Abstract
BACKGROUND AND PURPOSE: White matter changes such as periventricular hyperintensity (PVH) and deep white matter hyperintensity (DWMH) are associated with both periventricular edema and ischemic white matter degeneration. Their diagnostic and predictive value in normal pressure hydrocephalus (NPH) is unclear. To identify prognostically important changes, we classified PVH and DWMH at MR imaging in a large series of patients with NPH, before and after ventriculoperitoneal shunt surgery.
METHODS: Axial proton density– and T2-weighted turbo spin-echo sequences and coronal T1-weighted sequences were performed on a 0.5-T imager in 34 patients with NPH, before and 3 months after shunt surgery. PVH at the anterior, central, and posterior thirds of the lateral ventricles was assessed on transaxial images with a semiquantitative five-step scale describing the extension (in mm) and shape of the PVH. DWMH was quantified with a four-step scale. The number of cortical and subcortical lacunar infarctions, the flow void sign, and the width of the third and lateral ventricles were registered. Gait ability, need for sleep, urinary incontinence, living conditions, and psychometric test performance were assessed pre- and postoperatively.
RESULTS: After shunt surgery, 25 patients improved and nine did not. PVH, DWMH, and other MR imaging variables before shunting did not differ between groups, and no MR imaging variable could predict the clinical effect of shunt surgery. Postoperatively, the width of PVH was reduced in the improved patients, and clinical improvement correlated with reduction in PVH. Only the irregular type of PVH located at the frontal horns was reduced postoperatively. The presence of risk factors or MR imaging changes normally associated with cerebrovascular disease had no negative influence on the outcome of shunt surgery.
CONCLUSION: The presence of DWMH or subcortical lacunar infarctions in NPH did not predict a poor outcome from shunt surgery and should not be used as exclusion criteria for shunting. No MR imaging findings could predict outcome of shunt surgery in patients with NPH. Clinical improvement after surgery is associated with reduction in the irregular type of PVH located around the frontal horns.
Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, mental deterioration, and urinary incontinence in patients with an enlarged ventricular system and normal CSF pressure (1, 2). An important pathophysiological feature of NPH is dysfunctional CSF dynamics with reduced absorption through the arachnoid villi, compensatory CSF flow into the periventricular white matter, and transcapillary CSF resorption (3−6). The periventricular tissue is characterized pathologically by disruption of the ependyma, edema, neuronal degeneration, and gliosis (5, 7, 8). CSF studies in patients with NPH have shown neuronal degeneration and no major demyelination (9, 10). Symptom severity also has been related to CSF levels of neurofilament protein, a marker of neuronal degeneration (9).
A dilated ventricular system is a prerequisite for the diagnosis of NPH. The following changes at CT are considered prognostic indicators: flattening of the cortical sulci, widened temporal horns (11, 12), an enlarged third ventricle (13, 14), and periventricular hypoattenuation (15, 16). On MR images, marked aqueductal CSF flow (the flow void sign) has been described in NPH (17–22), correlating with a favorable outcome after shunt surgery (23, 24). These latter studies, by Bradley et al, used flow-sensitive techniques. The flow void sign also has been reported in healthy individuals (25), however, and has had little or no predictive value in large prospective, controlled series of patients with idiopathic NPH (26, 27) assessed by flow-compensated techniques.
The diagnostic and predictive value of white matter changes such as periventricular hyperintensity (PVH) and deep white matter hyperintensity (DWMH)—changes associated with both periventricular edema and ischemic white matter degeneration—in NPH is unclear. These changes are seen more frequently in patients with NPH than in age-matched control subjects, and patients with even relatively pronounced lesions of suspected vascular origin still can benefit from shunt surgery (18, 28). The degree of clinical improvement correlates negatively, however, with the extension of PVH and DWMH (28).
The aim of the present study was to improve the prognostic value of MR imaging in NPH. We tested a new, five-step, semiquantitative evaluation protocol that measures PVH, DWMH, lacunar infarctions, and the flow void sign, among other variables, and correlated the results with clinical improvement after shunt surgery.
Methods
Patients
Forty-six consecutive patients with a diagnosis of NPH who underwent surgery at the Hydrocephalus Research Unit, Sahlgrenska University Hospital, were considered for inclusion. Twelve patients whose MR images were impossible to evaluate were excluded, leaving 34 patients in the study. The Göteborg University Ethics Committee approved the study, and informed consent was obtained from all patients.
The mean age of the patients was 66 years (SD = 12 years; range, 28−82 years), and the mean duration of symptoms was 25 months (SD = 31 months; range, 2−120 months [Table 1]). The primary cause of NPH was subarachnoid hemorrhage (SAH) in 18% of the patients, trauma in 18%, cerebrovascular disorder in 26%, and idiopathy in 38%. Cerebrovascular disorder was considered when there was a temporal relationship between a hemorrhagic or ischemic incident other than SAH and development of NPH symptoms. There were no differences in clinical variables between the different etiologic groups.
