Abstract
BACKGROUND AND PURPOSE: MR techniques have proved useful in assessing brain injury from perinatal asphyxia when the injury is subacute or chronic. Recent advances in understanding the molecular mechanisms of brain injury have made medical intervention plausible, creating a need for assessment of the brain within the first few hours of life. We report the results of early (first 24 hours after birth) MR imaging in seven patients, including proton MR spectroscopy in six.
METHODS: MR studies were performed within the first 24 hours of life in seven consecutive patients who were encephalopathic after complicated deliveries. Standard T1-, T2-, and diffusion-weighted sequences were performed in all patients; single-voxel MR spectroscopy was performed in two locations in six of the seven patients. Follow-up MR studies were performed in four patients at ages 7, 8, 9, and 15 days, respectively.
RESULTS: T1-weighted images were normal in all seven patients. T2-weighted images were normal in three patients and showed T2 prolongation in the basal ganglia or white matter in the other four. Diffusion images showed small abnormalities in the lateral thalami or internal capsules in all seven patients. Comparison with clinical course in all seven patients and with follow-up MR studies in four showed that the diffusion images underestimated the extent of brain injury. Proton MR spectroscopy showed substantial lactate elevation in all six of the patients studied. Two patients died in the neonatal period and the other five were left with clinically significant neurologic impairment.
CONCLUSION: MR spectroscopy performed in the first 24 hours after birth is sensitive to the presence of hypoxic-ischemic brain injury, whereas diffusion imaging may help identify but underestimate the extent of the injury. Further studies are ongoing in an attempt to expand upon this observation.
MR techniques have had a considerable effect on the diagnosis of brain injury in the neonate. It has been demonstrated that standard MR imaging (1–7) and, more recently, MR diffusion imaging (8–11) and MR spectroscopy (12–19) enable identification of abnormalities that correspond with outcome. As techniques have improved, the goal of imaging studies has changed from merely identifying damage to identifying it at an early stage, when medical intervention might still be helpful. Diffusion imaging and MR spectroscopy are considered to have the greatest potential in this regard, yet to our knowledge the relative value of these techniques has not been studied. We recently had the opportunity to study seven encephalopathic neonates in the first 24 hours after birth; all seven were studied with diffusion imaging and six were studied with MR spectroscopy (patient 6 did not have MR spectroscopy because of a staff error). The results of those studies are reported in this article.
Methods
Seven patients at two institutions had clinically significant encephalopathy in the neonatal period after complicated deliveries; all seven neonates were admitted to our institutions over a period of 6 months. All were assessed while in the neonatal intensive care unit by experienced neonatologists and child neurologists who cared for or were consulted on the care of the infants. Apgar scores, initial umbilical cord blood gases, presence or absence of seizures, and neonatal neurologic abnormalities are listed in Table 1.
TABLE 1:
Clinical data
All subjects underwent MR imaging, and patients 1 through 6 were studied with single-voxel proton MR spectroscopy within the first 24 hours of life (range, 5 to 24 hours). The imaging study included sagittal spin-echo (SE) 4-mm-thick sections obtained at 500/14/0.75 (TR/TE/excitations), axial SE 4-mm-thick sections obtained at 3000/60,120/1, and axial SE 500/9/2 sections obtained by using a 256 × 192 imaging matrix and an 18-cm field of view (FOV). Diffusion-weighted images were acquired in the axial plane by using a single-shot echo-planar technique with a 128 × 256 matrix, a 36-cm FOV, a 4-mm section thickness, and a b value of 700 s/mm2. This b value was chosen because our experience and that of others have shown that the two- to threefold higher apparent diffusion coefficients (ADCs) in infants result in considerably increased signal-to-noise ratio with b values in this range without considerably affecting ADC values (personal communications, Petra Hüppi, Jeff Neil). The proton spectrum for each location was acquired using the GE PROton Brain Exam sequence with parameters of 2000/288 and 128 acquisitions. Voxel size was 5 cm3. The MR spectroscopy parameters and voxel locations were chosen to maximize the detection of lactate (20) and to minimize the spectral contamination from extracranial adipose tissue. The two spectra were obtained with the same parameters and voxel size; the first voxel was centered on the deep gray matter (lentiform nucleus or thalamus) and the second in the anterior watershed white matter. The spectra, diffusion images, ADC values (from the diffusion images), and imaging studies were compared with our database of more than 150 neonates of gestational ages ranging from 26 to 42 weeks postconceptual age and with published norms (13, 21–26).
