Abstract
BACKGROUND AND PURPOSE: The angle of insonation cannot be assessed with conventional transcranial Doppler sonography. Findings in healthy control subjects suggest that the angle is relatively small in routine clinical practice. Data regarding the angle in middle cerebral artery (MCA) stenosis are scarce. In this study, the angle and its effect on flow velocity measurements were assessed with transcranial color Doppler sonography in patients with MCA stenosis.
METHODS: Eighteen patients (median age, 53 y; age range, 22–72 y) who satisfied qualifying criteria (eg, angiographically revealed unilateral MCA stenosis of ≥50%) were selected from 149 consecutive patients enrolled in a prospective study of transcranial color Doppler sonography and cerebral digital subtraction angiography. All had active neurologic symptoms. The angle of insonation and peak systolic and mean flow velocities in both MCAs were measured from videotapes generated at sonography.
RESULTS: The mean angle of insonation was 47 ± 11° (range, 19–64°) on the stenotic side and 34 ± 18° on the contralateral side (P < .05). Angle-corrected velocities were higher than uncorrected ones. Differences between angle-corrected and uncorrected peak systolic and mean flow velocities on the stenotic side were 46.6% and 45.9%, respectively, of uncorrected values. Differences between corrected and uncorrected peak systolic and mean velocities were larger on the stenotic side compared with those on the contralateral side (P < .05).
CONCLUSION: In patients with moderate or severe MCA stenosis, the angle of insonation can be substantial and cause large errors when flow velocities are measured without angle correction.
Transcranial Doppler (TCD) sonography enables measurement of flow velocities in intracranial arteries (1, 2). A major limitation of the method is the sonographer's inability to visualize the artery being examined and to define the angle between the long axis of the vessel and the direction of the ultrasound beam. In ideal circumstances, the angle of insonation is 0°. In routine clinical practice, the angle is assumed to be relatively small; thus it may introduce a small and acceptable error in flow velocity measurements (3, 4). However, when the angle is large (5), the error in the measurement can be considerable.
In contrast to blind TCD sonography, transcranial color Doppler (TCCD) sonography allows outlining of parenchymal structures and visualization of vessels being examined. This outlining improves consistency and accuracy in placing the sample volume and allows measurement of angle-corrected velocities (6–8). Comparative studies of the two techniques in healthy control subjects show that angle-corrected middle cerebral artery (MCA) flow velocities are 10–20% higher than uncorrected values (4, 7). Studies of the angle of insonation in disease conditions are scarce. It is suspected that the angle frequently exceeds 30° when the MCA M1 segment is atherosclerotic or displaced by hemorrhagic or edematous brain tissue (9).
In this study, we assessed the effect of angle correction on blood flow velocity in patients with moderate or severe MCA stenosis.
Methods
Patients
A research program of TCCD measurement of intracranial arterial diameters and flow velocities in patients routinely referred for cerebral angiography for clinical reasons has been performed at the Bialystok Medical Academy since 1998. The program was approved by the Academy's ethics committee.
At the Bialystok Medical Academy Hospital, TCCD sonography is routinely performed in all patients with ischemic or hemorrhagic stroke. Some patients are subsequently referred for digital subtraction angiography. All patients included in the present investigation underwent TCCD sonography before angiography. In most cases, the patient was transferred to the angiography unit immediately after TCCD imaging.
Eighteen patients (10 men, eight women; age range, 22–72 y; median age, 53 y) were selected from the list of 149 patients enrolled in the program between June 1, 1999, and October 15, 2000. Selected patients satisfied the following criteria: transparency of both temporal windows, angiographically revealed unilateral MCA stenosis of more than 50% of its diameter; absence of extracranial internal carotid artery stenosis of more than 50% according to the North American Symptomatic Carotid Endarterectomy Trial criteria; and absence of aneurysm or arteriovenous malformation on cerebral angiograms.
Eleven patients had transient ischemic attacks or brain infarcts, one had epilepsy, and six had a deep-seated small or medium intraparenchymal hematoma. All neurovascular symptoms involved the territory supplied by the MCA of interest.
TCCD Sonography
Intracranial arteries were studied by using an SSH 140A scanner (Toshiba Medical System Division, Tokyo, Japan) equipped with a 2.5-MHz 90° phased-array probe. The MCAs were studied in the temporal bone acoustic window (10), and their M1 segments were insonated by placing a 3-mm–wide sample volume within a straight arterial segment approximately 10 mm from the carotid bifurcation (Fig 1). To determine the angle of insonation, a linear marker provided by the scanner software was placed, under visual guidance, on the color Doppler image of the arterial segment being insonated, and its direction was fitted to be oriented along the long axis of the segment. The angle between this marker and the ultrasound beam, displayed automatically on the screen of the scanner, was considered a two-dimensional approximation of the angle of insonation.