TABLE 1:
Baseline characteristics and results of shunt surgery, overall and by clinical outcome of surgery
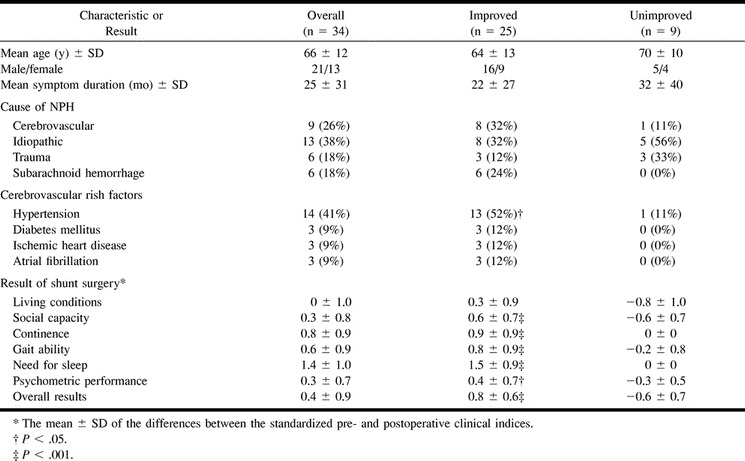
Fourteen patients had hypertension, three had diabetes mellitus, three had ischemic heart disease, and three had chronic atrial fibrillation. Two of the three patients with diabetes had no other cerebrovascular risk factor, and 16 patients (47%) had at least one risk factor for cerebrovascular disease.
All patients received a ventriculoperitoneal shunt, either a Sophy Programmable Pressure Valve (Sophysa, Orsay Cedex, France) or a Cordis Orbis-Sigma Valve (Cordis Corporation, Miami, USA). Follow-up examination, performed 3 months after shunt surgery, included all preoperative examinations except radionuclide cisternography (RC). The shunt was considered patent if the patient had improved or if the ventricular size was reduced on MR images. Otherwise the shunt function was tested.
Symptoms and signs were registered semiquantitatively according to Larsson et al (29) and Blomsterwall et al. (30), preoperatively and at follow-up examination 3 months after surgery. Gait ability (1, normal; 2, insecure; 3, insecure with cane; 4, bimanual support; 5, aided; 6, wheelchair), urgency incontinence (1, not present; 2, present), living conditions (1, independent; 2, at home but assisted; 3, senior citizen's retirement home; 4, nursing home; 5, hospital), daily need for sleep (1, normal; 2, increased by <2 hours; 3, increased by >2 hours), and psychometric tests were recorded before and after surgery and used in the calculation of five clinical indices. The mean of the differences (MoD) between the pre- and postoperative indices was used to measure the outcome of shunt surgery (see Statistical Analysis).
The psychometric tests used were Cronholm-Molander memory tests (learning and memory), the Identical Forms test (perceptual speed and accuracy) and Raven Colored Matrices (nonverbal, didactive reasoning) (9).
The diagnosis of NPH was based on the presence of the following: 1) gait disturbance; 2) mental deterioration, urinary incontinence, or both; 3) enlarged ventricles at MR imaging with an Evan's index (maximal width of frontal horns/maximal width of inner skull) >0.30; 4) lumbar CSF pressure <20 cm H2O; and 5) ventricular filling and block of convexity flow on RC. In some patients fulfilling these criteria, but with signs of other disorders, such as vascular lesions, the CSF tap test (31) and regional cerebral blood flow (rCBF) (32) measurement were performed to strengthen the indication for surgery. Only patients with improvement in the CSF tap test and a characteristic rCBF pattern (32, 33) were considered eligible for shunt surgery. The decision to perform surgery was based on the results of all examinations and the clinical presentation. The MR imaging criteria were limited to Evan's index and a more general visual evaluation, excluding cases of severe cortical atrophy. Cerebrovascular changes at MR imaging were deliberately not chosen as exclusion criteria.
RC was carried out in all patients by using 99mTc-diethylene-triamine-penta-acetic acid (DTPA) and conventional planar imaging. The rCBF was measured by using single-photon emission CT and 99mTc-hexamethylpropyleneamine oxime (32).
Imaging Protocol
All patients underwent MR imaging before and after shunt surgery. A 0.5-T magnet (Philips Gyroscan NT5, Eindhoven, the Netherlands) was used. Axial proton-density (PD)- and T2-weighted turbo spin-echo sequences were performed as well as a coronal T1-weighted sequence. Two observers (C.J., M.T.) judged the images according to a specially designed protocol while blinded to clinical symptoms and outcome of shunt surgery. White matter hyperintense areas were differentiated into two types: adjacent to the lateral ventricles (PVH) and isolated in the deep white matter without connection with the ventricular walls (DWMH). PVH in the anterior, central, and posterior thirds of the lateral ventricles was quantified on transaxial images with a semiquantitative five-step scale describing the extension (in millimeters) and form of the PVH (smooth or irregular) (Fig 1). DWMH was classified by using a four-step scale (Fig 1). The number of cortical infarcts in each hemisphere and subcortical lacunar infarcts in the thalami, basal ganglia, internal capsule, pons, and mesencephalon were registered. Ventricular index was calculated according to Hughes and Gado (34), and the width (in millimeters) of the third ventricle was measured. The temporal horns were judged as either normal or dilated. The protocol contained 24 subjective and objective variables.
fig 1.