Patients 4, 5, 6, and 7 underwent follow-up MR studies at ages 9, 15, 8, and 7 days, respectively. Imaging parameters were identical to those of the initial imaging study. Again, patient 6 was not studied with MR spectroscopy, and diffusion images were not obtained at the follow-up study of patient 6.
All imaging studies were reviewed by two neuroradiologists with extensive experience in imaging of neonates. Both authors independently reviewed the SE imaging studies, the spectra, and the diffusion images and were in agreement as to the presence or absence of every abnormality, the location of the abnormalities, and their severity. In addition, the spectra and diffusion data were reviewed by an MR scientist with more than 10 years' experience in in vivo MR spectroscopy (focusing particularly on neonates in the last 4 years). This author concurred with the findings on the MR spectra and diffusion studies as interpreted by the neuroradiologists.
Limited clinical follow-up was available in all seven patients, obtained from the neonatologists who last examined the infants.
Results
Initial MR Studies
Results are listed in Table 2. Routine T1-weighted images were normal for age in all patients. T2-weighted images were normal in four and showed mild edema (T2 prolongation) in the basal ganglia or cortex in the other three; no focal areas of T1 or T2 abnormality were present. Of interest, the diffusion-weighted images did not appear dramatically abnormal in any of the patients. The only area of reduced diffusion was in the posterior limb of the internal capsule (PLIC, Fig 1) in two patients and in the lateral thalamic nuclei and, perhaps, in the PLIC (Fig 2) in three patients. One patient had an apparently normal initial study (Fig 3) but further analysis showed a decrease of 15% to 20% in ADCs throughout the brain compared with that of healthy neonates imaged on our unit (unpublished results) and with published values (26).
TABLE 2:
MR imaging data
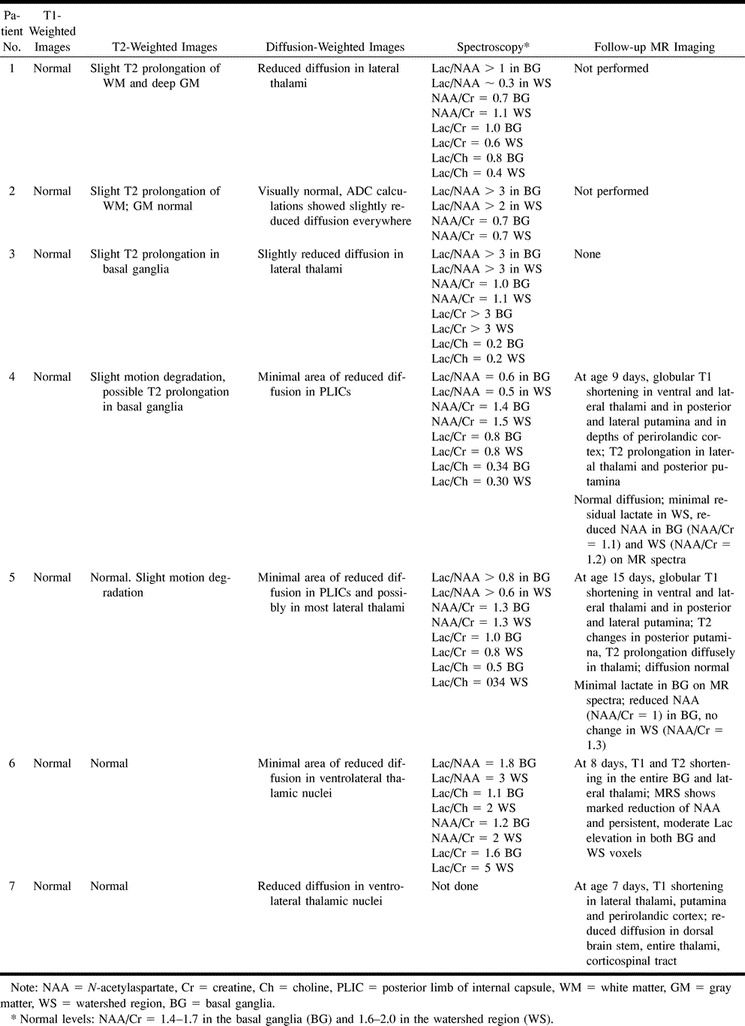
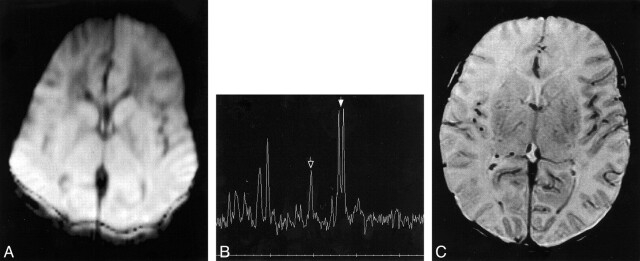
fig 1.
