Abstract
Summary: The complete form of the pli de passage fronto-pariétal moyen of Broca, a gyrus connecting the pre- and postcentral gyri at the level of the presumable primary motor (M1) hand area, represents a rare anatomic variation. By using functional MR imaging in a healthy subject incidentally found to harbor this configuration, we attempted to determine whether such an accessory gyrus would be functionally active and what effect it has on the complex somatotopic within-arm organization of M1. We found a specific and consistent activation pattern along the lateral and medial cortical boundaries of the pli de passage fronto-pariétal moyen. The gyrus completely segregated the M1 finger from the M1 elbow representation, one being laterally and the other medially located. Furthermore the M1 wrist representation was consistently split by the pli de passage fronto-pariétal moyen into a medial and lateral activation cluster. These findings demonstrate that this accessory gyrus not only contains functionally active neurons, but also leads to a functional separation of the motor homunculus at the level of the M1 wrist representation. This is a remarkable finding, because the region of within-arm representations in M1 was previously thought to be necessarily organized in a complex and intermingled fashion, without a topographic segregation between single body parts.
Broca (1) described in his detailed studies about the human cortical anatomy of the central region three folds that connect the pre- and postcentral gyri: 1) the pli de passage fronto-pariétal supérieur found at the interhemispheric fissure, corresponding to the paracentral lobule; 2) the pli de passage fronto-pariétal inférieur separating the central sulcus from the sylvian fissure, corresponding to the subcentral gyrus; and 3) the pli de passage fronto-pariétal moyen. The latter is described as a bulge into the central sulcus at the level of the middle knee of the central sulcus. It is always located within the sulcus, requires the gyri to be pulled apart to visualize it, and elevates the floor of the central sulcus. In 1892, Cunningham (2) confirmed the observations of Broca (1) by describing a deep annectant gyrus between the pre- and postcentral gyrus, which becomes apparent when the lips of the fissure of Rolando are drawn widely apart. All graduations between a mere shallowing with an interlocking of the adjacent walls of the fissure and the presence of a distinct deep annectant gyrus were described (2).
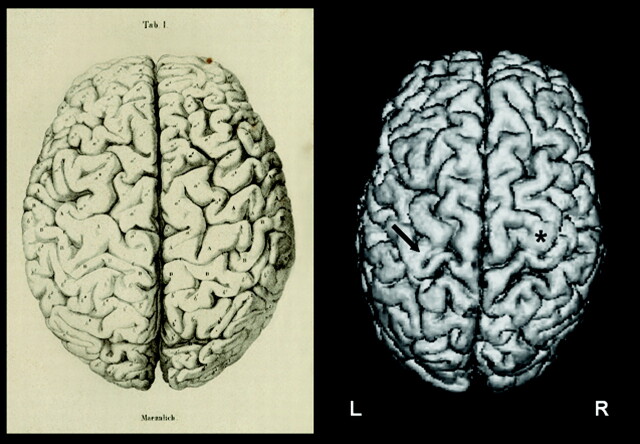
In rare cases, the deep annectant gyrus, or pli de passage fronto-pariétal moyen, arises completely to the cortical surface and cuts the fissure of Rolando into two separate parts. Whereas Cunningham did not observe this configuration in any of his anatomic specimens, Broca (1) found it to arise to the cortical surface in one brain, that of “an idiot, who was known to suffer from a large number of severe anomalies (page 72).” In fact, the first one to describe and to illustrate such an interruption of the Rolandic fissure appearing at the cortical surface was Wagner, in 1862 (3), who found this rare variation of the central region in the brain of a celebrated physician (Fig 1).
Fig 1.
The first description and illustration of the complete form of a pli de passage fronto-pariétal moyen was provided by Wagner in 1862 (3) (right hemisphere, left image). A superior view on the 3D surface reconstruction of the SPGR in our subject is shown on the right. Note the connecting gyrus (arrow) between the pre- and postcentral gyrus at the level of the middle genu of the central sulcus, as opposed to the common configuration of the central region in the opposite hemisphere (asterisk [*]). MR image is skipped for better comparison.