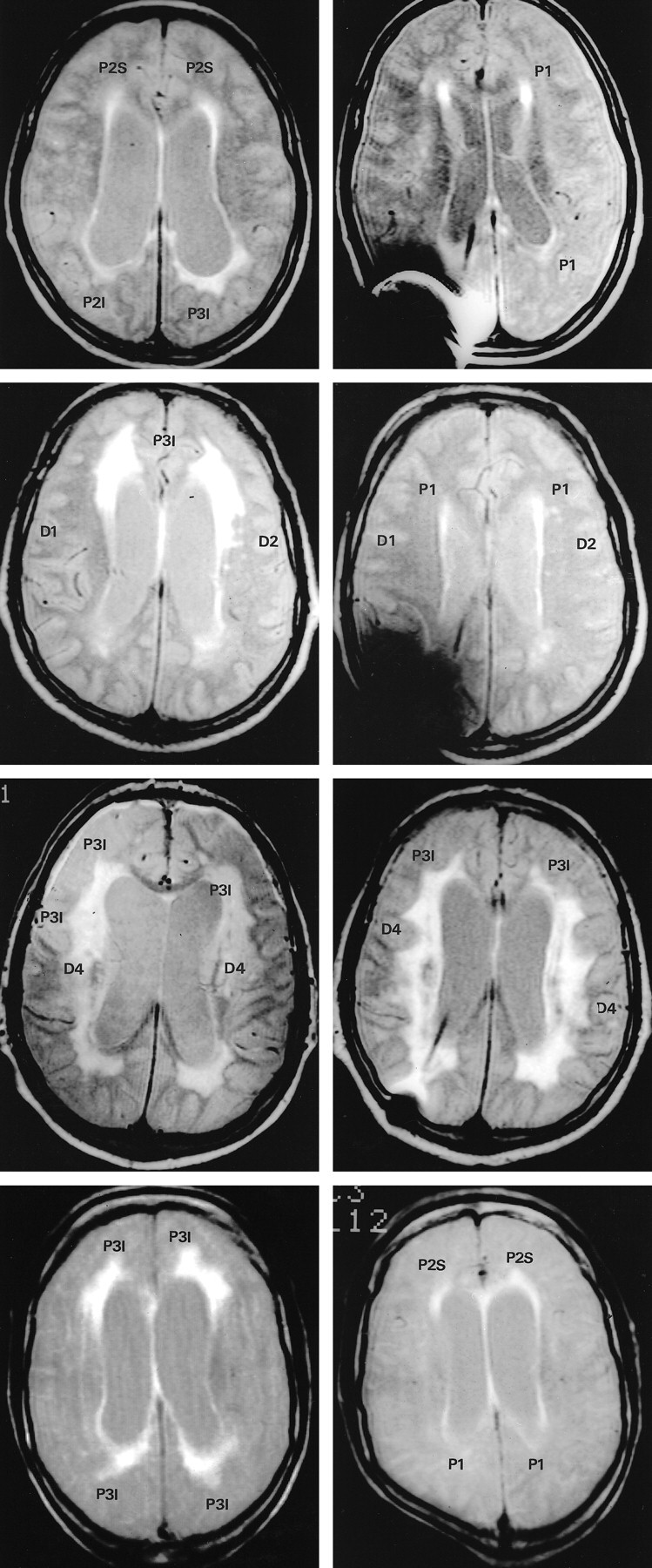
MR imaging of representative patients with NPH, before and after shunting.
Periventricular hyperintensity (PVH): P1 = normal; P2S = thin (5−10 mm) continuous PVH, smooth lateral borders; P2I = thin (5−10 mm) continuous PVH, irregular lateral borders; P3S = thick (>10 mm) continuous PVH, smooth lateral borders; P3I = thick (>10 mm) continuous PVH, irregular lateral borders. Deep white matter hyperintensity (DWMH): D1 = No DWMH or few (≤3) small discontinuous areas; D2 = several (>3) small (<10 mm) discontinuous areas of DWMH; D3 = several (>3) small and large (≥10 mm) areas of DWMH, beginning confluence; D4 = confluent DWMH.
Patient 1. Top row. Preoperative MR image (left) shows smooth PVH (P2S) at the anterior third and irregular PVH (P2I and P3I) at the posterior third of the lateral ventricles (TR/TE/excitations, 1960/60/6). Postoperative MR image (right) shows normalization (P1) of smooth PVH anteriorly and irregular PVH posteriorly. The patient improved (MoD, 0.34) after surgery (TR/TE/excitations, 2000/48/6).
Patient 2. Second row. Preoperative MR image (left) shows irregular PVH (P3I) at the anterior third of the lateral ventricles. There is no DWMH (D1) to the right and several small areas of DWMH (D2) to the left (TR/TE/excitations, 2035/60/4). Postoperatively (right), there is complete normalization of anterior PVH (P1), whereas DWMH remains unchanged. The patient improved (MoD, 0.73) after surgery (TR/TE/excitations, 1960/60/6).
Patient 3. Third row. Preoperative MR image (left) shows marked irregular PVH (P3I) at the anterior and central third of lateral ventricles and confluent DWMH (D4) bilaterally (TR/TE/excitations, 2168/30/1). Postoperative MR image (right) is unchanged. The patient deteriorated (MoD,−0.70) after surgery. Note the flow void sign indicating a patent shunt (TR/TE/excitations, 2034/60/4).
Patient 4. Fourth row. Preoperative MR image (left) shows marked irregular PVH (P3I) at the anterior and posterior third of the lateral ventricles (TR/TE/excitations, 1959/60/4). Postoperative MR image (right) shows smooth PVH at the anterior third (P2S) and normalization of PVH at posterior third (P1). The patient improved slightly (MoD, 0.52) after surgery (TR/TE/excitations, 1959/60/6).