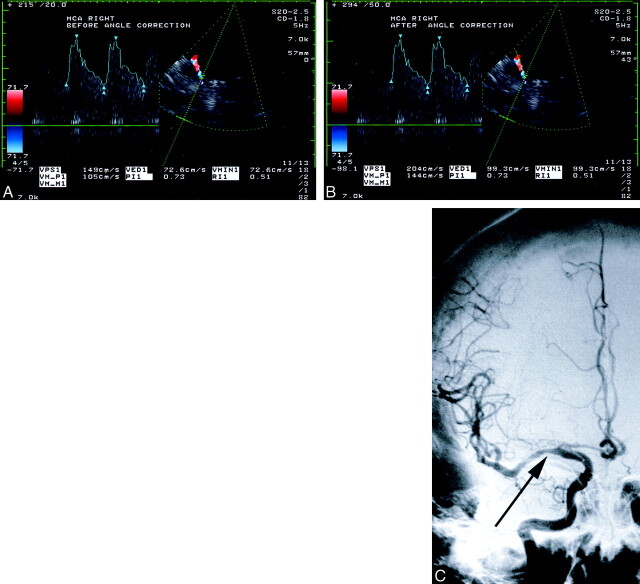
fig 1.
Comparison between angle-corrected and uncorrected flow velocities and angiographic findings in a 54-year-old woman with MCA stenosis.
A and B, At TCCD sonography, the sample volume is placed approximately 10 mm from the internal carotid artery bifurcation and within the color image of the right MCA. The velocity spectrum is adjacent to the left of the image of the artery. The uncorrected peak systolic velocity is 149 cm/s (B).
C, Angiogram shows a lesion causing a stenosis of more than 50% (arrow) in the right MCA M1 segment. The angle-corrected peak systolic velocity is 204 cm/s.
Mean-maximum, peak systolic, and end diastolic velocities were calculated by tracing the maximum envelope of the Doppler waveform over completed cycles. All examinations were recorded on videotape. For the purposes of the present study, MCA flow velocities of all 18 patients were remeasured with and without angle correction by using the videotapes.
Angiography
Intraarterial digital subtraction angiography was performed by using a femoral artery approach, with injections in both internal carotid arteries and at least one vertebral artery. Standard images included anteroposterior, lateral, and oblique views, all obtained with injection rates of 6 or 9 mL/s and filming rates of three frames per second. Two neuroradiologists who were unaware of the sonographic findings reviewed all angiograms. The view showing the most severe MCA narrowing was used for comparison with TCCD views. All measurements were performed by using a caliper (ARGOS 2M; Mecall, Milan, Italy). According to manufacturer specifications, the resolution of the caliper is 0.01 mm. The severity of stenosis was graded by using a three-point scale, as follows: no stenosis, 0–24%; mild stenosis, 25–49%; moderate or severe stenosis, 50% or greater. Angiograms were obtained during the acute stage of the illness and within 48 h of hospital admission.
For the purposes of this study, we selected the subgroup with moderate or severe angiographic stenosis. All 18 patients had unilateral (ipsilateral) MCA disease, and five also had mild MCA stenosis on the contralateral side.
Statistical Analyses
Data analysis was performed by using a personal computer equipped with statistical software (SYSTAT for Windows; SYSTAT, Evanston, IL). The paired t test was used. P values of .05 were considered to indicate statistically significant differences.
Results
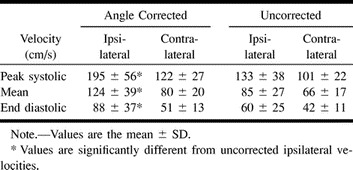
A significant difference was detected between the angles of insonation of the ipsilateral (47 ± 11°) and contralateral (34 ± 18°) MCAs. The range for the ipsilateral angle of insonation was 19–64°. The differences between angle-corrected and uncorrected ipsilateral peak systolic and mean flow velocities were 62 and 39 cm/s, respectively, and represented 46.6% and 45.9% of uncorrected values. On the contralateral side, the differences were 21 and 14 cm/s, and they represented 20.8% and 21.2%, respectively, of the uncorrected values. These differences in the ipsilateral peak systolic and mean flow velocities were significantly larger than the corresponding values on the contralateral side.
As expected, angle-corrected velocities were significantly higher than uncorrected ones, both ipsilaterally (Fig 1) and contralaterally. The main findings regarding flow velocities are presented in the Table.