Patient 5.
A, Diffusion-weighted image (b = 700 s/mm2) at age 16 hours. Small areas of reduced diffusion (arrows) are seen in posterior limbs of internal capsules.
B, Proton MR spectrum (2000/288) at age 16 hours from single voxel in thalamus/lentiform nucleus. Lactate peak (doublet at 1.33 ppm, indicated by solid arrow) is markedly elevated and NAA peak (singlet at 2.01 ppm, indicated by open arrow) is reduced.
C and D, Follow-up SE (517/8) images at age 15 days. Despite some motion degradation, globular T1 shortening (arrows) is seen in lateral putamen and posteromedial lentiform nucleus bilaterally and at the depths of the posterior sylvian cortex. Diffusion images were negative at this study.
E, Follow-up proton spectrum (2000/288) from the thalami/lentiform nuclei at age 15 days shows further diminution in the size of the NAA peak and almost complete disappearance of the lactate peak.
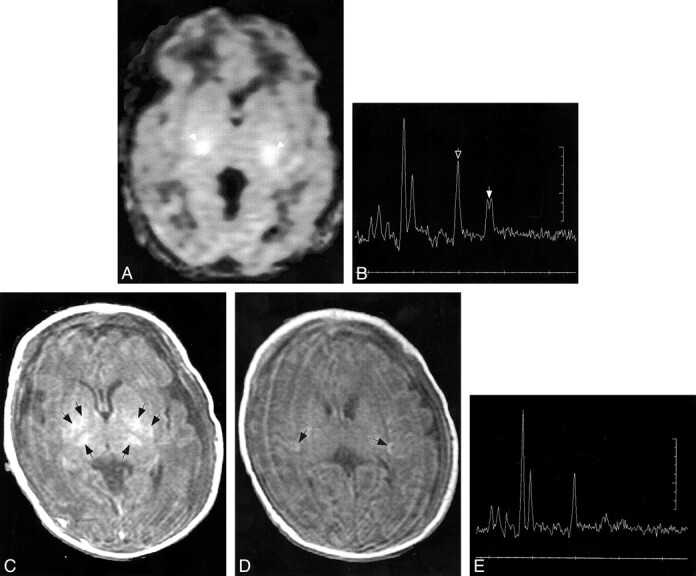
fig 2.
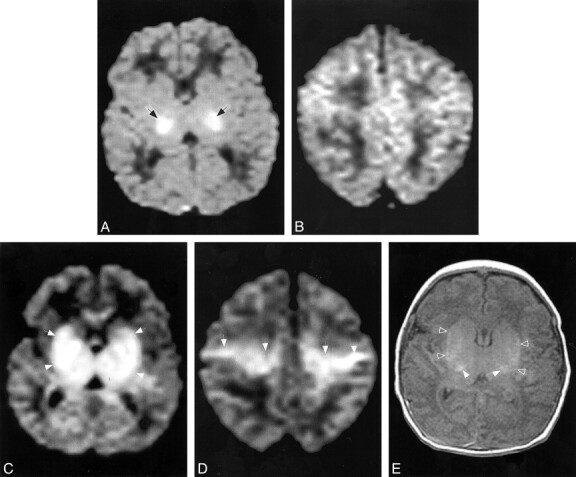
Patient 7.
A and B, Diffusion-weighted images (b = 700 s/mm2) at age 18 hours shows reduced diffusion, manifest as high signal intensity (arrows), in the lateral thalami and possibly in the posterior limbs of the internal capsules. No significant reduction in diffusion is seen in the centra semiovale (in B).
C and D, Follow-up diffusion-weighted images (b = 700, s/mm2) at age 7 days at the same levels show reduced diffusion (hyperintensity, arrows) within a much larger area, including the lentiform nuclei and thalami (in C) and along the corticospinal tracts (in D).
