patients with brain tumors, symptoms and signs (often even neuroradiologic findings) are not pathognomonic. However, because of the variable clinical course and prognosis of the diseases that must be included in the differential diagnosis—untreated brain abscesses, for example, can be rapidly fatal—an early and correct diagnosis is necessary for choosing the appropriate treatment. The introduction of CT has led to a dramatic decrease in the mortality rate from brain abscesses (7) because of early and improved diagnosis with exact localization of the mass. Although there are various CT features that help differentiate brain abscesses from primary or secondary solid brain tumors, infarction, and noninfectious inflammatory mass lesions, determining the true nature of ring-enhancing cerebral lesions remains difficult. Even with the advent of MR imaging, this diagnostic dilemma did not disappear. The differential diagnosis of ring-enhancing cerebral lesions continues to be a challenge, as such lesions include glioblastoma, metastasis, pyogenic abscess, subacute ischemic infarction, resolving hematoma, and demyelinating disease.
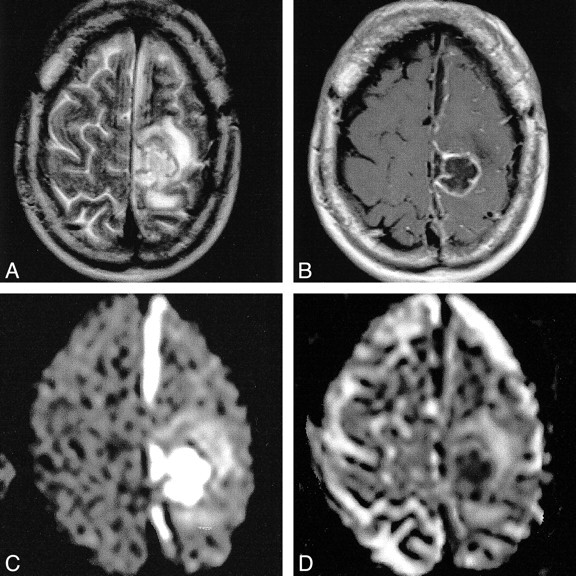
Recent experience suggests that DW MR imaging might be decisive in the differential diagnosis of ring-enhancing cerebral lesions. In 1996, Ebisu et al (2) reported finding restricted diffusion within the cavity of a brain abscess. This phenomenon might be related to the high viscosity and cellularity of pus, which causes restriction of water proton mobility. However, in 1997, Krabbe et al (8) reported the opposite: increased diffusion within an abscess. Further studies confirmed restricted diffusion in abscesses with high signal intensity in the central cavity and correspondingly low ADC values (1, 3–5). Yet, the ADC values between and within these studies varied widely. It is possible that variable concentrations of inflammatory cells and bacteria, different etiologic organisms, and the age of the abscess with variable viscosity of the abscess fluid were responsible for these conflicting data. Nevertheless, these studies strongly suggest that the diagnosis of a brain abscess can be made if there is restricted diffusion within a cystic ring-enhancing cerebral lesion with low ADC values. Our example of a postoperative abscess that developed within the resection cavity and showed unrestricted diffusion within the larger nondependent portion, with a small area of restricted diffusion within the smaller dependent portion (Fig 5), underlines the usefulness of DW imaging in the differential diagnosis of ring-enhancing lesions.
fig 5.
