Abstract
Summary: The blood-brain barrier disruption (BBBD) procedure is an established strategy to enhance drug delivery to brain tumors. Complication rates associated with this procedure are usually low, but when complications do occur, they usually mandate discontinuation of treatment. Orbital pseudotumor is an inflammatory condition of one or more extraocular muscles that produces limitation of ocular motility. Patients usually experience sudden diplopia associated with orbital pain, conjunctival chemosis and injection, and proptosis. Imaging of the orbit shows diffuse enlargement of the extraocular muscles, exophthalmia, and, rarely, sinusal or intracranial infiltration. On pathologic examinations, the soft tissues of the orbit are infiltrated with a mixture of eosinophils, lymphocytes, and plasma cells. Many etiologies can induce this syndrome, including the intracarotid infusion of platinum molecules. As part of a phase II study, a total of 110 patients were treated for malignant brain tumors with intra-arterial carboplatin, enhanced by the BBBD procedure, at the Sherbrooke University Hospital. Here we report on three patients who developed orbital pseudotumor ipsilateral to the carotid infused a few hours to days after the procedure. After the occurrence of this syndrome in the first patient, we developed a technical modification to the procedure that enabled uninterrupted treatment in the other two patients. This modification was as follows: after the mannitol infusion, and before carboplatin, the catheter was changed for a 3.5 tracker and was repositioned just above the emergence of the ophthalmic artery.
Birch-Hirschfel first described the syndrome of orbital pseudotumor in 1905. Orbital pseudotumor is an inflammatory condition of one or more extraocular muscles that produces limitation of ocular motility; it may be bilateral and recurrent. Patients usually experience painful eye movements, lid edema, conjunctival hyperemia and chemosis, poor extraocular motility, diplopia due to restriction of extraocular muscle movement, and proptosis (1–3). Many diseases are associated with orbital myositis, including Crohn disease, Lyme disease, Wegener granoulomatosis, paraneoplastic syndrome in non-Hodgkin lymphoma, psoriatic arthropathy, systemic lupus erythematosus, and rheumatoid arthritis (4, 5). The major differential diagnosis includes thyroid ophthalmopathy, orbital cellulitis, metastasis, Tolosa-Hunt syndrome, trochleitis, and infectious myositis due to trichinosis (4).
Imaging of the orbit typically shows diffuse enlargement of the extraocular muscles, exophthalmia, and, rarely, sinusal or intracranial infiltration. Pathologic examination depicts orbital soft tissues (fat, muscles, lacrimal glands, and optic nerve) infiltration with a mixture of eosinophils, lymphocytes, and plasma cells. Proliferating fibrous tissue is also seen (5). Typically, a rapid clinical response to steroids is observed. Cases resistant to steroids can be treated with radiation therapy and chemotherapy.
To date, five cases of orbital myositis related to chemotherapy have been described in the literature: one case each related to nimustine/carmustine, and a combination of carboplatin and etoposide, and two cases related to cisplatin (6–8).
Platinum salts are broad-spectrum alkylating agents composed of a central platinum atom attached to two ammonia groups and one or more leaving groups, which confer their properties to the molecule (9–11). The antineoplastic effects of platinum compounds are exerted through induction of DNA and RNA alkylation and cytoplasmic proteins modifications. Carboplatin has been shown to have activity in the treatment of malignant gliomas in numerous clinical studies (12–18). The effectiveness of carboplatin is dose-dependent, however, and this drug does not cross the intact blood-brain barrier (BBB) well when administered intravenously.
The BBB disruption (BBBD) procedure is an established strategy to enhance transport of a drug across the BBB. Williams et al have shown that intra-arterial administration with BBBD increases delivery of carboplatin to the tumor, brain around tumor, and brain distant to tumor (19). Moreover, Doolittle et al have reported on the safety of this approach in a multi-institutional setting (20).