E, Axial SE (516/8) image through the basal ganglia shows T1 shortening in the lateral thalami (open arrows) and lentiform nuclei (solid arrows), confirming the injury seen in C.
fig 3.
Patient 2.
A, Diffusion-weighted image (b = 700 s/mm2) at the level of the basal ganglia performed at age 16 hours shows diffusely reduced diffusion in cortex and deep gray nuclei. On initial evaluation, this image was thought to be normal; however, subsequent analysis revealed a reduction in the ADC of about 15% throughout the brain. The distortion and hyperintensity of the back of the head results from chemical blankets used to keep the body temperature at 37°C.
B, Proton MR spectrum (2000/288) from the thalami and basal ganglia at age 16 hours shows marked reduction of NAA (singlet at 2.01 ppm, indicated by open arrow) and marked elevation of lactate (doublet at 1.33 ppm, indicated by solid arrow).
C, Axial SE (3000/120) image at age 18 hours shows some mildly diffuse T2 prolongation.
Findings at MR spectroscopy were abnormal in the six patients in whom it was performed. All had significant (>0.3) elevation of lactate/N-acetylaspartate (NAA) ratios in both voxels sampled (17, 27). In addition, the NAA appeared reduced, as the NAA/creatine (Cr) ratios were substantially lower than normal (our normal values in neonates range from 1.4 to 1.7 in the basal ganglia and from 1.6 to 2.0 in the frontal watershed white matter).
Follow-up MR Studies
All follow-up studies in the four patients in whom they were obtained showed definite brain injury (Table 2). All had much more extensive brain injury than was apparent on the initial diffusion images. In patients 4, 5, and 6, who initially had diffusion abnormality in only small regions of the PLIC, the follow-up studies at 9, 15, and 8 days, respectively, showed involvement of most of the thalami, the posterior putamina, and the depth of the perirolandic cortex, respectively (Fig 2). The diffusion abnormalities had resolved in the two in whom follow-up diffusion studies were obtained (patients 4 and 5), and the amount of lactate was definitely reduced at MR spectroscopy. NAA/Cr was further decreased in the basal ganglia. Patient 7, who initially had reduced diffusion in the ventrolateral thalamic nuclei (and possibly in the PLIC), had abnormal T1 shortening and reduced diffusion throughout the dorsal brain stem, basal ganglia, and corticospinal tracts at 7 days (Fig 2).
Clinical Follow-up
Limited follow-up was available in these infants. Two of the patients (patients 2 and 3) died after mechanical ventilation was discontinued, owing to persistent profound neurologic impairment. The other five were hypotonic, with feeding difficulties and poor airway protection at discharge from the hospital; their long-term prognosis is considered poor. Indeed, patients 1 and 7 had abnormal tone and impaired gag reflexes at their 5-month follow-up examinations.
Discussion
Over the past decade, a number of publications have described the utility of MR techniques in the assessment of hypoxic-ischemic brain injury in neonates (4–7, 10, 13, 14, 16, 19, 27–37). These studies have shown that the subsequent clinical deficit is related to the severity of brain injury, as assessed by imaging or metabolic assessment (spectroscopy). Recent work has suggested that, in the near future, the extent of hypoxic-ischemic brain injury may be reduced by a variety of new strategies (38, 39). For the purposes of such therapy, the ability to detect subacute or chronic injury and prognosticate outcome becomes less important than the ability to detect damage in the early postnatal period, at a time when therapy may be effective. A few publications have addressed the detection of acute neonatal hypoxic-ischemic injury in the early postnatal period, using both diffusion imaging (8, 9, 10, 11) and proton MR spectroscopy (14, 40). This is the first study, to our knowledge, to compare diffusion imaging and spectroscopy in the first 24 hours after birth.
