Abstract
Lipopolysaccharide (LPS) from oral pathogenic bacteria is an important factor leading to alveolar bone absorption and the implant failure. The present study aimed to evaluate the modulation of berberine hydrochloride (BBR) on the LPS-mediated osteogenesis and adipogenesis imbalance in rat bone marrow-derived mesenchymal stem cells (BMSCs). Cell viability, osteoblastic and adipogenic differentiation levels were measured using the Cell Counting Kit-8 assay, alkaline phosphatase (ALP) staining and content assay, and oil red O staining, respectively. Reverse transcription-quantitative PCR and immunoblotting were used to detect the related gene and protein expression levels. In undifferentiated cells, BBR increased the mRNA expression levels of the osteoblastic genes (Alp, RUNX family transcription factor 2, osteocalcin and secreted phosphoprotein 1) but not the adipogenic genes (fatty acid binding protein 4, Adipsin and peroxisome proliferator-activated receptorγ). LPS-induced osteoblastic gene downregulation, adipogenic gene enhancement and NF-κB activation were reversed by BBR treatment. In osteoblastic differentiated cells, decreased ALP production by LPS treatment was recovered with BBR co-incubation. In adipogenic differentiated cells, LPS-mediated lipid accumulation was decreased by BBR administration. The mRNA expression levels of the pro-inflammatory factors (MCP-1, TNF-α, IL-6 and IL-1β) were increased by LPS under both adipogenic and osteoblastic conditions, which were effectively ameliorated by BBR. The actions of BBR were attenuated by compound C, suggesting that the role of BBR may be partly due to AMP-activated protein kinase activation. The results demonstrated notable pro-osteogenic and anti-adipogenic actions of BBR in a LPS-stimulated inflammatory environment. This indicated a potential role of BBR for bacterial infected-related peri-implantitis medication.
Keywords: berberine, bone marrow-derived mesenchymal stem cells, osteogenesis, adipogenesis, lipopolysaccharide
Introduction
To improve the quality of life in patients with dentition defects and delay the absorption of the alveolar bone, osteointegrated dental implants have been developed and have been widely used since the 1980s (1,2). With the popularization of the dental implant, inflammation in the mucosa and bone tissues around the implant has become a new problem (3). The formation of the bacterial biofilm, on the surface of the implant and in the peripheral gingival crevicular fluid, stimulates the inflammatory reaction and finally leads to alveolar bone absorption. In serious cases, the implant has been found to loosen and fall off (3,4). A meta-analysis based on 15 articles of 11 studies during 2006 to 2014, found that the incidence of peri-implantitis was ~22% (5,6). At present, the primary clinical treatment aim of peri-implantitis is to control the pathogens and reduce the periodontal pockets around the implant, using subgingival curettage, antibiotics and laser treatment (7,8). However, it is difficult to reverse the process of bone absorption. Therefore, it is important to identify a drug or material that can inhibit the pathogenic bacteria-induced inflammation and improve the bone regeneration capability.
Several of the major pathogens of peri-implantitis belong to Gram-negative bacterium, which produce lipopolysaccharide (LPS) in their cell wall (9). LPS predominantly activates the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway and induces pro-inflammatory cytokine expression, including TNF-α, IL-6, IL-1β and monocyte chemoattractant protein-1 (MCP-1) (10). Enhancement of LPS-induced receptor activator of NF-κB ligand (RANKL) and autophage activation was found to promote osteoclastogenesis (11,12). Furthermore, LPS could adhere to the surface of the titanium implant, which had no effect on cell attachment but impaired osteoblast differentiation (13). Therefore, LPS is a contributing factor in both soft-tissue destruction and alveolar bone absorption, affecting the implant success rate (14).
As a key factor during the development of peri-implantitis, the balance of alveolar bone absorption and reconstruction was found to be damaged (15). Alveolar bone regeneration includes two aspects: The osteoclastogenesis of monocyte macrophages and the osteogenesis of mesenchymal stem cells (15). Bone marrow-derived mesenchymal stem cells (BMSCs) are a type of multipotent cell, which are considered to be common progenitors for osteoblasts and adipocytes (16). BMSCs have been applied for bone repair, due to their osteogenic ability (17). Thus, in the current study, BMSCs were used to investigate the regulation of osteogenesis. As aforementioned, LPS was found to impair osteoblast differentiation; in addition, a previous study has reported that LPS could affect the potential differentiation tendency of BMSCs (18). Therefore, it is important to investigate the regulation of drugs on LPS-induced osteogenesis/adipogenesis imbalance and inflammation in BMSCs.
Berberine is a type of quaternary ammonium alkaloid, extracted from varieties of plants species, such as Coptis chinensis (19). Berberine hydrochloride (BBR) is typically used in a clinical setting, due to its numerous pharmacological activities, including anti-microbial, glucose/cholesterol regulatory and immune modulatory properties (19). Previous studies have revealed that it is implicated in bone biology. For instance, BBR inhibited adipogenesis and promoted osteogenesis in the C3H10T1/2 cell line (20). In addition, berberine suppressed RANKL-induced osteoclast formation and protected cell survival (21,22). However, the role of BBR on osteogenesis and adipogenesis under inflammatory conditions remains unknown. The aim of the present study was to demonstrate the modulating effect of BBR on the osteogenesis/adipogenesis balance in LPS-mediated inflammatory response in BMSCs, which may provide novel evidence for peri-implantitis medication.
Materials and methods
Materials
BBR, LPS (extracted from Escherichia coli O111:B4 strain), L-ascorbic acid, β-glycerophosphate disodium, dexamethasone (Dex), 3-isobutyl-1-methylxanthine (IBMX) and indomethacin were purchased from Sigma-Aldrich (Merck KGaA). Compound C was obtained from CSNpharm, Inc. The Cell Counting Kit (CCK)-8 was purchased from Dojindo Molecular Technologies, Inc. The alkaline phosphatase (ALP) content assay kit, the ALP stain kit, BeyoECL Moon (ECL chemiluminescence kit), protease inhibitor cocktail, phosphatase inhibitor cocktail and insulin were purchased from Beyotime Institute of Biotechnology. The oil Red O Stain kit was purchased from Cyagen Biosciences, Inc., and the PrimeScript™ RT kits was obtained from Takara Bio, Inc. The Kapa SYBR® Fast QPCR Master Mix (2X) Universal kit was purchased from Roche Diagnostics and the primers were synthesized by Sangon Biotech Co., Ltd. The RIPA lysis buffer and TRIzol® reagent were purchased from Thermo Fisher Scientific, Inc., while the antibodies against phosphorylated (p)-p65 (cat. no. 3033), total p65 (cat. no. 8242), (p)-IκBα (cat. no. 2859), total IκBα (cat. no. 9243) and anti-rabbit IgG HRP-linked (cat. no. 7074) were purchased from Cell Signaling Technology, Inc. The anti-GAPDH antibody (cat. no. 10494-1-AP) was purchased from ProteinTech Group, Inc. Basic α-modified Eagle medium (α-MEM), FBS and penicillin-streptomycin antibiotics were purchased from Thermo Fisher Scientific, Inc. Other reagents were of analytical grade and used without further purification.