We report on a healthy subject with an incidentally found complete form of the pli de passage fronto-pariétal moyen of Broca appearing at the cortical surface and dividing the central sulcus into two parts. The functional role of such an accessory gyrus, which represents an anatomic connection between the primary motor (M1) and the primary somatosensory cortex (S1) has not yet been determined. We therefore used functional MR imaging 1) to investigate whether such a gyrus would be functionally active and 2) to determine what effect such an anatomic configuration would have on the somatotopic organization of the within-arm representations of M1.
Case Report
A healthy, 31-year-old, right-handed man without a history of neurologic or psychiatric illness underwent structural and functional MR imaging. T1-weighted whole-brain anatomic reference volume data sets with an isotropic spatial resolution of 1.2 mm were acquired with a 3D spoiled gradient echo sequence ([SPGR] TR/TE, 50/9 ms; flip angle, 45°). A 3D surface reconstruction of the SPGR image is provided in Figure 1. It demonstrates the pli de passage fronto-pariétal moyen of Broca in the left hemisphere as a connecting gyrus between the pre- and postcentral gyri at the level of the middle knee of the central sulcus, appearing at the cortical surface, and dividing the central sulcus into two separate parts. As an additional more common anatomic variant, the superior frontal sulcus intersects the central sulcus medially to the pli de passage fronto-pariétal moyen.
Functional MR Imaging Methods.
Blood oxygen level–dependent functional MR imaging (field strength, 1.5 T; pulse sequence, gradient-echo echo-planar; resolution, 2 × 2 × 4 mm) was performed in this subject and findings were compared with the functional MR imaging results of 12 healthy volunteers (4). All 12 controls harbored the common anatomic configuration with an omega-shaped precentral knob at the level of the middle knee of the precentral gyrus (5). After motion correction of the data, single subject analyses by using the linear model were computed. Further details about the anatomic definitions of M1, the functional MR imaging methods, and postprocessing of the data are described elsewhere (4, 6). Three series of simple, self-paced movements with closed eyes at a rate of 0.5 Hz were performed: repetitive, sequential finger-to-thumb opposition of the digits 2, 3, 4 and 5 of the right hand; repetitive flexion and extension of the right wrist; and flexion and extension movements of the right elbow. To test for consistency of the functional MR imaging data, all experiments were repeated within 1 week.
Functional MR Imaging Results.
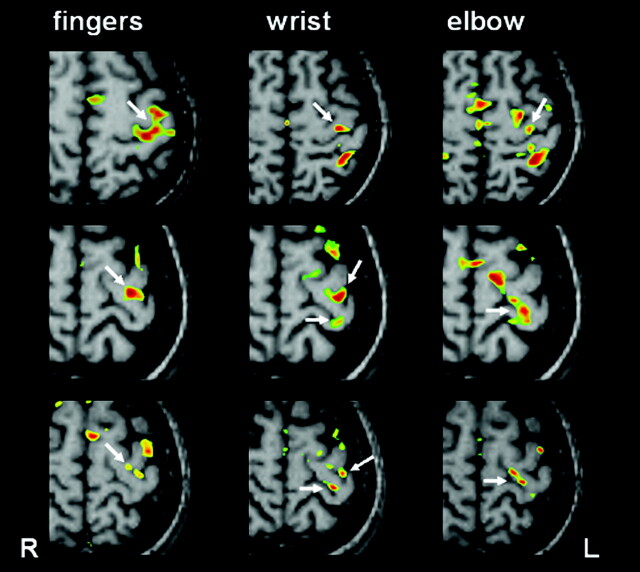
A representative example of a control subject showing the typical M1 activation patterns for the finger, wrist, and elbow movements is provided in Figure 2 (upper row). The M1 activation during finger movements is located along the omega-shaped knob of the precentral gyrus. The M1 wrist and elbow representations were located more medially, posteriorly, and superiorly along the course of the precentral gyrus, as compared with the M1 finger representation. Despite the clear small-scale somatotopic gradient, there is a significant overlap of the individual within-forearm representations (4). Figure 2 also demonstrates the M1 activation patterns obtained in the first (middle row) and second functional MR imaging session (lower row) in the subject harboring the pli de passage fronto-pariétal moyen. When compared with the controls, the following and consistent changes were detected: 1) Activation was present both along the lateral and medial cortex of the pli de passage fronto-pariétal moyen, depending on which movements tested. This indicates that this accessory gyrus may contain functionally active neurons. 2) The M1 finger representation was located laterally and anteriorly at the junction of the pli de passage fronto-pariétal moyen with the lateral part of the precentral gyrus. 3) The M1 wrist representation was consistently split into two parts, one located on the lateral and more anterior cortex and the other on the medial and more posterior cortex of the pli de passage fronto-pariétal moyen. 4) The M1 elbow representation is located entirely along the medial cortical wall of the pli de passage fronto-pariétal moyen. 5) This leads to an overlap of M1 finger and wrist representations on the lateral aspect and to an overlap of M1 elbow and wrist representations on the medial aspects of the pli de passage fronto-pariétal moyen. 6) The M1 finger and elbow representations are completely separated from each other, a finding never observed in any of the 12 controls (4).