Although the diffusion-weighted images were at least slightly abnormal in all of our patients, the discrepancy between the extent of the abnormality on the first set of images and the second set of images was striking; clearly, the topologic extent of injury was underestimated on the initial studies. In all four patients in whom follow-up MR imaging was obtained, tissue damage was substantially more extensive than was suggested by the initial images (Figs 1 and 2). A similar underestimation of damage in asphyxiated neonates by early diffusion-weighted imaging has been noted by others (10, 41). This underestimation could have serious consequences if therapy were withheld because minimal or relatively minor injury was suspected. In patients 4, 5, 6, and 7, the images obtained on day 1 suggested minor injury when, in fact, subsequent studies on days 9, 15, 8, and 7, respectively, showed much more extensive injury. The reason for this underestimation is not clear. In embolic and thrombotic stroke, both in adults and children, diffusion imaging seems to be sensitive to the early detection of ischemic injury. In adults with embolic or thrombotic infarctions, the enlargement of strokes is often seen and is attributed to an ischemic penumbra that is hypoperfused but viable at the time of diffusion imaging (42–44). It is generally accepted that the penumbra suffers definitive infarction after the initial diffusion image has been acquired. However, neonatal injury is neither thrombotic nor embolic. The brain is hypoperfused, then reperfused. No classical penumbra exists in this situation. How, then, do we explain this discrepancy between the day 1 diffusion study and the final injured territory? Could it result from the same processes that caused false-negative diffusion-weighted imaging findings in the study by Robertson et al (10). In all likelihood, both are the result of delayed energy failure (also called biphasic energy failure), a well-known phenomenon in neonatal hypoxic-ischemic injury (45–48). In biphasic energy failure, an initial reduction of energy substrate results in an initial impairment of cell metabolism. Reperfusion results in a transient return to apparently normal cell metabolism that lasts for a variable period of time, generally in the range of 24 hours (45–47), after which the cell dies. The delay in cell death is postulated to result from the duration of a cascade of intracellular molecular events that lead to mitochondrial dysfunction (38, 39). Imaging confirmation of this delay was identified by Rumpel et al (45), who found a biphasic function for the evolution of cytotoxic edema, as determined by ADC maps and histologic examination, after hypoxic-ischemic injury in neonatal rats. At the end of hypoxia-ischemia, these authors found the ADC in the ipsilateral cortex to be substantially decreased. Upon reoxygenation, it returned transiently to normal, followed by a secondary, although less pronounced, decline after 8 to 48 hours. After this, the ADC rose steadily. After 8 hours of recovery, the proportion of vasogenic edema steadily increased, as indicated by the T2 prolongation; at 21 hours, the majority of glial cells were enlarged, whereas the neurons were apoptotic. These results indicate that delayed cerebral injury is accompanied by late glial swelling in conjunction with an enlarged interstitial space due to cell damage; they also help to explain why findings on diffusion-weighted studies may be falsely negative when performed in the first hours after neonatal hypoxic-ischemic injury.
Our results suggest that proton MR spectroscopy depicts the severity of injury more accurately than does diffusion-weighted imaging. MR spectroscopy performed within minutes of the diffusion-weighted studies showed abnormally elevated lactate and diminished NAA in all patients in our series. We obviously cannot state that there was no diminution in lactate during the interval between the first and second phases of energy failure. If cell function and, in particular, mitochondrial function normalized during reperfusion, we would expect a resumption of adenosine triphosphate production via the tricarboxylic acid cycle and a decrease in lactate production. Indeed, Penrice et al (49) showed in a pig model that lactate levels decrease after an initial increase, with a nadir at about 24 hours after birth, before beginning to rise again. However, it appears that mitochondrial function does not completely normalize, as lactate levels remain elevated. More important from an imaging perspective, it seems that any reduction of lactate is less significant than, or occurs later than, the normalization of diffusion, as all six patients in our study in whom MR spectroscopy was performed had lactate levels predictive of moderate to severe injury, according to the work of Hanrahan et al (14) and Amess et al (40). The clinical outcome and the follow-up MR studies indicate that the predictions based on these measurements were accurate.
We compared the topologic extent of the diffusion abnormality, as assessed by diffusion imaging, with the degree of biochemical change in specified topological sites, as assessed by MR spectroscopy. This comparison was made because these are currently the means by which studies are evaluated in the literature (7, 10, 13, 14, 15, 36, 40) and by which norms are established. A comparison of diffusion imaging with spectroscopic imaging might be useful in the future, as spectroscopic imaging is now commercially available on some MR units. Another important point is that diffuse injury might not be initially apparent on diffusion images. As Figure 3A illustrates, a diffusion image of a patient with reduction of diffusion fairly equally throughout the brain looks nearly normal. Thus, ADCs must be calculated and compared with those of age-matched control subjects before a study can be definitively read as normal or abnormal.