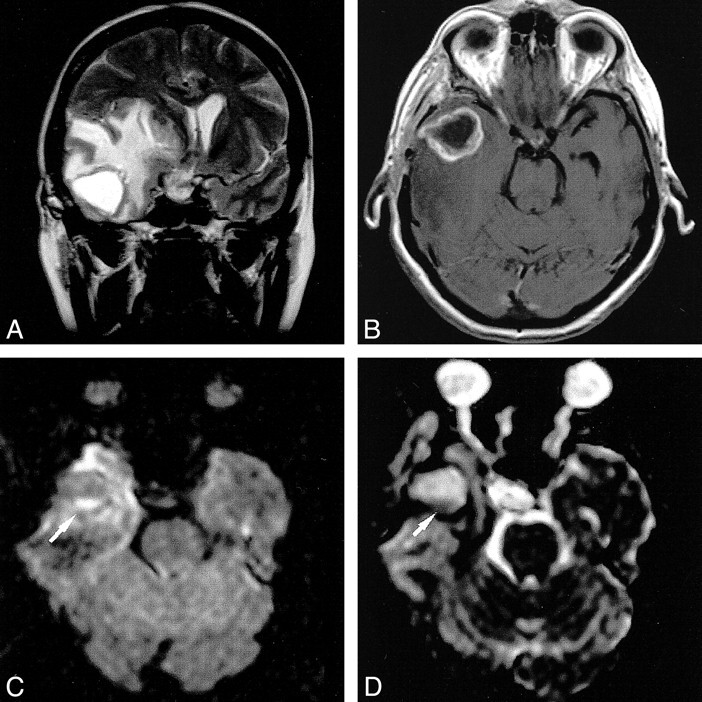
Postoperative brain abscess. The T2-weighted image (A) shows a hyperintense cavity surrounded by massive edema. The contrast-enhanced T1-weighted image (B) shows ring enhancement. With diffusion weighting (C), the gravity-dependent portion was markedly hyperintense (arrow) and showed markedly hypointense signal on the ADC map, (arrow), indicating restricted diffusion (D). The DW imaging intensity of the nondependent portion was hypointense with corresponding marked hyperintensity on the ADC map
On the other hand, our example of a metastatic adenocarcinoma with restricted diffusion within the necrosis shows that low ADC values within a ring- enhancing lesion are not pathognomonic for brain abscess. The reason for restricted diffusion within the metastasis in our study is unclear. Increased protein concentration in the form of highly viscous mucin might cause restricted diffusion. This, however, contradicts the results of Krabbe et al (8), who found increased ADC values (mean, 2.62 ± 0.31 × 10−3 cm2/s) in four metastases from adenocarcinoma. The adenocarcinoma “outlier” in our study contained, in addition to tumor cells, additional geographic necrosis, lymphocytes, and connective tissue, but no mucin was found during histopathologic analysis (Fig 1E). Thus, we conclude that low ADC values are not pathognomonic for metastatic adenocarcinoma. Small hemorrhages or hemosiderin deposits are unlikely, as one could expect marked hypointensity because of susceptibility effects on the DW images, and we excluded lesions with possible subacute blood products that were hyperintense on noncontrast T1-weighted images. On the other hand, the exclusion of these lesions from our study is a potential limitation. From diffusion measurements in intracranial hematomas, we know that intracellular hemoglobin states (intracellular oxy-, intracellular deoxy-, and intracellular methemoglobin) cause restricted diffusion in hyperacute, acute, and early subacute hematomas despite their marked differences on conventional MR images (9). Therefore, bloody lesions would be displayed as identical to pyogenic abscess, based on signal intensity, and these findings may be confounding.
Conclusion
In patients with ring-enhancing cerebral mass lesions, low ADC values are not 100% specific for brain abscess, as restricted water diffusion also might be found in brain metastases. The dilemma concerning the differential diagnosis of ring-enhancing cerebral mass lesions continues.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Atlanta, April 2000.
Address reprint requests to Marius Hartmann, MD, Department of Neuroradiology, University of Heidelberg Medical School, Im Neuenheimer Feld 400, D-69120 Heidelberg, Germany.
References
- 1.Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteux M. Use of diffusion-weighted MR imaging in the differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol 1999;20:1252-1257 [PMC free article] [PubMed] [Google Scholar]
- 2.Ebisu T, Tanaka C, Umeda M, et al. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging 1996;14:1113-1116 [DOI] [PubMed] [Google Scholar]
- 3.Kim YJ, Chang K-H, Song IC, et al. Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol 1998;171:1487-1490 [DOI] [PubMed] [Google Scholar]
- 4.Noguchi K, Watanabe N, Nagayoshi T, et al. Role of diffusion-weighted echo-planar MRI in distinguishing between brain abscess and tumour: a preliminary report. Neuroradiology 1999;41:171-174 [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya K, Yamakami N, Hachiya J, Saito I, Kobayashi H. Multiple brain abscesses: differentiation from cerebral metastases by diffusion-weighted magnetic resonance imaging. Int J Neuroradiol 1998;4:258-262 [Google Scholar]
- 6.Heid O, Weber J. Diffusion tensor pulse sequences (abstr). Proceedings of the fifth annual meeting of the International Society for Magnetic Resonance in Medicine 1997;5:224 [Google Scholar]
- 7.Rosenblum ML, Hoff JT, Norman D, Weinstein PR, Pitts L. Decreased mortality from brain abscesses since the advent of computerized tomography. J Neurosurg 1978;49:658-688 [DOI] [PubMed] [Google Scholar]
- 8.Krabbe K, Gideon Ü, Wagn P, Hansen U, Thomsen C, Madsen F. MR diffusion imaging of human intracranial tumors. Neuroradiology 1997;39:483-489 [DOI] [PubMed] [Google Scholar]
- 9.Atlas SW, DuBois PH, Singer MB, Lu D. Diffusion measurements in intracranial hematomas: implications of MR imaging of acute stroke. AJNR Am J Neuroradiol 2000;21:1190-1194 [PMC free article] [PubMed] [Google Scholar]