We use carboplatin as part of a tridrug regimen in a phase II study on BBBD to enhance delivery of chemotherapy. In this regimen, the carboplatin is administered intra-arterially after osmotic opening of the BBB, with the goal of significantly increasing its regional area under the curve, while minimizing streaming (21, 22). In the context of this phase II study, a total of 110 patients with different histologic malignant brain tumors subtypes were exposed to this treatment technique at our institution (23).
In this technical note, we report ocular toxicity in the form of orbital pseudotumor in three patients treated with BBBD and intra-arterial carboplatin. Although treatment was discontinued in the first patient, we designed a simple modification to the procedure that allowed treatment continuation in the two subsequent patients.
BBBD Technique and Carboplatin Infusion
The procedure has been described extensively elsewhere and is conducted in the angiography suite (24). In brief, the patient is placed under general anesthesia. A 5F transfemoral intra-arterial catheter is placed in the parent vessel responsible for most the tumor irrigation (right or left internal carotid artery, dominant vertebral artery). The catheter is placed at the level of C1–C2 for the carotid circulation, and at the level of C5–C6 for the vertebral circulation. Via this catheter, BBBD is performed by the infusion of warmed mannitol (25%) administered at a predetermined flow rate to completely fill the vascular tree for 30 seconds. Five minutes after the completion of the mannitol infusion, intracarotid carboplatin (400 mg/m2) is infused at a rate of 0.2 mL/s to limit streaming. The other chemotherapy agents are infused intravenously.
Case Reports and Technical Modification
Patient 1
A 41-year-old white male patient was referred to our center 10 years after having been diagnosed with a temporal low-grade astrocytoma and after undergoing partial resection. The patient was reoperated in August 2000, and a diagnosis of right-sided anaplastic oligodendroglioma was reported. The samples from the first surgery were requested and reanalyzed, and the diagnosis was changed to that of an oligodendroglioma. The patient qualified for BBBD and intra-arterial carboplatin administration and, after giving consent, was enrolled in the phase II study.
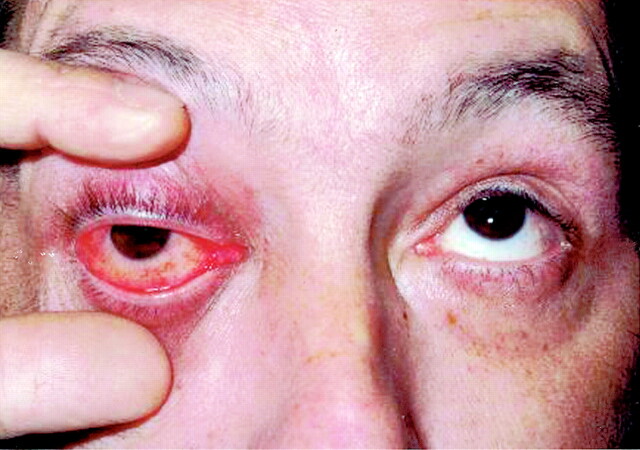
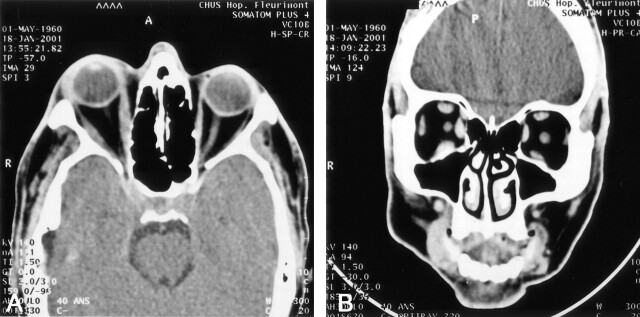
The first cycle was well tolerated. Approximately 36–48 hours after the second treatment, upon awakening, he presented a painful swelling of the right orbit, with blurred vision, ptosis, chemosis, and limitation of ocular motility. The examination depicted a visual acuity of 20/200, lid swelling with a complete ptosis, exophtalmia, relative afferent papillary defect, and complete paresis of outward and upward movements of the right eye (Fig 1). Imaging of the orbit with CT demonstrated a right-sided exophtalmia, fat infiltration, swelling of intraorbital muscles and lacrimal gland, confirming the diagnosis of orbital myositis (Fig 2). The patient was treated with a 3-day course of 1 g intravenous solumedrol followed by 100 mg prednisone for 14 days and then gradual weaning. Conjunctival biopsy at day 2 revealed inflammatory changes. Intra-arterial carboplatin was discontinued, and the patient was offered oral temozolomide. On 5-week follow-up, the patient had recovered well. His right eye vision improved to 20/40, color vision was 10/10, and no proptosis or ophthalmoplegia was observed. The patient has discontinued temozolomide after three cycles because of poorly tolerated side effects. The patient is still alive but presents a gradual decline in his neurologic condition.