An understanding of these MR spectroscopic and diffusion imaging findings is difficult with the limited knowledge available about the temporal evolution of neonatal hypoxic-ischemic injury. The generation of spectra with more accurate lactate peaks, obtained by using the technique of lactate editing, which eliminates contributions from other protons that might resonate at the same frequency as lactate, would help to increase our accuracy in assessing these patients. Our understanding could be further improved by the generation of ADC-versus-time and lactate-versus-time curves that would show when ADC values and lactate levels reach their highest and lowest levels with respect to the injury. Such curves could be useful for determining the reliability of lactate and diffusion levels at a particular postnatal time for predicting the severity of injury. Forbes et al (8) recently published data indicating that proton diffusion normalizes more quickly in the basal nuclei of the asphyxiated neonate than in the cortex. However, their patients were all imaged at least 2 days after birth, and their analysis dealt with timing in days rather than hours. For our analyses of images obtained during the first day of life, the difficulty in determining precisely when an injury occurred with respect to the time of delivery can substantially alter the temporal analysis. Therefore, we believe it is more useful, at present, to rely on time-independent values and ratios, such as those generated by Hanrahan et al (14) and Amess et al (40) for lactate levels, rather than on time-dependent numbers.
What, then, is the optimal MR examination for an encephalopathic, possibly asphyxiated, neonate who is only a few hours old? Until more information is available, it appears that proton MR spectroscopy, performed with a long TE to maximize sensitivity to lactate (20), is the single most useful study. Regarding the location of the voxel, we found the highest levels of lactate in the thalamic/basal ganglia voxel in this study. Amess et al (40) and Hanrahan et al (14) also used voxels centered over the deep gray nuclei for their studies and found a good correlation between metabolite ratios and outcome. These results most likely reflect the fact that acute, profound diminution of blood flow in the neonate seems to severely affect the deep gray nuclei (30, 50–52). Therefore, it seems that if a single voxel is to be sampled, the thalamus/putamen is the best location. Keeping in mind that patients with watershed injury may have higher lactate levels in the intervascular boundary zones (13), it is probably reasonable to sample a second voxel in the watershed region if time permits. The optimal solution may be to perform a spectroscopic imaging sequence, if available, that allows multiple smaller regions to be sampled individually. We continue to perform diffusion imaging because it is fast and supplies confirmatory information. Also, we continue to perform standard T1- and T2-weighted SE sequences to look for underlying hemorrhage, malformations, and patterns of injury that suggest metabolic disorders or infection, as disorders other than hypoxia-ischemia can cause neonatal encephalopathy.
Conclusion
This small series suggests that MR spectroscopy performed in the first 24 hours after birth is sensitive to the severity of hypoxic-ischemic brain injury, whereas diffusion imaging depicts and localizes, but underestimates, the extent of the injury. If MR imaging is to contribute to decisions regarding medical intervention in encephalopathic neonates, it is critical to better understand the optimal time at which the MR studies should be performed.
Footnotes
Supported by NIH grant NS35902 and NIH Clinical Research Center grant MO1RR01271.
Address reprint requests to A. James Barkovich, MD, Neuroradiology Section, Room L-371, UCSF, 505 Parnassus Ave, San Francisco, CA 94143.
References
- 1.Barkovich AJ, Westmark KD, Ferriero DM, Sola A, Partridge JC. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol 1995;16:427-438 [PMC free article] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Hallam D. Neuroimaging in perinatal hypoxic-ischemic injury. MRDD Res Rev 1997;3:28-41 [Google Scholar]
- 3.Barkovich AJ, Sargent SK. Profound asphyxia in the preterm infant: imaging findings. AJNR Am J Neuroradiol 1995;16:1837-1846 [PMC free article] [PubMed] [Google Scholar]