Primary cell isolation and culture
Female Sprague-Dawley rats (age, 3 weeks; weight, 80-100 g; 20-26°; relative humidity 40-70%; 12-h light/dark cycle; diet and drinking water ad libitum) were sacrificed using carbon dioxide (2 l/min in a 10-l box). The femur and tibia of the rats were isolated and the bone marrow cavity was washed with α-MEM to obtain the primary BMSCs. The cells were collected and centrifuged at 1,000 × g for 10 min at room temperature. After removing the supernatant, the aspirates were re-suspended with BMSC growth medium (α-MEM containing 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin) and seeded in a 10-cm dish for 3 days at 37°C, and then the non-adherent cells were removed. Adherent BMSCs were further cultured and expanded. Cells that passaged 3-6 times were used for further experimentation.
Cell viability assay
The BMSCs cells were seeded into 96-well plates (5,000 cells per well) and cultured overnight, then treated with BBR at different concentrations (0, 1, 5, 10, 50 and 100 µM) at 37°C for 72 h. Cell viability was measured using the CCK-8 kit according to the manufacturer's protocol. After removing the supernatant, fresh growth medium containing CCK-8 (Vcck-8:Vmedium, 1:10) was added into each well and incubated at 37°C for 2 h. The absorbance of the samples was measured at 450 nm using a Synergy 2 microplate reader (Agilent Technologies, Inc.).
RNA isolation and reverse transcription-quantitative (RT-q) PCR analysis
Total RNA was extracted using TRIzol®, and subsequently isolated with isopropanol and chloroform according to the manufacturer's protocol. RT reactions and qPCR detections were performed according to the manufacturer's instructions. The reverse transcription reaction was: 37°C for 15 min, 85°C for 15 sec. The program of the qPCR thermocycling was: First step: 95°C for 3 min; second: 95°C for 3 sec and 60°C for 30 sec, repeated for 40 cycles. The reactions were performed using Eppendorf Mastercycler Pro and an ABI 7900 Real-time PCR system (Thermo Fisher Scientific, Inc.). The relative mRNA expression levels were normalized to GAPDH using the comparative 2−∆∆Cq method (23). The PCR primer pairs are shown in Table I.
Table I.
Primer sequences for reverse transcription-quantitative PCR.
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| ALP | AACGTGGCCAAGAACATCATCA | TGTCCATCTCCAGCCGTGTC |
| RUNX2 | GCACCCAGCCCATAATAGA | TTGGAGCAAGGAGAACCC |
| OCN | TGAGGACCCTCTCTCTGCTC | GGGCTCCAAGTCCATTGTT |
| SPP1 | ATCTGAGTCCTTCACTG | GGGATACTGTTCATCAGAAA |
| FABP4 | GCGTAGAAGGGGACTTGGTC | TTCCTGTCATCTGGGGTGATT |
| ADIPSIN | CACGTGTGCGGTGGCACCCTG | CCCCTGCAAGTGTCCCTGCGGT |
| PPARγ | CCTTTACCACGGTTGATTTCTC | GGCTCTACTTTGATCGCACTTT |
| MCP-1 | TGCTGTCTCAGCCAGATGCAGTTA | AGAAGTGCTTGAGGTGGTTGTGGA |
| TNF-α | CCCAATCTGTGTCCTTCTAACT | CAGCGTCTCGTGTGTTTCT |
| Il-6 | GGTTTGCCGAGTAGACCTCA | GTGGCTAAGGACCAAGACCA |
| Il-1β | AAAGAAGGTGCTTGGGTCCT | CAGGAAGGCAGTGTCACTCA |
| GAPDH | CAGGGCTGCCTTCTCTTGT | TCCCGTTGATGACCAGCTTC |
ALP, alkaline phosphatase; Runx2, RUNX family transcription factor 2; Ocn, osteocalcin; Spp1, secreted phosphoprotein 1; Fabp4, fatty acid binding protein 4; Pparγ, peroxisome proliferator-activated receptorγ; MCP-1, monocyte chemoattractant protein-1.
Western blot analysis
BMSCs were lysed with RIPA lysis buffer containing protease inhibitor cocktail and phosphatase inhibitor cocktail. Cell lysate was collected, and its total protein concentration was determined using a BCA kit (Thermo Fisher Scientific, Inc.). Total protein (~20 µg) was loaded in each lane and separated using SDS-PAGE (12.5%) and then transferred onto PVDF membranes. The membranes were blocked by 5% non-fat milk in PBST buffer (PBS containing 0.1% Tween-20) for 1 h at room temperature. Then the membranes were incubated with the corresponding primary antibodies (the dilution of p-p65, t-p65, p-IκBα and t-IκBα antibodies, 1:1,000; GAPDH antibody, 1:5,000) at 4°C overnight. The membranes were washed 3 times with PBST buffer, 10 min each time, and then incubated with HRP-linked anti-Rabbit IgG (1:2,000) for 2 h at room temperature. The membranes were then washed 3 times, as previously. An ECL chemiluminescence kit (cat. no. P0018FS; Beyotime Institute of Biotechnology) was used for HRP detection. GE Amersham Imager 600 (Cytiva) was used for signal collection. Semi-quantification of band intensity was performed using ImageJ v1.44p software (National Institute of Health). Data are presented as the mean ± SD from three independent experiments.