Fig 2.
M1 activation patterns in a control subject harboring the common configuration of the central region (upper row) and in the subject harboring the pli de passage fronto-pariétal moyen (first functional MR imaging session: middle; second session: lower row). Activation was present for each movement tested along the lateral and/or medial border of the accessory gyrus (arrows). The M1 wrist representation was consistently split into two parts, one being laterally, the other medially located. The M1 finger and elbow representations were completely separated from each other.
Discussion
Embryology of the Central Sulcus
According to Retzius (7), the primordium of the central sulcus appears between the 5th and 6th month of fetal life. It develops in two separate and distinct portions, the lower appearing in the form of a shallow oblique groove, and the upper appearing in the form of a deep pit or depression (2). An eminence separates these two parts of the sulcus; however, soon a faint furrow runs over the summit of this elevated intervening piece of cortex, and the two primitive portions of the sulcus are partially united to each other. As development goes on, the more complete the union becomes, and the more fully is the intervening eminence borne down into the sulcus. The intervening portion is never entirely obliterated, but is still to be discerned in the bottom of the sulcus; ie, the pli de passage fronto-pariétal moyen of Broca (1) or deep annectant gyrus of Cunningham (2). In very rare cases, as in the subject of the present study, the two original portions of the fissure of Rolando remain distinct throughout life, and the intervening bridge of cortex remains on the surface and is not depressed by the fusion of the upper and lower divisions of the sulcus. The presence of an annectant gyrus appearing to the cortical surface might be therefore an expression of the remains of a fetal condition.
Function and Structure of the Central Region
The human central sulcus harbors M1 in its anterior wall (Brodmann area 4), and a major portion of S1 (Brodmann area 3) in its posterior wall. Despite some interindividual and interhemispheric variations in the superficial appearance of the central sulcus, the cytoarchitectonic boundaries that demarcate these areas from each other show a considerable consistency in the distribution along the central sulcus with respect to the crests of the pre- and postcentral gyri and the fundus of the sulcus (8). Neural elements subserving motor hand functions have been shown to be located in a characteristic, omega-shaped precentral knob, which becomes evident on an axial plane through the central sulcus at the level of the interdigitation of the pre- and postcentral gyri (5). This is exactly that portion of the central sulcus, where in the fundus the pli de passage fronto-pariétal moyen as an annectant gyrus becomes apparent (8, 9).
Since the original observations of Jackson (10), the debate over whether various body parts activate topographically separate subregions within the contralateral M1 in a somatotopic fashion has continued. Whereas a large-scale somatotopy within M1 is no longer questioned, the topographic organization of the within-arm representations in humans has triggered many investigations. A body of experimental evidence suggested alternative organizational principles, most indicating a major spatial overlap with no evidence for somatotopy of finger, hand, and wrist representations (11, 12). In a recent functional MR imaging study readdressing this issue, however, a fine-scale somatotopic organization in the forearm representations with statistically significant differences in the geometric centers of activation was found, despite a considerable overlap of the activated volumes (4). This indicated a clear fine-scale somatotopic gradient for the wrist, elbow, fingers, and hand and suggested that such a topography would be functionally relevant to smoothly and promptly organize the muscle synergies required by most movements.