- 4.Steinlin M, Dirr R, Martin E, et al. MRI following severe perinatal asphyxia: preliminary experience. Pediatr Neurol 1991;7:164-170 [DOI] [PubMed] [Google Scholar]
- 5.Keeney S, Adcock EW, McArdle CB. Prospective observations of 100 high-risk neonates by high field (1.5 Tesla) magnetic resonance imaging of the central nervous system, II: Lesions associated with hypoxic-ischemic encephalopathy. Pediatrics 1991;87:431-438 [PubMed] [Google Scholar]
- 6.Rutherford M, Pennock J, Schwieso J, et al. Hypoxic ischaemic encephalopathy: early and late magnetic resonance findings in relation to outcome. Arch Dis Child 1996;75:145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143-150 [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes KPN, Pipe JG, Bird R. Neonatal hypoxic-ischemic encephalopathy: detection with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2000;21:1490-1496 [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion weighted magnetic resonance imaging. Neuropediatrics 1994;25:172-175 [DOI] [PubMed] [Google Scholar]
- 10.Robertson R, Ben-Sira L, Barnes P, et al. MR line scan diffusion weighted imaging of term neonates with perinatal brain ischemia. AJNR Am J Neuroradiol 1999;20:1658-1670 [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford MA, Cowan FM, Manzur AY, et al. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. J Comput Assist Tomogr 1991;15:188-198 [DOI] [PubMed] [Google Scholar]
- 12.Auld KL, Ashwal S, Holshouser B, et al. Proton magnetic resonance spectroscopy in children with acute central nervous system injury. Pediatr Neurol 1995;12:323-334 [DOI] [PubMed] [Google Scholar]
- 13.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy in the evaluation of asphyxiated term neonates. AJNR Am J Neuroradiol 1999;20:1399-1405 [PMC free article] [PubMed] [Google Scholar]
- 14.Hanrahan JD, Cox IJ, Azzopardi D, et al. Relation between proton magnetic resonance spectroscopy within 18 hours of birth asphyxia and neurodevelopment at 1 year of age. Dev Med Child Neurol 1999;41:76-82 [DOI] [PubMed] [Google Scholar]
- 15.Hanrahan JD, Sargentoni J, Azzopardi D, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res 1996;39:584-590 [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Buchli R, Ritter S, et al. Diagnostic and prognostic value of cerebral 31-P magnetic resonance spectroscopy in neonates with perinatal asphyxia. Pediatr Res 1996;40:749-758 [DOI] [PubMed] [Google Scholar]
- 17.Penrice J, Cady EB, Lorek A, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants and early changes after perinatal hypoxia-ischemia. Pediatr Res 1996;40:6-14 [DOI] [PubMed] [Google Scholar]
- 18.Shu SK, Ashwal S, Holshouser BA, Nystrom G, Hinshaw DB Jr. Prognostic value of 1-H MRS in perinatal CNS insults. Pediatr Neurol 1997;17:309-318 [DOI] [PubMed] [Google Scholar]
- 19.Ashwal S, Holshouser BA, Tomasi LG, et al. Proton magnetic resonance spectroscopy-determined cerebral lactate and poor neurological outcomes in children with central nervous system disease. Ann Neurol 1997;41:470-481 [DOI] [PubMed] [Google Scholar]
- 20.Holshouser BA, Ashwal S, Shu S, Hinshaw DB. Proton MR spectroscopy in children with acute brain injury: comparison of short and long echo time acquisitions. J Magn Reson Imaging 2000;11:9-19 [DOI] [PubMed] [Google Scholar]
- 21.Barkovich AJ. MR of the normal neonatal brain: assessment of deep structures. AJNR Am J Neuroradiol 1998;19:1397-1403 [PMC free article] [PubMed] [Google Scholar]
- 22.Barkovich AJ. Normal development of the neonatal and infant brain, skull, and spine. In: Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins; 2000:13–71
- 23.Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 1988;166:173-180 [DOI] [PubMed] [Google Scholar]
- 24.Hüppi P, Barnes P. Magnetic resonance techniques in the evaluation of the newborn brain. Clin Perinatol 1997;24:693-723 [PubMed] [Google Scholar]
- 25.Hüppi PS, Fusch C, Boesch C, et al. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res 1995;37:145-150 [DOI] [PubMed] [Google Scholar]
- 26.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998;209:57-66 [DOI] [PubMed] [Google Scholar]
- 27.van der Grond J, Veenhoven RH, Groenendaal F, de Vries LS, Mali WP. MR spectroscopy in full-term infants with perinatal asphyxia (abstract). Radiology 1992;185(P):185 [Google Scholar]