Osteogenic and adipogenic differentiation
BMSCs were seeded into 12-well plates at a density of 2×104 cells/cm2 and cultured until confluent. To identify the effects of drug administration, LPS (1 µg/ml), BBR (1, 5 and 10 µM) and compound C (1 µM) were used, unless otherwise specified. For osteogenic differentiation, 10 nM Dex, 100 mg/l L-ascorbic acid and 10 mM β-glycerophosphate disodium were added to the BMSC growth media, co-incubated with given drug simultaneously. The osteoblastic media with drugs was replaced every 3 days. On day 7, the cells were harvested for ALP staining, intracellular ALP content assay and gene expression level analysis. For adipogenic differentiation, 1 µM Dex, 0.5 mM IBMX, 10 µg/ml insulin and 100 µM indomethacin were added to the BMSC growth media. The cells were incubated with the adipogenic media and corresponding drugs at 37°C for 12 days, and the media was replaced every 3 days. Then, the cells were harvested for oil red O staining.
ALP stain and intracellular content assay
Following treatment with osteogenic media and corresponding drugs for 7 days, the BMSCs were washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Following which, the cells were washed three times with PBS to remove the fixing liquid. The ALP staining solution was mixed according to the manufacturer's instructions, and added to the wells of the culture plate. After 30-min incubation at room temperature, the cells were washed with sterile water three times and images were obtained using a light microscope at ×200 magnification (Leica Microsystems GmbH).
For the ALP content assay, cells were treated as aforementioned, then washed with PBS and collected using a lysis buffer. The concentration of the samples was detected using a BCA kit (Thermo Fisher Scientific, Inc.). Each sample was diluted to 2 mg/ml, and the ALP content was measured according to the manufacturer's instructions. The absorbance was measured at 405 nm.
Oil red O stain
The oil red O working solution was prepared by diluting the stock solution with sterile water (Vstock:Vwater, 3:2). After differentiation for 12 days, the BMSCs were washed with PBS, fixed with 4% formaldehyde at room temperature for 15 min and then stained with freshly diluted oil red O working solution for 60 min at room temperature. Following which, the supernatant was removed and the cells were washed with water three times. Images of the oil red O-positive cells were collected using a bright-field microscope at ×200 magnification.
Statistical analysis
Data are presented as the mean ± SD from three separate experiments in each group. Statistical differences between groups were analyzed using one-way ANOVA, multiple comparisons between the groups were performed using Tukey's test with GraphPad Prism version 6 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
BBR reverses the LPS-induced decrease in osteogenic gene expression and increase in adipogenic gene expression levels
Firstly, to investigate the potential cytotoxicity of BBR, BMSCs were treated with different concentrations of BBR for 3 days and the cell viability was measured. As shown in Fig. 1A, BBR had no significant cytotoxic effect on BMSCs; therefore low concentrations, at 1-10 µM were selected for subsequent experiments. Additionally, the cytotoxic effects of LPS at different concentrations were also detected. At the dose of 1 µg/ml, LPS had no significant cytotoxic effect; however, 10 µg/ml LPS exhibited weak cytotoxicity (data not shown).
Figure 1.
BBR alters osteogenic and adipogenic gene expression levels in undifferentiated bone marrow-derived mesenchymal stem cells. (A) Cells were incubated with DMSO or BBR (1, 5, 10, 50 or 100 µM) for 3 days, and then cell viability was determined using a Cell Counting Kit-8 assay. The cells were treated with BBR (1, 5 or 10 µM) alone or simultaneously with LPS (1 µg/ml), and then collected for reverse transcription-quantitative PCR analysis. The mRNA expression levels of the osteogenic genes (B) Runx2, (C) Spp1 and (D) Ocn, and the adipogenic genes (E) Fabp4, (F) Adipsin and (G) Pparγ were measured. Their relative changes were normalized to the GAPDH expression levels. The data are presented as the mean ± standard deviation of three independent experiments. #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 vs. control group; **P<0.01, ***P<0.001, ****P<0.0001 vs. LPS group. BBR, berberine hydrochloride; LPS, lipopolysaccharide; Runx2, RUNX family transcription factor 2; Ocn, osteocalcin; Spp1, secreted phosphoprotein 1; Fabp4, fatty acid binding protein 4; Pparγ, peroxisome proliferator-activated receptorγ.
As LPS-related adverse effects on bone reconstruction are a key factor in the development of peri-implantitis (14), the potential role of BBR on osteogenic and adipogenic gene expression in undifferentiated BMSCs was investigated. The cells were co-incubated with BBR at different concentrations (0, 1, 5 and 10 µM) and with or without 1 µg/ml LPS for 3 days. The results demonstrated that BBR significantly promoted the mRNA expression levels of osteogenic genes, RUNX family transcription factor 2 (Runx2), osteocalcin (Ocn) and secreted phosphoprotein 1 (Spp1), in a dose-dependent manner (Fig. 1B-D). Notably, LPS significantly decreased the Runx2 and SPP1 mRNA expression levels, and these effects were partly or totally reversed by BBR. The Ocn mRNA expression level was not changed by LPS; however, it was increased after BBR treatment under the LPS-stimulated condition. For the adipogenic genes, compared with the negative control, LPS induced a two-fold increase in both fatty acid binding protein 4 (Fabp4) and Adipsin gene expression levels (Fig. 1E and F); however, the peroxisome proliferator-activated receptorγ (Pparγ) expression level decreased by half (Fig. 1G). BBR itself had little effect on the adipogenic genes, except for Adipsin, and a small reduction in the increase of Fabp4 expression levels induced by LPS. Moreover, 10 µM BBR lowered Adipsin gene expression level, and further dose-dependently reduced the increase stimulated by LPS.