Statistical Analysis
Simple descriptive statistics were produced by using univariate analysis. Nonparametric one-way analysis of variance and Wilcoxon's rank-sum test were used for group comparisons (SAS software, SAS Institute Inc., Cary, NC). The Spearman rank test was used for correlation analyses. Unless otherwise specified, P ≤ .05 was defined as significant. The clinical indices were constructed by calculating the mean of the variables included in each index. Standardization was used to enable comparison of scores (29). The preoperative variables were standardized to a mean of 0 and an SD of 1. In the postoperative statistics, the mean and SD of the preoperative variables were used in the standardization calculation. The difference in each index between the pre- and postoperative values was calculated, and the MoD between the pre- and postoperative indices for each patient was calculated as the overall result after shunt surgery. Values are listed as mean ± SD. Improvement was arbitrarily defined as a change in MoD ≥ 0.05 and deterioration as ≤−0.05.
Results
Three months after surgery, 25 patients (73%) had improved (mean MoD, 0.78; range, 0.08−2.8), four (12%) were unchanged (mean MoD,−0.01; range,−0.04−0), and five (15%) had deteriorated (mean MoD,−0.98; range−1.4−−0.08) despite a patent shunt (Table 1).
Patients who improved after surgery did so in all calculated indices except living conditions, particularly in wakefulness, gait ability, and continence. There was no correlation between age or duration of symptoms and the result of surgery. Improvement after surgery positively correlated with the need for sleep before surgery (r = 0.53; P ≤ .05). Patients with cerebrovascular risk factors (hypertension, diabetes, ischemic heart disease, or atrial fibrillation) improved more after shunt surgery (MoD, 0.78 ± 0.70) than those without such risk factors (MoD, 0.11 ± 0.91) (P ≤ .01). The improvement in the patients with cerebrovascular cause was similar to that in the other etiologic groups (Table 1).
In all, 88% of the patients had PVH around the lateral ventricles and 73% had DWMH. In 27% of the patients, PVH and DWMH could not be separated and were accordingly classified as continuous. Fifty-five percent had subcortical lacunar infarctions, predominantly located in the basal ganglia and internal capsule, and 21% had cortical infarctions, but no patient had more than one. Twenty-two percent had PVH around the third ventricle, with a marked flow void signal in 30%. In a single patient, the cortical sulci were widened; otherwise, they appeared normal or obliterated. Two patients had normal temporal horns. In eight patients, the aqueduct was dilated. The third ventricle was balloon-shaped and dilated in all patients. No patient had PVH around the aqueduct (Table 2). MR imaging variables did not differ between patients with and those without cerebrovascular disease or risk factors, except for cortical and subcortical infarcts, which were more frequent in patients with cerebrovascular risk factors (P ≤ .05).
TABLE 2:
MR imaging results
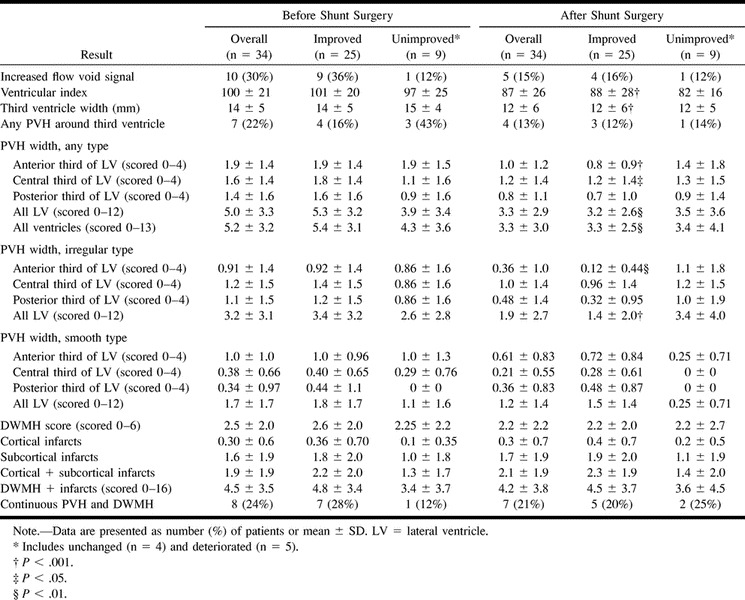
The width of PVH around the entire ventricular system and the total amount of white matter changes (PVH + DWMH) correlated with a poor psychometric test index (r =−0.58; P ≤ .01). Ventricular index (r = 0.49; P ≤ .02) and flow void (r = 0.45; P ≤ .03) correlated positively with an increased need for sleep. We saw no other correlation between the width or type of PVH or other MR imaging variables and any of the standardized clinical variables.
There were no significant differences in MR imaging variables between improved and unimproved patients (Table 2). Thus, the appearance of PVH and DWMH and the number of cortical and subcortical infarctions were equal in the two groups. No preoperative MR imaging variable correlated with the degree of improvement after surgery. Although some patients with lacunar infarctions did improve after surgery, the number of lacunar infarctions correlated negatively with the overall result after surgery in the improved patients (r =−0.47; P ≤ .05).