Comparison of angle-corrected and uncorrected blood flow velocities in 18 patients with MCA stenosis of ≥50%
Discussion
These results indicate at least two important points. First, they show that the mean angle of insonation for a stenotic MCA M1 segment is larger than that of an unaffected normal artery. This angle results in higher peak systolic and mean flow velocity measurements and larger errors when only uncorrected velocity values are used. This “stenosis factor” effect was not addressed in previous investigations in which comparative analyses of the conventional TCD and TCCD measurements were performed (4, 7, 8), and its magnitude suggests that the error in uncorrected velocity values might be larger than previously thought. The 47° angle in this study also exceeds the 30° limit generally considered acceptable (5). The significant difference between ipsilateral and contralateral values indicates that this effect is independent of age, because contralateral arteries served as controls in the comparison. Rather, the effect may have been secondary to the tortuosity associated with atherosclerosis and stenosis—an effect observed with cerebral and peripheral arteries as well. An additional factor not addressed in this study relates to mass effect and M1 segment displacement associated with infarction or intraparenchymal hemorrhage in the basal ganglia and insula (9, 11) (Fig 2). This effect may be particularly relevant in patients with acute stroke, in whom the mass effect changes over days.
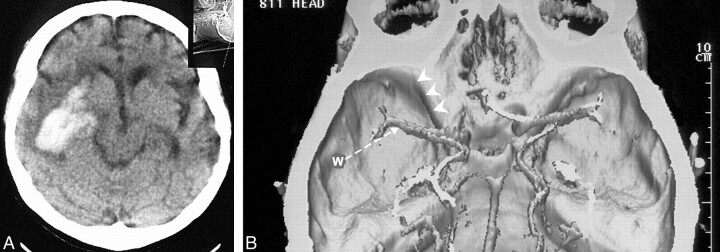
fig 2.
Mass effect and arterial displacement from intracerebral hemorrhage.
A, Brain CT scan in a 50-year-old man shows an intraparenchymal hematoma with a relatively limited mass effect on the basal cerebral cisterns.
B, A 3D CT reconstruction illustrates the relationships between the MCA, the temporal sonographic window (W) and direction of the ultrasound beam direction (arrow), and the lesser sphenoid wing (arrowheads). When compared with the contralateral MCA, the hematoma has displaced the ipsilateral MCA toward the skull base.
Second, these findings differ from those of previous investigations in that the angle of insonation for the contralateral unaffected MCA M1 segment also was substantial. Previous investigators evaluated mostly healthy and relatively young volunteers (age range, 20–40 y) (4, 7) and reported angles of insonation of 23.8–27.4°, as opposed to the 34° in this study. Wider angles were reported in one investigation (12). Patients included in the present study had neurologic conditions, and their median age was 53 y. The course of the M1 segment usually differs between young and elderly subjects (9, 10). The angle tends to be favorable in the young, but in older patients, the M1 segment often bows ventrally, coursing even closer to the edge of the sphenoid wing edge (9). Differences in arterial course may be secondary to changes in elasticity associated with aging (13).
These findings are particularly relevant in the clinical setting when patients with stroke undergo screening with TCD sonography to detect intracranial atherosclerotic lesions. Without angle correction, the error in velocity determinations can be substantial, because uncorrected velocities are lower than corrected ones. Furthermore, in view of the variability in the angle of insonation among patients, the use of a fixed velocity threshold to diagnose the presence of a stenotic lesion can be misleading, especially when the severity of stenosis is mild. Angle correction can help limit the error in velocity measurements.
Technical difficulties associated with angle correction are recognized (14), especially when the study population, like the present one, is small. Limitations are related to the unknown relationship of the insonated artery to the scan plane and to the difficulty in obtaining an accurate measurement of the insonation angle. Other factors that affect flow velocities include normal aging (15), occlusion of the ipsilateral internal carotid artery (16), increased intracranial pressure (17), vasoconstriction of cerebral microvasculature (18, 19), and embolic occlusion of distal MCA branches. The site of M1 segment sampling also can affect velocity measurements. Disturbed flow near the M1 origin might prevent reliable determination of the insonation angle, whereas sampling near its end has the risk of causing insonation of an MCA branch (20). A tortuous distal M1 segment frequently escapes even TCCD visualization.
Intracranial structures cannot be imaged with conventional TCD sonography, and consequently, the angle of insonation cannot be measured. Thus, the sonographer cannot verify whether the assumption of a narrow angle of insonation is correct. The findings of this and other studies indicate that the angle of insonation can be wide in elderly patients, in patients with intracranial arterial stenosis, and when the mass effect is present. The TCD sonographic finding of even mildly increased flow velocities when these conditions are present or suspected should prompt further imaging with other technologies, particularly if therapeutic decisions hinge on criteria based on velocity. The routine use of TCCD sonography provides a solution to this problem.
In summary, the angle of insonation cannot be ignored when transcranial sonography is used to detect MCA stenosis.