BBR promotes BMSC osteogenic differentiation under LPS treatment
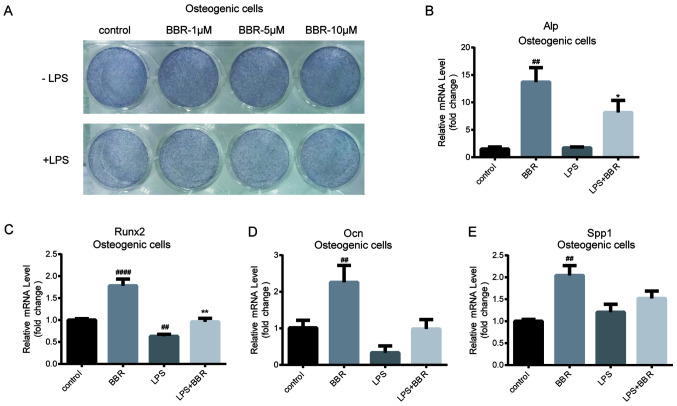
As aforementioned, it was determined that BBR could ameliorate the LPS-induced inhibition of BMSCs osteogenic gene expression. Considering that ALP is a key protein during the differentiated process, particularly in the early and middle stages (24), ALP protein expression was determined using ALP staining. As shown in Fig. 2A (upper panel), cells treated with BBR during the differentiation process exhibited increased staining compared with that in the control cells, in a dose-dependent manner. Furthermore, staining of cells treated with LPS was markedly decreased compared with that in the control cells. Notably, BBR reversed the LPS-induced reduction (Fig. 2A; lower panel). In addition, intracellular Alp mRNA expression level exhibited a similar result (Fig. 2B). Compared with Alp mRNA expression in the control cells, there was a 13.7-fold increase induced by BBR treatment and an 8.1-fold change induced by BBR and LPS co-incubation. The gene expression level of Runx2, the key transcriptional factor during osteogenesis (25), was higher in cells treated with BBR and lower in LPS-treated cells. Furthermore, co-incubation with BBR and LPS reversed the LPS-mediated reduction of Runx2 mRNA expression level (Fig. 2C). Similar results were also found for Ocn mRNA expression levels (Fig. 2D), which is one of the essential osteogenic marker genes (26). The regulation of BBR on SPP1 mRNA expression was similar with that on Ocn mRNA expression; however, the result was not significantly different (Fig. 2E). These findings indicated that BBR could accelerate osteogenic differentiation and assist with restricting LPS-induced adverse effects in BMSCs.
Figure 2.
BBR promotes osteogenesis in bone marrow-derived mesenchymal stem cells. The cells were incubated with BBR (0, 1, 5 or 10 µM) alone or simultaneously with LPS (1 µg/ml) in osteogenic media for 7 days. Then, the cells were detected using ALP stain and reverse transcription-quantitative PCR analysis. (A) Images of the ALP stained cells were obtained using a camera. The mRNA expression levels of the (B) Alp, (C) Runx2, (D) Ocn and (E) Spp1 osteogenic genes were measured. Their relative changes were normalized to the GAPDH expression levels. The data are presented as the mean ± standard deviation of three independent experiments. ##P<0.01, ####P<0.0001 vs. control group; *P<0.05, **P<0.01 vs. LPS group. BBR, berberine hydrochloride; LPS, lipopolysaccharide; Runx2, RUNX family transcription factor 2; Ocn, osteocalcin; Spp1, secreted phosphoprotein 1; ALP, alkaline phosphatase.
BBR reduces adipogenic differentiation of BMSCs under LPS treatment
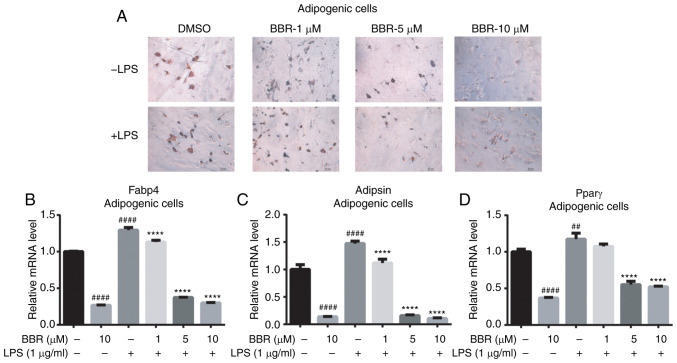
Basic effects of BBR on adipogenic gene expression were weak in undifferentiated BMSCs; however, it was hypothesized that BBR could serve a role in the adipogenic process. The maturity of adipose-like cells, which were treated with different concentrations of BBR in combination with or without LPS during the differentiated process, was investigated. As illustrated in the oil red O staining images, cells treated with a higher BBR dose exhibited fewer positive stained cells (Fig. 3A; upper panel). LPS increased the number of mature cells further, while BBR co-treatment reversed the effect in a dose-dependent manner (Fig. 3A; lower panel). It was found that BMSCs administrated with BBR combined with LPS had more positive cells compared with those treated with BBR alone. This suggested that BBR partly blocked differentiation.
Figure 3.
BBR inhibits adipogenesis in bone marrow-derived mesenchymal stem cells. The cells were incubated with BBR (0, 1, 5 or 10 µM) alone or simultaneously with LPS (1 µg/ml) in adipogenic media for 12 days. Then, the cells were detected using oil red O stain and reverse transcription-quantitative PCR analysis. (A) Images of the ALP stained cells were obtained using a microscope, at ×200 magnification. The mRNA expression levels of the (B) Fabp4, (C) Adipsin and (D) Pparγ adipogenic genes were measured. Their relative changes were normalized to the GAPDH expression levels. The data are presented as the mean ± standard deviation of three independent experiments. ##P<0.01, ####P<0.0001 vs. control group; ****P<0.0001 vs. LPS group. BBR, berberine hydrochloride; LPS, lipopolysaccharide; Fabp4, fatty acid binding protein 4; Pparγ, peroxisome proliferator-activated receptorγ; ALP, alkaline phosphatase.
The gene expression levels in differentiated BMSCs were also detected. As presented in Fig. 3B-D, the mRNA expression levels of all of the three adipogenic genes were decreased by BBR and increased by LPS. Furthermore, BBR combined with LPS decreased the LPS-stimulated Fabp4, Adipsin and Pparγ gene expression levels to a lower level in a dose-dependent manner. Taken together, it was found that BBR inhibited BMSCs adipogenesis and partly prevented the LPS-mediated effect.
BBR ameliorates LPS-stimulated NF-κB signaling activation and enhancement of pro-inflammatory factor expression
As LPS activates cells and the expression of the pro-inflammatory cytokines via the TLR4-NF-κB signaling pathway, the effects of BBR on the primary proteins of the NF-κB signaling pathway were investigated. It is known that p-IκBα and p-NF-κB p65 subunits are markers of TLR4/NF-κB pathway activation (27). BMSCs were cultured and treated with BBR and LPS, alone or in combination, for another 2h. It was found that the LPS-treated group exhibited darker p-p65 and p-IκBα bands compared with those in the control group and in the BBR-treated group (Fig. 4A). There was no effect from BBR alone; however, BBR decreased the LPS-stimulated activated protein expression levels. Densitometry analysis of the bands is shown in Fig. 4B and C. Notably, LPS treatment significantly increased the phosphorylation of the NF-κB p65 subunit and IκBα, and these increments were reversed when exposed to BBR simultaneously.
Figure 4.