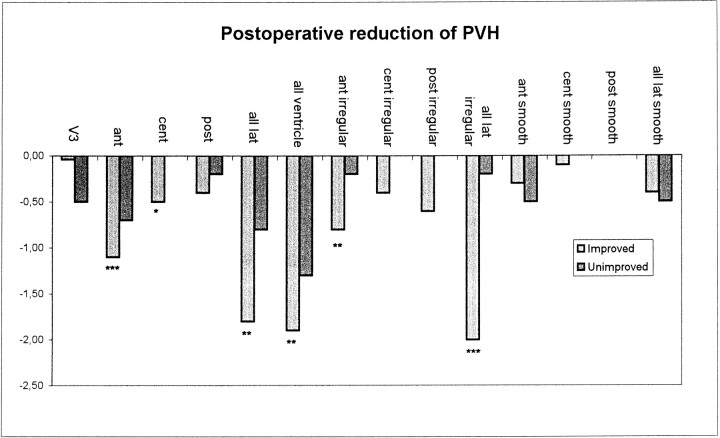
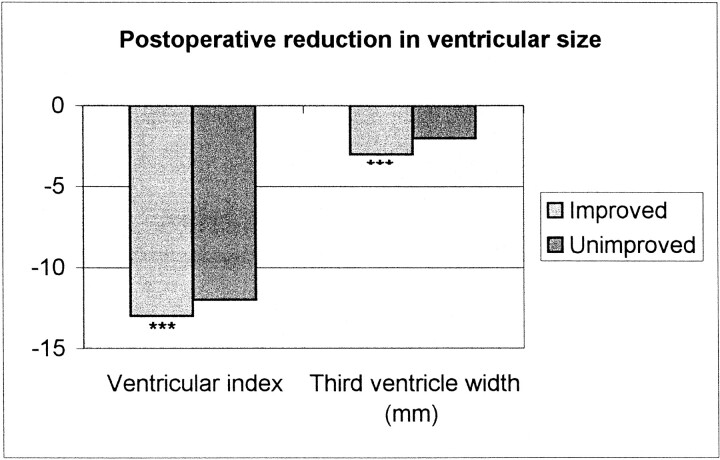
After shunt surgery, the width of PVH around the entire ventricular system decreased in the improved patients. The smooth type of PVH remained unchanged, whereas the irregular type of PVH around the frontal horns diminished (P ≤ .01) (Table 2, Fig 2). Further improved patients had significant reductions in ventricular index and the width of the third ventricle (2.6 mm) (Table 2, Fig 3) and showed a trend toward reduced flow void (P = .07). Unimproved patients, on the other hand, showed no significant change at follow-up MR imaging. However, the width of the third ventricle, ventricular index, and number of patients with PVH around the third ventricle were reduced numerically to the same extent as in improved patients and statistically at a trend level (Figs 2 and 3).
fig 2.
Postoperative reduction in PVH. Note the reduction in irregular but not smooth PVH around the frontal horns in improved patients
fig 3.
Postoperative reduction in ventricular size is numerically equal in both improved and unimproved patients, although significant only in the larger group of improved patients
The reduction in the PVH width around the lateral ventricles correlated with improvement in gait performance (r = 0.78; P ≤ .01), improved continence (r = 0.58; P ≤ .05), and reduced need for sleep (r = 0.67; P ≤ .05) in all patients and those improved after surgery. The reduction in PVH width around the posterior horns correlated with improved psychometric performance (r = 0.45; P ≤ .05), particularly in the Identical Forms test (r = 0.54, P ≤ .05). The regression coefficient was 0.78 between improvement in gait and reduction in irregular PVH around the lateral ventricles and 0.73 between reduction in PVH (regardless of type) and total result. Reduced ventricular size correlated with a reduced need for sleep, improved continence, and, at trend level, with a positive overall outcome (MoD ≥ 0.05) (P = .07). Reduced width of the third ventricle correlated with improvement in Raven Colored Matrices (P ≤ .05). Continence improved in patients where the flow void sign disappeared (P ≤ .05).
Discussion
PVH at MR imaging is considered a hallmark of NPH, but its relationship with clinical symptoms and its prognostic implications remain unsettled (18, 28, 35). Fazekas et al (36) found a good correlation between signal abnormalities on MR images and pathologic changes, indicating that the potential diagnostic value of MR imaging has not yet been fully realized. In the present study, we used a protocol designed to distinguish between hypothetically different types of white matter changes to predict the result of shunt surgery in NPH patients.
Forty-six consecutive patients were considered, subjected to a thorough investigation, and judged against strict diagnostic criteria. We excluded 12 patients, resulting in a smaller patient cohort than planned. Seventy-three percent of the patients improved after shunt surgery, a result comparable with success rates reported from other centers. All clinical symptoms were standardized, to allow comparison of different symptom scores and to create a continuous rating scale of symptoms and results of shunt surgery. We therefore believe that our diagnostic criteria are valid, that the group is representative of NPH, and that the reported clinical changes after surgery are reliable. One of the purposes of the study was to find white matter changes on MR images that had negative predictive value. We therefore carefully registered risk factors and causes of cerebrovascular disease and deliberately avoided excluding patients with such disease.
Probably the most important differential diagnosis for NPH is subcortical arteriosclerotic encephalopathy (SAE). Patients with SAE often have enlarged ventricles and symptoms and signs similar to those seen in NPH. Pathologically, SAE is characterized by continuous, irreversible ischemic degeneration of the periventricular and deep white matter. Microinfarctions and demyelination appear on MR images as extensive PVH and DWMH and enlarged ventricles. There is clinical (37, 38), pathoanatomic (39), and MR imaging (18, 40) evidence of coexistence of NPH and SAE. In our opinion, so-called vascular changes are common in NPH. The prognostic value of these ischemia-related changes should be negative, although probably not excluding a positive shunt effect.