- 28.Volpe JJ. Hypoxic-ischemic encephalopathy: clinical aspects. In: Neurology of the Newborn. 3rd ed. Philadelphia: Saunders; 1995:314–369
- 29.Rutherford MA, Pennock JM, Dubowitz LMS. Cranial ultrasound and magnetic resonance imaging in hypoxic-ischemic encephalopathy: a comparison with outcome. Dev Med Child Neurol 1994;36:813-825 [DOI] [PubMed] [Google Scholar]
- 30.Pasternak JF, Gorey MT. The syndrome of acute near total intrauterine asphyxia in the term infant. Pediatr Neurol 1998;18:391-398 [DOI] [PubMed] [Google Scholar]
- 31.Menkes J, Curran JG. Clinical and magnetic resonance imaging correlates in children with extrapyramidal cerebral palsy. AJNR Am J Neuroradiol 1994;15:451-457 [PMC free article] [PubMed] [Google Scholar]
- 32.McArdle CB, Richardson CJ, Hayden CK, et al. Abnormalities of the neonatal brain: MR imaging, II: Hypoxic-ischemic brain injury. Radiology 1987;163:395-403 [DOI] [PubMed] [Google Scholar]
- 33.Martin E, Barkovich AJ. Magnetic resonance imaging in perinatal asphyxia. Arch Dis Child 1995;72:F62-F70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuenzle C, Baenziger O, Martin E, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 1994;25:191-200 [DOI] [PubMed] [Google Scholar]
- 35.Keeney SE, Adcock EW, McArdle CB. Prospective observations of 100 high-risk neonates by high field (1.5 Tesla) magnetic resonance imaging of the central nervous system, I: Intraventricular and extracerebral lesions. Pediatrics 1991;87:421-430 [PubMed] [Google Scholar]
- 36.Groenendaal F, Veenhoven EH, van der Grond J, Jansen GH, Witkamp TD, de Vries L. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in-vivo using proton magnetic resonance spectroscopy. Pediatr Res 1994;35:148-151 [DOI] [PubMed] [Google Scholar]
- 37.Cady EB. Metabolite concentrations and relaxation in perinatal cerebral hypoxic-ischemic injury. Neurochem Res 1996;21:1043-1052 [DOI] [PubMed] [Google Scholar]
- 38.Robertson NJ, Edwards AD. Recent advances in developing neuroprotective strategies for perinatal asphyxia. Curr Opin Pediatr 1998;10:575-580 [DOI] [PubMed] [Google Scholar]
- 39.Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997;100:1004-1014 [DOI] [PubMed] [Google Scholar]
- 40.Amess PN, Penrice J, Wylezinska M, et al. Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 1999;41:436-445 [PubMed] [Google Scholar]
- 41.Soul JS, Robertson RL, du Plessis AJ, Volpe JJ. Time course of changes in the apparent diffusion coefficient in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult (abstract). Neurology 2000;54: (Suppl 3) A248. [DOI] [PubMed] [Google Scholar]
- 42.Beauchamp NJ, Barker PB, Wang PY, van Zijl PCM. Imaging of acute cerebral ischemia. Radiology 1999;212:307-324 [DOI] [PubMed] [Google Scholar]
- 43.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology 1999;53:1528-1537 [DOI] [PubMed] [Google Scholar]
- 44.Latchaw R. The roles of diffusion and perfusion MR imaging in acute stroke management. AJNR Am J Neuroradiol 1999;20:957-958 [PMC free article] [PubMed] [Google Scholar]
- 45.Rumpel H, Nedelcu J, Aguzzi A, et al. Late glial swelling after acute cerebral hypoxia-ischemia in the neonatal rat: a combined magnetic resonance and histochemical study. Pediatr Res 1997;42:54-59 [DOI] [PubMed] [Google Scholar]
- 46.Miyasaka N, Nagaoka T, Kuriowa T, et al. Histopathologic correlates of temporal diffusion changes in a rat model of cerebral hypoxia/ischemia. AJNR Am J Neuroradiol 2000;21:60-66 [PMC free article] [PubMed] [Google Scholar]
- 47.Hope PL, Costello AM, Cady EB, et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 1984;2:366-370 [DOI] [PubMed] [Google Scholar]
- 48.Azzopardi D, Wyatt JS, Cady EB, et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res 1989;25:445-451 [DOI] [PubMed] [Google Scholar]
- 49.Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 1997;41:795-802 [DOI] [PubMed] [Google Scholar]
- 50.Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. AJNR Am J Neuroradiol 1992;13:959-972 [PMC free article] [PubMed] [Google Scholar]
- 51.Azzarelli B, Caldemeyer KS, Phillips JP, DeMyer WE. Hypoxic-ischemic encephalopathy in areas of primary myelination: a neuroimaging and PET study. Pediatr Neurol 1996;14:108-116 [DOI] [PubMed] [Google Scholar]
- 52.Roland EH, Poskitt K, Rodriguez E, Lupton BA, Hill A. Perinatal hypoxic-ischemic thalamic injury: clinical features and neuroimaging. Ann Neurol 1998;44:161-166 [DOI] [PubMed] [Google Scholar]