BBR protects bone marrow-derived mesenchymal stem cells from LPS-induced NF-κB activation under undifferentiated condition. Cells were incubated with DMSO (as the control), BBR (10 µM), LPS (1 µg/ml) or both BBR and LPS for 2h. Then, the cells were harvested for western blot analysis. (A) Bands of the p-and t- (B) NF-κB p65 subunit, (C) IκBα and GAPDH protein expression levels are shown and the results were semi-quantified. The data are presented as the mean ± standard deviation of three independent experiments. #P<0.05, ####P<0.0001 vs. control group; **P<0.01 vs. LPS group. T, total, p, phosphorylated; BBR, berberine hydrochloride; LPS, lipopolysaccharide; p-, phosphorylated; t-, total.
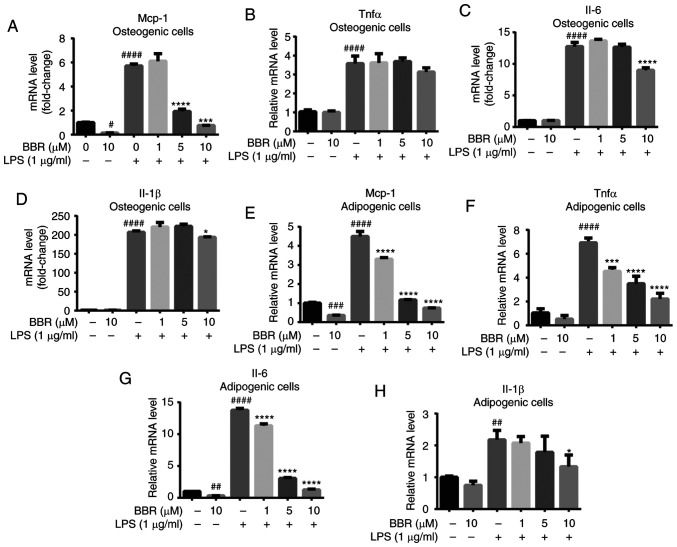
Subsequently, the product of the activated NF-κB signaling pathway was investigated using RT-qPCR, including the mRNA expression levels of TNF-α, MCP-1, Il-6 and Il-1β. BMSCs were differentiated and treated as aforementioned, then mature osteoblast cells and adipose-like cells were harvested and detected. In osteogenic BMSCs (Fig. 5A-D), BBR alone had little effect on the pro-inflammatory factors except MCP-1; however, LPS significantly increased TNF-α, MCP-1, Il-6 and Il-1β gene expression to a great extent. Moreover, the reversal effect of BBR against LPS was not strong. However, a dose-dependent change was still found, particularly on MCP-1 mRNA expression levels.
Figure 5.
BBR alleviates LPS-induced inflammatory factor expression in both osteogenic and adipogenic differentiated bone marrow-derived mesenchymal stem cells. The cells were incubated with BBR (0, 1, 5 or 10 µM) or simultaneously with LPS (1 µg/ml) in osteogenic media for 7 days or in adipogenic media for 12 days. Then, the cells were harvested for reverse transcription-quantitative PCR analysis. The mRNA expression levels of the pro-inflammatory factors (A) MCP-1, (B) TNF-α, (C) Il-6 and (D) Il-1β were measured in differentiated osteoblastic cells. (E) MCP-1, (F) TNF-α, (G) Il-6 and (H) Il-1β expression levels were also measured in adipogenic differentiated cells. Their relative changes were normalized to GAPDH levels. The data are presented as the mean ± standard deviation of three independent experiments. #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 vs. control group; *P<0.05, ***P<0.001, ****P<0.0001 vs. LPS group. BBR, berberine hydrochloride; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1.
The regulation of BBR was notable in adipogenic BMSCs. The gene expression levels of MCP-1, Il-6 and Il-1β were alleviated with BBR treatment. LPS stimulated a 4.5-, 6.9-, 13.7- and 2.1-fold increase in the MCP-1, TNF-α, Il-6 and Il-1β mRNA expression levels, respectively. Furthermore, BBR co-treatment caused a significant and dose-dependent decrease in these expression levels (Fig. 5E-H). At 10 µM concentration, BBR almost reversed the mRNA expression levels of MCP-1 and Il-6 back to the basic level.
All the aforementioned results suggested that BBR efficiently decreased the mRNA expression levels of pro-inflammatory factors in both types of cells and may act more sensitively in adipogenic cells.
BBR regulates the balance of osteogenic/adipogenic differentiation partly via AMP-activated protein kinase (AMPK) activation
As BBR typically acts as an adenosine AMPK agonist (28), it was hypothesized whether its regulation of osteogenesis/adipogenesis balance was due to AMPK activation. The efficiency of BMSC osteogenic and adipogenic differentiation with respective compound treatments was subsequently investigated.
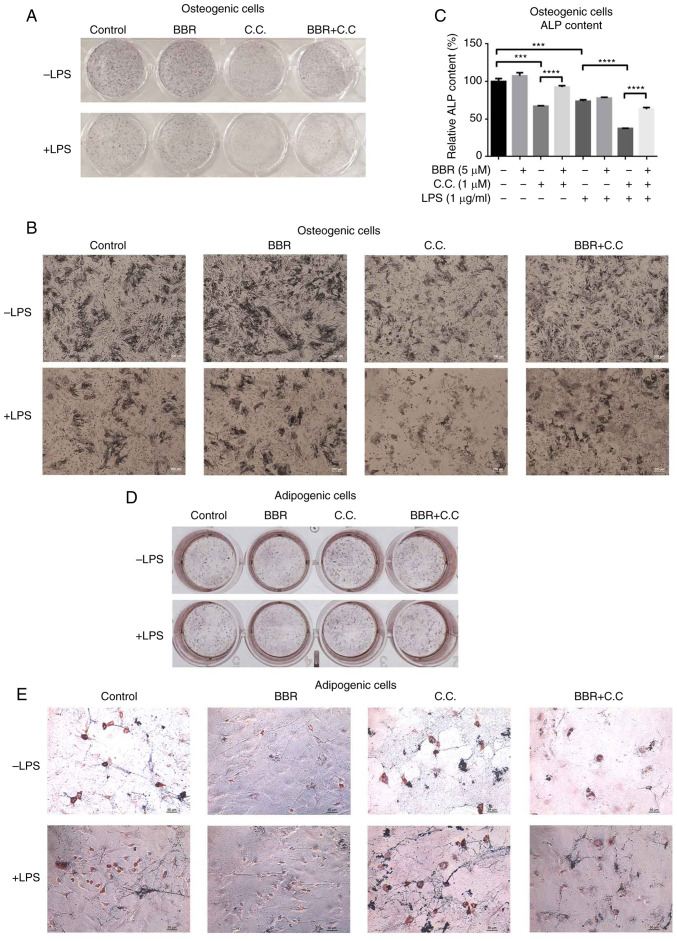
For osteogenic differentiation, compared with that in the control cells, cells incubated with BBR stained darker, while compound C (AMPK antagonist)-treated cells stained lighter (Fig. 6A and B). The color of the stain in BBR and compound C co-incubated cells was darker compared with that in compound C only treated cells, and lighter compared with that in cells treated with BBR only treated cells (Fig. 6A and B; upper panel). Similar results were detected under the LPS-mediated inflammatory condition (Fig. 6A and B; lower panel). In addition, the intracellular ALP content was measured and the results are presented in Fig. 6C. The ALP content was weakly increased in the BBR group, while cells treated with compound C and LPS had decreased ALP content. Compared with that in the compound C group, the content of ALP in the BBR +compound C group was significantly higher, suggesting the recovery of ALP expression. Under inflammatory conditions, the LPS + compound C group showed a lower ALP level compared with that in the LPS, and LPS + BBR + compound C groups. Both the ALP staining and intracellular content demonstrated that altered AMPK activation affected osteogenic differentiation, and BBR could block the effect of compound C.