There was no statistically significant difference between the improved and unimproved patients in any preoperative MR imaging variable. Of interest, the number of lacunar infarctions was higher in the improved than in unimproved patients, although lacunar infarctions had a negative predictive effect. Two very important conclusions that should be drawn in this regard are that 1) lacunar infarctions are common findings in a general NPH population and 2) they should never be considered a contraindication to shunt surgery. They do imply less pronounced improvement, however, in accordance with previous reports (41). The fact that up to 73% of patients in this prospective, consecutive NPH cohort had DWMH strongly indicates that so-called ischemic white matter changes are either part of the NPH state or that NPH is strongly associated with arteriosclerotic small vessel disease. Previous reports (23, 28) have indicated a positive prognostic value of the flow void sign and PVH in NPH. Our results support this assumption, as the flow void sign was more common, and PVH (both smooth and irregular) more extensive, in improved patients, albeit not significantly. Of note, the imaging protocol was optimized to evaluate white matter changes but had reduced sensitivity to detect flow void. The reduced sensitivity with our imaging technique should not have affected the specificity, however.
After shunt surgery, the width of the PVH (regardless of type) was decreased around the lateral ventricles, indicating that MR imaging does indeed reflect a reversible process in the periventricular white matter. Contrary to what we expected, the smooth PVH remained unchanged, whereas the irregular PVH, which we thought represented the ischemic white matter change, was significantly reduced around the frontal horns after surgery in improved patients. The conclusions that must be drawn are that the irregular PVH shown in Figure 1 represents prognostically important changes in the periventricular region of NPH patients, and future MR imaging studies should focus on this alteration. The frontal location has support in the literature (32). The reduction in smooth PVH after surgery was not equally pronounced, but we do not exclude a prognostic value of the smooth PVH in NPH. PVH and possibly also DWMH may represent microvascular disturbances caused by the pathologic periventricular CSF diffusion in NPH with tissue edema and ischemia. Accordingly, the pathophysiological mechanism would correspond to certain MR imaging changes and the clinical picture.
The reductions in ventricular index and width of the third ventricle were numerically equal in improved and unimproved patients, although the reductions were statistically significant only in the larger group of improved patients. Normalizing CSF dynamics and intracranial pressure can restore the size and form of the ventricular system, probably through the CSF diversion itself, for the most part. Normalization of size, however, does not correlate with clinical improvement and thus does not relate to the reversible process in NPH. Ventricular size and form probably cannot be used to predict the shunt effect.
PVH around the third ventricle was more frequent in unimproved patients. Its frequency was reduced after surgery in this group, albeit not statistically significantly so. It is very difficult to judge PVH in this region, and the use of this variable in future studies is not recommended under the present circumstances.
Another important result is the correlation between clinical improvement and reduction of certain MR imaging changes, further supporting the view that MR imaging reflects the pathophysiological process with potentially reversible neuronal dysfunction and minor or no demyelination (10). The importance of irregular PVH and its relation to symptoms is further emphasized by the high regression coefficient between reduction in irregular PVH and improvement in gait. Of interest, we found that the decrease in posterior PVH correlated with improvement in psychometric tests that measure function of the parietal-association cortex. Further reductions in the frontal PVH correlated with a reduced need for sleep, in accordance with stimulation of the thalamic-activation system in the brain stem.
Our rating scale was constructed to evaluate ischemia-related deep white matter changes and lacunar infarctions of presumably negative prognostic value, by a semiquantitative, four-step grading of DWMH and counting of lacunar infarctions. We also evaluated NPH-related continuous periventricular changes. NPH-related PVH has been described as homogeneous, continuous caps or rims or a periventricular halo (42−44). Tamaki et al (45), in an MR imaging study, also reported periventricular edema in NPH. The caps and rims may have irregular features, possibly explained by ischemic white matter changes. We chose to categorize PVH as one of two types on the basis of its appearance—either smooth or irregular—thus including the possibility of differentiating between PVH thought to be related to NPH edema and PVH related to ischemia. We used a five-step scale and classified PVH around the anterior, central, and posterior thirds of the lateral ventricles on consecutive transaxial MR imaging sections while blinded to the clinical data. We classified PVH <5 mm as a normal feature. This judgment is based on previous reports (35), and our own observations in clinical routine, of thin PVH as a common sign in normal subjects of the same age as the patients studied. The study was performed as a retrospective analysis of a prospectively controlled group, with MR images evaluated by investigators blinded to the clinical variables. The use of MR imaging data to diagnose NPH is a bias to the study, but patients were included only on the basis of ventricular width and after ascertainment that no severe cortical atrophy was present. Patients who were excluded because of failed MR imaging did not differ in any respect from those included in the study.
DWMH and subcortical infarcts were common findings in our patients. In the preoperative, diagnostic MR imaging criteria, we deliberately avoided including an evaluation of vascular white matter changes compatible with SAE. Therefore, the diagnostic criteria are limited by the inability to distinguish mixed cases of NPH and SAE. Some of the included patients, especially in the unimproved group, may have represented such cases. This reflects the clinical situation, however—presumed mixed cases are common in our unit.
Conclusion
The main conclusion of the present study is that it is impossible to predict the outcome of shunt surgery by MR imaging. Lacunar infarctions and DWMH must not be used to exclude NPH patients from surgery, although the presence of lacunar infarctions has negative prognostic value. Cerebrovascular cause and the presence of risk factors associated with cerebrovascular disease do not negatively influence the outcome of shunt surgery. After shunt surgery, irregular PVH seems to be the key reversible white matter change at MR imaging. Future studies should focus on this variable.