Footnotes
Address reprint requests to Jaroslaw Krejza, MD, PhD, Department of Radiology, Bialystok Medical Academy, M. Sklodowskiej Curie 24a, 15–224 Bialystok, Poland.
References
- 1.Lupetin AR, Davis DA, Beckman I, Dash N. Transcranial Doppler sonography, II: evaluation of intracranial and extracranial abnormalities and procedural monitoring. Radiographics 1995;15:193-209 [DOI] [PubMed] [Google Scholar]
- 2.Sloan MA. Detection of vasospasm following subarachnoid hemorrhage. In: Babikian VL, Wechsler LR, eds. Transcranial Doppler Ultrasonography. St. Louis, Mo: Mosby; 1993;105–127
- 3.Lupetin AR, Davis DA, Beckman I, Dash N. Transcranial Doppler sonography, I: principles, technique, and normal appearances. Radiographics 1995;15:179-191 [DOI] [PubMed] [Google Scholar]
- 4.Eicke BM, Tegeler CH, Dalley G, Myers LG. Angle correction in transcranial Doppler sonography. J Neuroimaging 1994;4:29-33 [DOI] [PubMed] [Google Scholar]
- 5.Santalucia P, Feldmann E. The basic transcranial Doppler examination: technique and anatomy. In: Babikian VL, Wechsler LR, eds. Transcranial Doppler Ultrasonography 2nd ed. Boston, Mass: Butterworth Heidemann; 1999;13–31
- 6.Baumgartner RW, Mathis J, Sturzenegger M, Mattle PH. A validation study on the intraobserver reproducibility of transcranial color-coded duplex sonography velocity measurements. Ultrasound Med Biol 1994;20:233-237 [DOI] [PubMed] [Google Scholar]
- 7.Schöning M, Bucholz RD, Walter J. Comparative study of transcranial color duplex sonography and transcranial Doppler sonography in adults. J Neurosurg 1993;78:776-784 [DOI] [PubMed] [Google Scholar]
- 8.Martin PJ, Evans DH, Naylor AR. Measurement of blood flow velocity in the basal cerebral circulation: advantages of transcranial color-coded sonography over conventional transcranial Doppler. J Clin Ultrasound 1995;23:21-26 [DOI] [PubMed] [Google Scholar]
- 9.Hacker H. Abnormal middle cerebral artery. In: Newton TH, Potts GD, eds. Radiology of the Skull and Brain. Great Neck, NY: C.V. Mosby; 1974;1479–1526
- 10.Krejza J, Mariak Z, Melhem ER, Bert RJ. A guide to the identification of major cerebral arteries with transcranial color Doppler sonography. AJR Am J Roentgenol 2000;174:1297-1303 [DOI] [PubMed] [Google Scholar]
- 11.Ring BA. The middle cerebral artery. In: Newton TH, Potts GD, eds. Radiology of the Skull and Brain. Great Neck, NY: C.V. Mosby; 1974:1442–1478
- 12.Tsuchiya T, Yasaka M, Yamaguchi T, Kimura K, Omae T. Imaging of the basal cerebral arteries and measurement of blood velocity in adults by using transcranial real-time color flow Doppler sonography. AJNR Am J Neuroradiol 1991;12:497-502 [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi K, Handa H, Nagasawa A, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomechanics 1980;13:175-184 [DOI] [PubMed] [Google Scholar]
- 14.Giller CA. Is angle correction correct? J Neuroimaging 1994;4:51-52 [DOI] [PubMed] [Google Scholar]
- 15.Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol 1999;172:213-218 [DOI] [PubMed] [Google Scholar]
- 16.Babikian VL. Transcranial Doppler evaluation of patients with ischemic cerebrovascular disease. In: Babikian VL, Wechsler LR, eds. Transcranial Doppler Ultrasonography. St. Louis, Mo: Mosby; 1993;97–104
- 17.Klingelhöfer J, Sander D, Holzgrafe M, Bischoff C, Conrad B. Cerebral vasospasm evaluated by transcranial Doppler ultrasonography at different intracranial pressures. J Neurosurg 1991;75:752-758 [DOI] [PubMed] [Google Scholar]
- 18.Scheel P, Puls I, Becker G, Schöning M. Volume reduction in cerebral blood flow in patients with vascular dementia. Lancet 1999;354:2137. [DOI] [PubMed] [Google Scholar]
- 19.Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain, II: the distribution of amyloid vascular changes. Stroke 1983;14:924-928 [DOI] [PubMed] [Google Scholar]
- 20.Krejza J, Mariak Z, Walecki J. Usefulness of transcranial color-coded sonography in the diagnosis of cerebral vasospasm. Stroke 1999;30:2240-2241 [DOI] [PubMed] [Google Scholar]