Figure 6.
BBR regulates osteogenic and adipogenic differentiation via AMPK activation. The cells were incubated with BBR (10 µM), C.C (1 µM) or both, in the presence or absence of LPS (1 µg/ml), in osteogenic media for 7 days or in adipogenic media for 12days. Then, the cells were collected for ALP staining, ALP content assay or oil red O staining. The images of the ALP stained cells were obtained using a (A) camera and (B) microscope at ×200 magnification. (C) Relative ALP content levels of osteogenic cells were measured and normalized to control levels. The images of the oil red O stained cells were obtained using a (D) camera and (E) microscope at ×200 magnification. The data are presented as the mean ± standard deviation of three independent experiments. ***P<0.001, ****P<0.0001. C.C, compound C; BBR, berberine hydrochloride; LPS, lipopolysaccharide; ALP, alkaline phosphatase.
For adipogenic differentiation, the number of positive cells in the BBR group was reduced compared with that in the control group, while the compound C group had more positive cells compared with that in the control group (Fig. 6D and E). The BBR + compound C group had a higher number of positive cells compared with that in the control group, but fewer compared with that in the compound C group. On the other hand, compared with that in the control group, LPS increased the number of positive cells, and the combination group with compound C had even higher numbers (Fig. 6D and E). The LPS + BBR + compound C group had less stained cells compared with that in the LPS + compound C group. It was suggested that BBR reversed the pro-adipogenic differentiation of LPS, and compound C disturbed the anti-adipogenic function of BBR.
Discussion
Similar to periodontitis of teeth, peri-implantitis of implanted teeth can occur. Inflammatory responses caused by bacteria have been found to stimulate pro-inflammatory factor upregulation and contribute to the pathogenesis of both periodontitis and peri-implantitis (29). Therefore, controlling the inflammatory reaction is an active strategy to solve the peri-implant problem, by either reducing bacteria growth or alleviating the accumulation of pro-inflammatory cytokines. Notably, it would be beneficial to have both options. Previous studies have reported that BBR was a broad-spectrum anti-bacterial agent (30), and also exhibited anti-inflammatory properties (28), thus, presenting as a suitable candidate for peri-implantitis therapy. Although BBR decreased the LPS-mediated inflammatory response in several types of cells (28,31,32), the anti-inflammatory effect of BBR in BMSCs is yet to be fully elucidated. The present study demonstrated that BBR not only attenuated the LPS-induced NF-κB p65 and IκBα activation in undifferentiated BMSCs, but also downregulated the pro-inflammatory factor expression levels in differentiated cells. These results were consistent with the expected effect, confirming BBR as a suitable candidate.
Besides anti-inflammation, bone regeneration is an important aspect for peri-implantitis therapy. Thus, it should be investigated whether a candidate would improve bone regeneration. Firstly, previous studies have reported the pro-osteogenesis effect of BBR (20,33); however, it was not clear whether the action was sufficient to resist the effect by LPS. Secondly, LPS could cause the imbalance of chondrogenesis and adipogenesis in multi-potential BMSCs (18). The function of LPS in regulating osteogenesis and adipogenesis balance requires further investigation. In the present study, there was no significant cytotoxic effect of LPS at 1 µg/ml concentration but weak cytotoxicity was induced at the dose of 10 µg/ml. Notably, the properties of LPS may vary at different concentrations. For instance, LPS promoted adipogenesis at a dose of 10 µg/ml, but attenuated adipogenic differentiation at a concentration of 100 ng/ml (18,34). Thus, in the current study, in order to avoid the effect of cytotoxicity caused by high concentration of LPS on cell differentiation, the dose of 1 µg/ml was selected for subsequent experimentation. In a LPS-mediated inflammatory condition, the regulation of BBR on the complex roles of LPS-induced differentiation promotion or attenuation should also be investigated.
To answer these questions, the changes in undifferentiated and differentiated cells treated with BBR alone or in combination with LPS were investigated. Understanding the basic effect of BBR in undifferentiated cells may assist with predicting osteogenic or adipogenic differentiation tendency in the complex differentiated environment. In the present study, it was found that BBR reversed the LPS-induced low mRNA expression levels of osteogenic genes to a higher level (compared with that in the control group) in undifferentiated cells; however, the changes in the differentiated osteoblast were not as effective. Up to a 10 µM concentration, BBR was not able to completely neutralize the inhibition of LPS. Furthermore, the changes in the ALP protein staining were moderate compared with that observed for the mRNA expression level. Analogously, the resistant role of BBR against LPS in regulating adipogenic gene expression level was weak in the undifferentiated BMSCs; however, it was stronger in the mature adipose-like cells. The oil red O stain assay further identified the high efficiency of BBR. The extent of the variation maybe different; however, the tendency of pro-osteogenic and anti-adipogenic effects of BBR were found to be similar, regardless of whether the BMSCs were differentiated or not. In other words, LPS-mediated osteogenic and adipogenic differentiation was rectified by BBR.