Footnotes
Supported by research grants from the Edit Jacobson Foundation, the John and Brit Wennerström Foundation, the Hjalmar Svensson Foundation, the Rune and Ulla Ahlmlöv Foundation, the Göteborg Medical Society, and the Swedish Association of Neurologically Disabled.
Presented in part as a poster at the First International Congress on Vascular Dementia, Geneva, Switzerland, October 3−6, 1999.
Address reprint requests to Mats Tullberg, Institute of Clinical Neuroscience, Sahlgrenska University Hospital, SE 413 45 Göteborg, Sweden.
References
- 1.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal fluid pressure: a treatable syndrome. N Engl J Med 1965;273:117-126 [DOI] [PubMed] [Google Scholar]
- 2.Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965;2:307-327 [DOI] [PubMed] [Google Scholar]
- 3.Milhorat TH, Clark RG, Hammock MK, McGrath PP. Structural, ultrastructural, and permeability changes in the ependyma and surrounding brain favoring equilibration in progressive hydrocephalus. Arch Neurol 1970;22:397-407 [DOI] [PubMed] [Google Scholar]
- 4.Milhorat TH. The third circulation revisited. J Neurosurg 1975;42:628-645 [DOI] [PubMed] [Google Scholar]
- 5.Miyagami M, Shibuya T, Tsubokawa T. Subependymal CSF absorption in hydrocephalic edema. In: Matsumoto S, Tamaki N, eds. Hydrocephalus, Pathogenesis and Treatment. Tokyo: Springer Verlag; 1981
- 6.Deo-Narine V, Gomez DG, Vullo T, et al. Direct in vivo observation of transventricular absorption in the hydrocephalic dog using magnetic resonance imaging. Invest Radiol 1994;29:287-293 [DOI] [PubMed] [Google Scholar]
- 7.Weller RO, Wisniewski H, Shulman K, Terry RD. Experimental hydrocephalus in young dogs: histological and ultrastructural study of the brain tissue damage. J Neuropathol Exp Neurol 1971;30:613-626 [PubMed] [Google Scholar]
- 8.Akai K, Uchigasaki S, Tanaka U, Komatsu A. Normal pressure hydrocephalus: neuropathological study. Acta Pathol Jpn 1987;37:97-110 [PubMed] [Google Scholar]
- 9.Tullberg M, Rosengren L, Blomsterwall E, Karlsson JE, Wikkelso C. CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology 1998;50:1122-1127 [DOI] [PubMed] [Google Scholar]
- 10.Tullberg M, Månsson J-E, Fredman P, et al. CSF sulfatide distinguishes between normal pressure hydrocephalus and subcortical arteriosclerotic encephalopathy. J Neurol Neurosurg Psych 2000;69:74-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tans JT. Differentiation of normal pressure hydrocephalus and cerebral atrophy by computed tomography and spinal infusion test. J Neurol 1979;222:109-118 [DOI] [PubMed] [Google Scholar]
- 12.LeMay M, Hochberg FH. Ventricular differences between hydrostatic hydrocephalus and hydrocephalus ex vacuo by computed tomography. Neuroradiology 1979;17:191-195 [DOI] [PubMed] [Google Scholar]
- 13.Pappada G, Poletti C, Guazzoni A, Sani R, Colli M. Normal pressure hydrocephalus: relationship among clinical picture, CT scan and intracranial pressure monitoring. J Neurosurg Sci 1986;30:115-121 [PubMed] [Google Scholar]
- 14.Wikkelso C, Andersson H, Blomstrand C, Matousek M, Svendsen P. Computed tomography of the brain in the diagnosis of and prognosis in normal pressure hydrocephalus. Neuroradiology 1989;31:160-165 [DOI] [PubMed] [Google Scholar]
- 15.Yamada F, Fukuda S, Samejima H, Yoshii N, Kudo T. Significance of pathognomonic features of normal–pressure hydrocephalus on computerized tomography. Neuroradiology 1978;16:212-213 [DOI] [PubMed] [Google Scholar]
- 16.Di Chiro G, Arimitsu T, Brooks RA, et al. Computed tomography profiles of periventricular hypodensity in hydrocephalus and leukoencephalopathy. Radiology 1979;130:661-666 [DOI] [PubMed] [Google Scholar]
- 17.Bradley WG, Jr, Kortman KE, Burgoyne B. Flowing cerebrospinal fluid in normal and hydrocephalic states: appearance on MR images. Radiology 1986;159:611-616 [DOI] [PubMed] [Google Scholar]
- 18.Bradley WG Jr, Whittemore AR, Watanabe AS, Davis SJ, Teresi LM, Homyak M. Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible origin of normal-pressure hydrocephalus. AJNR Am J Neuroradiol 1991;12:31-39 [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR Jr, Mokri B, Laws ER Jr, Houser OW, Baker HL Jr, Petersen RC. MR findings in normal-pressure hydrocephalus: significance and comparison with other forms of dementia. J Comput Assist Tomogr 1987;11:923-931 [DOI] [PubMed] [Google Scholar]
- 20.Kunz U, Heintz P, Ehrenheim C, Stolke D, Dietz H, Hundeshagen H. MRI as the primary diagnostic instrument in normal pressure hydrocephalus? Psychiatry Res 1989;29:287-288 [DOI] [PubMed] [Google Scholar]
- 21.Schroth G, Klose U. MRI of CSF flow in normal pressure hydrocephalus. Psychiatry Res 1989;29:289-290 [DOI] [PubMed] [Google Scholar]