It is known that the presence of striking topographic variations may obscure and divert structural-functional relationships in the human brain (2). The particular anatomic configuration in the subject reported herein represents therefore a good paradigm to test what effect a macroanatomic separation of the central sulcus at the level of the presumable M1 arm and hand representation would have on its functional organization. Indeed, our study revealed functional variations in the M1 somatotopy, in that the entire M1 finger area was located laterally, and the entire M1 elbow area medially to the pli de passage fronto-pariétal moyen, without any overlap between these two representations. Moreover, the M1 wrist representation was consistently divided by the pli de passage fronto-pariétal moyen into a lateral and medial part, only the corresponding parts showing an overlap with the adjacent finger or elbow representations.
Van Essen (13) hypothesized in his theory of brain morphogenesis, that the tension along axons in the white matter can explain the characteristic folding of the cerebral cortex. For example a gyrus may develop within a region of functionally related cortex to allow for efficient axonal connectivity between its opposite cortical walls (13). The observed parallel activation of both the opposite lateral and medial cortex of the pli de passage fronto-pariétal moyen during the movement of a single body part possibly reflects these connections across the gyrus. These may even develop in case of an anatomic variation to allow for smooth and organized movements.
Acknowledgments
This work was supported by the Swiss National Foundation (grant NRP 38 4038–052837/1) and by the National Center of Competence in Research on Neural Plasticity and Repair.
References
- 1.Broca P. Description elémentaires des circonvolutions cérébrales de l’homme. Mémoires d’Anthropologie. Memo Paris: C. Reinwald;1888. :707–804
- 2.Cunnigham DJ. The fissure of Rolando. Contributions to the Surface Anatomy of the Cerebral Hemispheres. Cunnigham Memoirs No. 7. Dublin: Academy House;1892. :161–193
- 3.Wagner R. Vorstudien zu Einer Wissenschaftlichen Morphologie und Physiologie des Menschlichen Gehirns als Seelenorgan. Vol 2 . Göttingen: Verlag der Dieterichschen Buchhandlung;1862. :1–105 [Google Scholar]
- 4.Alkadhi H, Crelier GR, Hotz Boendermaker S, et al. Reproducibility of primary motor cortex somatotopy under controlled conditions. AJNR Am J Neuroradiol 2002;23:1524–1532 [PMC free article] [PubMed] [Google Scholar]
- 5.Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain 1997;120:141–157 [DOI] [PubMed] [Google Scholar]
- 6.Kollias SS, Alkadhi H, Jaermann T, et al. Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res Rev 2001;36:185–195 [DOI] [PubMed] [Google Scholar]
- 7.Retzius G. Das Menschenhirn: Studien in der Makroskopischen Morphologie. Stockholm: PA Norstedt;1896. :1–167
- 8.White LE, Andrews TJ, Hulette C, et al. Structure of the human sensorimotor system. I. Morphology and cytoarchitecture of the central sulcus. Cereb Cortex 1997;7:18–30 [DOI] [PubMed] [Google Scholar]
- 9.Boling W, Olivier A, Bittar RG, Reutens D. Localization of hand motor activation in Broca’s pli de passage moyen. J Neurosurg 1999;91:903–910 [DOI] [PubMed] [Google Scholar]
- 10.Hughlings Jackson J. Convulsive spasms of the right hand and arm preceding epileptic seizures. Med Times Gaz 1863;1:589 [Google Scholar]
- 11.Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science 1993;261:489–492 [DOI] [PubMed] [Google Scholar]
- 12.Sanes JN, Donoghue JP, Thangaraj V, et al. Shared neural substrates controlling hand movements in human motor cortex. Science 1995;268:1775–1777 [DOI] [PubMed] [Google Scholar]
- 13.Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997;385:313–318 [DOI] [PubMed] [Google Scholar]