Given that BBR is an activator of AMPK, it was hypothesized that BBR could modulate the balance of osteogenic and adipogenic differentiation by activating AMPK. Therefore, an AMPK inhibitor, compound C, was used. The present results identified that compound C attenuated the effects of BBR; however, its strong regulation of anti-osteogenesis and pro-adipogenesis was notable. It was suggested that up- and down-regulation of AMPK could enhance the pro-osteogenic and pro-adipogenic effects, accordingly. BBR has been revealed to competitively bind with myeloid differentiation protein (MD-2) and interfere with the binding of the TLR4/MD-2 complex with LPS (35,36); thus, BBR acts as a high-affinity LPS antagonist. However, these molecular docking data were the result of computer simulation, and not from experimental detection. In the present study, BBR reversed the effects of LPS; however, this does not suggest a direct interaction between BBR and LPS. Until now, the mechanism of action was unknown, regarding whether it was direct, indirect or mixed. Based on the aforementioned results, further investigation is required to elucidate the direct competitive antagonism. Elimination of LPS function by knockout or mutation of the MD-2 gene may assist with identification or exclusion of the direct method. Until this is identified, it can only be hypothesized that BBR attenuated the LPS-mediated effects, at least partly, via the activation of AMPK. Furthermore, BBR is a multi-target molecule. For instance, ubiquitin-like with PHD and RING Finger domains 1 is also a BBR target protein (37), which could negatively regulate Pparγ in colorectal cancer (38). Whether the ubiquitin-dependent proteasome system serves a role in the osteogenic differentiated regulation in BMSCs also requires confirmation.
In the present study, there was an interesting finding that LPS stimuli decreased Pparγ mRNA expression level in undifferentiated BMSCs but increased its gene expression level in the adipogenic cells. Simonin et al (39) reported a decrease in Pparγ expression induced by 10 µg/ml LPS in rat synovial fibroblasts. Moreover, LPS (0.5 µg/ml) was found to downregulate Pparγ mRNA and protein expression levels in vascular smooth muscle cells (40). The effects of LPS on Pparγ gene expression in these cells were consistent with the current findings in the undifferentiated BMSCs. On the other hand, similar to the present results, another research team reported that LPS increased Pparγ mRNA expression under the adipogenic condition (13). Sun et al (41) revealed that mineralization of ST2 cells is inhibited by PPARγ agonist troglitazone, while osteogenic differentiation of BMSCs from osteoblast-targeted PPARγ knock-out mice increases compared with wild type mice. Cortical bone formation in the knock-out mice is also increased. In fact, Pparγ is a protein with complex mechanism, which could inhibit certain gene expression levels in untreated cells and enhance these in LPS-stimulated cells (42). Thus, it was suggested that the inhibition of Pparγ gene expression by LPS in undifferentiated BMSCs may reflect a potential osteogenic role. However, under adipogenic conditions, Pparγ expression and activation was increased, and LPS further promoted Pparγ expression via other mechanisms. This mechanism requires further study.
In summary, the present study demonstrated that BBR ameliorated the LPS-induced imbalance of osteogenic and adipogenic differentiation in BMSCs and promoted osteogenesis. The current study provided a potential drug candidate, BBR, to solve the peri-implantitis problem, with regards to its multiple role in anti-bacterial capacity (43), anti-inflammation action and osteogenesis.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the Natural Science Foundation of Shanghai (grant no. 19ZR1439600) and the Natural Science Talent Cultivation Foundation of Shanghai Tenth People's Hospital (grant no. 040318055).
Funding
This work was supported by the Natural Science Foundation of Shanghai (grant no. 19ZR1439600) and the Natural Science Talent Cultivation Foundation of Shanghai Tenth People's Hospital (grant no. 040318055).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YX, SQ, RZ and FC designed the research. RZ and HL carried out the PCR analysis. FC and XZ performed the cell differentiation and staining. XW isolated the primary cells. FY and GS supervised the project and contributed to data analysis. YX, SQ and RZ contributed to the writing of the manuscript. XZ and XW helped with manuscript revision and SQ and YX confirmed the authenticity of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures performed in this work were approved by the Animal Ethics Committee of Shanghai Tenth People's Hospital, Shanghai, China (approval no. 2018-4487).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, Glöckner S, Krause G, Stiesch M. Epidemiology and risk factors of peri-implantitis: A systematic review. J Periodontal Res. 2018;53:657–681. doi: 10.1111/jre.12562. [DOI] [PubMed] [Google Scholar]
- 2.Heitz-Mayfield LJ. Diagnosis and management of peri-implant diseases. Aust Dent J. 2008;53(Suppl 1):S43–S48. doi: 10.1111/j.1834-7819.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 3.Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemming TF. Prevalence and predictive factors for peri-implant disease and implant failure: A cross-sectional analysis. J Periodontol. 2015;86:337–347. doi: 10.1902/jop.2014.140438. [DOI] [PubMed] [Google Scholar]
- 4.Mishler OP, Shiau HJ. Management of peri-implant disease: A current appraisal. J Evid Based Dent Pract. 2014;14(Suppl):53–59. doi: 10.1016/j.jebdp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017;96:31–37. doi: 10.1177/0022034516667484. [DOI] [PubMed] [Google Scholar]
- 6.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–S171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 7.Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014;66:255–273. doi: 10.1111/prd.12049. [DOI] [PubMed] [Google Scholar]