- 22.Mascalchi M, Ciraolo L, Bucciolini M, Inzitari D, Arnetoli G, Dal Pozzo G. Fast multiphase MR imaging of aqueductal CSF flow: 2. Study in patients with hydrocephalus. AJNR Am J Neuroradiol 1990;11:597-603 [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology 1991;178:459-466 [DOI] [PubMed] [Google Scholar]
- 24.Bradley WG Jr, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 1996;198:523-529 [DOI] [PubMed] [Google Scholar]
- 25.Sherman JL, Citrin CM. Magnetic resonance demonstration of normal CSF flow. AJNR Am J Neuroradiol 1986;7:3-6 [PMC free article] [PubMed] [Google Scholar]
- 26.Krauss JK, Regel JP, Vach W, Jungling FD, Droste DW, Wakhloo AK. Flow void of cerebrospinal fluid in idiopathic normal pressure hydrocephalus of the elderly: can it predict outcome after shunting? Neurosurgery 1997;40:67-73 [DOI] [PubMed] [Google Scholar]
- 27.Hakim R, Black PM. Correlation between lumbo-ventricular perfusion and MRI-CSF flow studies in idiopathic normal pressure hydrocephalus. Surg Neurol 1998;49:14-19 [DOI] [PubMed] [Google Scholar]
- 28.Krauss JK, Droste DW, Vach W, et al. Cerebrospinal fluid shunting in idiopathic normal–pressure hydrocephalus of the elderly: effect of periventricular and deep white matter lesions. Neurosurgery 1996;39:292-299 [DOI] [PubMed] [Google Scholar]
- 29.Larsson A, Wikkelso C, Bilting M, Stephensen H. Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol Scand 1991;84:475-482 [DOI] [PubMed] [Google Scholar]
- 30.Blomsterwall E, Bilting M, Stephensen H, Wikkelso C. Gait abnormality is not the only motor disturbance in normal pressure hydrocephalus. Scand J Rehabil Med 1995;27:205-209 [PubMed] [Google Scholar]
- 31.Wikkelso C, Andersson H, Blomstrand C, Lindqvist G, Svendsen P. Normal pressure hydrocephalus: predictive value of the cerebrospinal fluid tap-test. Acta Neurol Scand 1986;73:566-573 [DOI] [PubMed] [Google Scholar]
- 32.Larsson A, Bergh AC, Bilting M, et al. Regional cerebral blood flow in normal pressure hydrocephalus: diagnostic and prognostic aspects. Eur J Nucl Med 1994;21:118-123 [DOI] [PubMed] [Google Scholar]
- 33.Graff-Radford NR, Rezai K, Godersky JC, Eslinger P, Damasio H, Kirchner PT. Regional cerebral blood flow in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 1987;50:1589-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes CP, Gado M. Computed tomography and aging of the brain. Radiology 1981;139:391-396 [DOI] [PubMed] [Google Scholar]
- 35.Zimmerman RD, Fleming CA, Lee BC, Saint-Louis LA, Deck MD. Periventricular hyperintensity as seen by magnetic resonance: prevalence and significance. AJR Am J Roentgenol 1986;146:443-450 [DOI] [PubMed] [Google Scholar]
- 36.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993;43:1683-1689 [DOI] [PubMed] [Google Scholar]
- 37.Graff-Radford NR, Godersky JC. Idiopathic normal pressure hydrocephalus and systemic hypertension. Neurology 1987;37:868-871 [DOI] [PubMed] [Google Scholar]
- 38.Koto A, Rosenberg G, Zingesser LH, Horoupian D, Katzman R. Syndrome of normal pressure hydrocephalus: possible relation to hypertensive and arteriosclerotic vasculopathy. J Neurol Neurosurg Psychiatry 1977;40:73-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bech RA, Juhler M, Waldemar G, Klinken L, Gjerris F. Frontal brain and leptomeningeal biopsy specimens correlated with cerebrospinal fluid outflow resistance and B-wave activity in patients suspected of normal-pressure hydrocephalus. Neurosurgery 1997;40:497-502 [DOI] [PubMed] [Google Scholar]
- 40.Krauss JK, Regel JP, Vach W, Droste DW, Borremans JJ, Mergner T. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke 1996;27:24-29 [DOI] [PubMed] [Google Scholar]
- 41.Boon AJ, Tans JT, Delwel EJ, et al. Dutch Normal-Pressure Hydrocephalus Study: the role of cerebrovascular disease. J Neurosurg 1999;90:221-226 [DOI] [PubMed] [Google Scholar]
- 42.Bydder GM, Steiner RE, Young IR, et al. Clinical NMR imaging of the brain: 140 cases. AJR Am J Roentgenol 1982;139:215-236 [DOI] [PubMed] [Google Scholar]
- 43.Bradley WG Jr, Waluch V, Yadley RA, Wycoff RR. Comparison of CT and MR in 400 patients with suspected disease of the brain and cervical spinal cord. Radiology 1984;152:695-702 [DOI] [PubMed] [Google Scholar]
- 44.Brant-Zawadzki M, Badami JP, Mills CM, Norman D, Newton TH. Primary intracranial tumor imaging: a comparison of magnetic resonance and CT. Radiology 1984;150:435-440 [DOI] [PubMed] [Google Scholar]
- 45.Tamaki N, Shirakuni T, Ehara K, Matsumoto S. Characterization of periventricular edema in normal-pressure hydrocephalus by measurement of water proton relaxation times. J Neurosurg 1990;73:864-870 [DOI] [PubMed] [Google Scholar]