- 8.Hentenaar DFM, De Waal YCM, Van Winkelhoff AJ, Meijer HJA, Raghoebar GM. Non-surgical peri-implantitis treatment using a pocket irrigator device; clinical, microbiological, radiographical and patient-centred outcomes-A pilot study. Int J Dent Hyg. 2020;18:403–412. doi: 10.1111/idh.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/S1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 10.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Noh AL, Kang JH, Sim JS, Lee DS, Yim M. Peroxiredoxin II negatively regulates lipopolysaccharide-induced osteoclast formation and bone loss via JNK and STAT3. Antioxid Redox Signal. 2015;22:63–77. doi: 10.1089/ars.2013.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sul OJ, Park HJ, Son HJ, Choi HS. Lipopolysaccharide (LPS)-induced autophagy is responsible for enhanced osteoclastogenesis. Mol Cells. 2017;40:880–887. doi: 10.14348/molcells.2017.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonsignore LA, Anderson JR, Lee Z, Goldberg VM, Greenfield EM. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone. 2013;52:93–101. doi: 10.1016/j.bone.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nouneh RA, Wataha JC, Hanes PJ, Lockwood PE. Effect of lipopolysaccharide contamination on the attachment of osteoblast-like cells to titanium and titanium alloy in vitro. J Oral Implantol. 2001;27:174–179. doi: 10.1563/1548-1336(2001)027<0174:EOLCOT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Insua A, Monje A, Wang HL, Miron RJ. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J Biomed Mater Res A. 2017;105:2075–2089. doi: 10.1002/jbm.a.36060. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen KY, Chung CM, Chen YS, Bau DT, Yao CH. Rat bone marrow stromal cells-seeded porous gelatin/tricalcium phosphate/oligomeric proanthocyanidins composite scaffold for bone repair. J Tissue Eng Regen Med. 2013;7:708–719. doi: 10.1002/term.1461. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Tang H, Zhang Z, Zhang Y, Qiu C, Zhang L, Huang P, Li F. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol. 2017;43:236–242. doi: 10.1016/j.intimp.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449–461. doi: 10.1016/j.atherosclerosis.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Lee HW, Suh JH, Kim HN, Kim AY, Park SY, Shin CS, Choi JY, Kim JB. Berberine promotes osteoblast differentiation by Runx2 activation with p38 MAPK. J Bone Miner Res. 2008;23:1227–1237. doi: 10.1359/jbmr.080325. [DOI] [PubMed] [Google Scholar]
- 21.Han SY, Kim YK. Berberine Suppresses RANKL-induced osteoclast differentiation by inhibiting c-Fos and NFATc1 expression. Am J Chin Med. 2019;47:439–455. doi: 10.1142/S0192415X19500228. [DOI] [PubMed] [Google Scholar]
- 22.Hu JP, Nishishita K, Sakai E, Yoshida H, Kato Y, Tsukuba T, Okamoto K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-kappaB and Akt pathways. Eur J Pharmacol. 2008;580:70–79. doi: 10.1016/j.ejphar.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Shanbhag S, Mohamed-Ahmed S, Lunde THF, Suliman S, Bolstad AI, Hervig T, Mustafa K. Influence of platelet storage time on human platelet lysates and platelet lysate-expanded mesenchymal stromal cells for bone tissue engineering. Stem Cell Res Ther. 2020;11:351. doi: 10.1186/s13287-020-01863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Hou Q, Su H, Chen D, Luo Y, Jiang T. miR-488 negatively regulates osteogenic differentiation of bone marrow mesenchymal stem cells induced by psoralen by targeting Runx2. Mol Med Rep. 2019;20:3746–3754. doi: 10.3892/mmr.2019.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YC, Chan YH, Hsieh SC, Lew WZ, Feng SW. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model. Int J Mol Sci. 2019;20:5015. doi: 10.3390/ijms20205015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu WL, Shi JY, Jia Q, Li YM. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin. 2017;38:157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Q, Li Y, Cheng N, Sun W, Shi B. Effect of retinoic acid on the function of lipopolysaccharide-stimulated bone marrow stromal cells grown on titanium surfaces. Inflamm Res. 2015;64:63–70. doi: 10.1007/s00011-014-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Shan Y, Wu Y, Xu C, Yu X, Zhao J, Yan J, Shang W. Berberine suppresses LPS-induced inflammation through modulating Sirt1/NF-κB signaling pathway in RAW264.7 cells. Int Immunopharmacol. 2017;52:93–100. doi: 10.1016/j.intimp.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Fan C, Yan X, Lu X, Jiang H, Di S, Ma Z, Feng Y, Zhang Z, Feng P, et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother Res. 2019;33:130–148. doi: 10.1002/ptr.6206. [DOI] [PubMed] [Google Scholar]
- 33.Tao K, Xiao D, Weng J, Xiong A, Kang B, Zeng H. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol Lett. 2016;240:68–80. doi: 10.1016/j.toxlet.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Fiedler T, Salamon A, Adam S, Herzmann N, Taubenheim J, Peters K. Impact of bacteria and bacterial components on osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Exp Cell Res. 2013;319:2883–2892. doi: 10.1016/j.yexcr.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Chu M, Ding R, Chu ZY, Zhang MB, Liu XY, Xie SH, Zhai YJ, Wang YD. Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement Altern Med. 2014;14:89. doi: 10.1186/1472-6882-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 37.Gu C, Yin Z, Nie H, Liu Y, Yang J, Huang G, Shen J, Chen L, Fei J. Identification of berberine as a novel drug for the treatment of multiple myeloma via targeting UHRF1. BMC Biol. 2020;8:33. doi: 10.1186/s12915-020-00766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabatino L, Fucci A, Pancione M, Carafa V, Nebbioso A, Pistore C, Babbio F, Votino C, Laudanna C, Ceccarelli M, et al. UHRF1 coordinates peroxisome proliferator activated receptor gamma (PPARG) epigenetic silencing and mediates colorectal cancer progression. Oncogene. 2012;31:5061–5072. doi: 10.1038/onc.2012.3. [DOI] [PubMed] [Google Scholar]
- 39.Simonin MA, Bordji K, Boyault S, Bianchi A, Gouze E, Bécuwe P, Dauça M, Netter P, Terlain B. PPAR-gamma ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am J Physiol Cell Physiol. 2002;282:C125–C133. doi: 10.1152/ajpcell.2002.282.1.C125. [DOI] [PubMed] [Google Scholar]
- 40.Qu R-N, Qu W. Metformin inhibits LPS-induced inflammatory response in VSMCs by regulating TLR4 and PPAR-γ. Eur Rev Med Pharmacol Sci. 2019;23:4988–4995. doi: 10.26355/eurrev_201906_18090. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH. Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling. Stem Cells. 2013;31:2183–2192. doi: 10.1002/stem.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sergeeva MG, Aleshin SE, Grabeklis S, Reiser G. PPAR activation has dichotomous control on the expression levels of cytosolic and secretory phospholipase A2 in astrocytes; inhibition in naïve, untreated cells and enhancement in LPS-stimulated cells. J Neurochem. 2010;115:399–410. doi: 10.1111/j.1471-4159.2010.06931.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Wang P, Li T, Tian X, Guo W, Xu B, Huang G, Cai D, Zhou F, Zhang H, et al. Self-assemblies based on traditional medicine berberine and cinnamic acid for adhesion-induced inhibition multidrug-resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2020;12:227–237. doi: 10.1021/acsami.9b17722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.