Fig 1.
Photograph of patient 1, taken 12 hours after he presented with the first stigmata of the pseudotumor syndrome. Note the exophthalmia, hyperemia, and chemosis of the right eye.
Fig 2.
Axial (A) and coronal (B) CT images, demonstrating the right-sided exophthalmia, fat infiltration, and swelling of intraorbital muscles and lacrimal gland in patient 1. CT was performed 14 hours after the initiation of the pseudotumor syndrome.
Patient 2 and Technical Modification
A 67-year-old white female patient with a left frontal metastasis of a B-cell systemic lymphoma was referred to our institution after not responding to radiation therapy and high-dose systemic methotrexate. This patient showed excellent response throughout nine cycles of BBBD and intra-arterial carboplatin. Forty-eight hours after administration of the 9th cycle, she observed a slight swelling of the left eye that eventually progressed to a painful swelling and ptosis. The neurologic examination demonstrated, in addition to the ptosis and swelling of the eyelid, a visual acuity of 20/200 (20/30 right eye), a complete downward paresis, and moderate abduction paresis of the left eye. Brain MR imaging obtained 12 hours after the onset of this syndrome demonstrated a left-sided exophtalmia with swelling and enhancement of all left extraocular muscles (Fig 3). She was treated with steroids and responded equally well.
Fig 3.
MR characteristics of the pseudotumor syndrome as displayed in patient 2. A, Axial T1-weighted brain MR imaging highlighting the left-sided exophthalmia and swelling of the extraocular muscles. B, Contrast-enhanced T1-weighted MR study in the same patient depicting enhancement of the extraocular muscles of the left orbital cavity.
The excellent response of the frontal metastasis to our therapeutic regimen (Fig 4), combined with the lack of alternatives, prompted us to continue therapy and attempt supraophthalmic infusion of carboplatin to bypass the emergence of the ophthalmic artery. To do so, the BBBD procedure was performed as described above. After mannitol infusion, and before carboplatin, however, the catheter was changed for a 3.5 tracker and was repositioned just above the emergence of the ophthalmic artery (Fig 5). The patient underwent her 10th and 11th cycles without any ocular adverse events. Two weeks after the 11th cycle, she was admitted in another institution for a sudden onset of severe dyspnea and died of a massive pulmonary embolism.
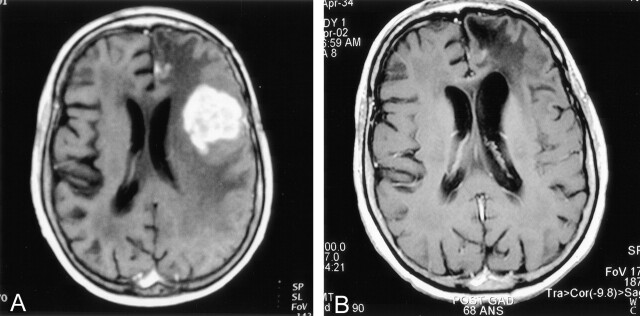
Fig 4.
Response obtained in the treatment of a 67-year-old patient with a primary CNS lymphoma (patient 2). Before study accrual, the patient had been treated with high-dose intravenous methotrexate and radiation and had not responded to these modalities. A, Axial T1-weighted brain MR image obtained at study accrual in April 2001. The highly and homogeneously left frontal enhancing lesion is obvious, as well as the surrounding vasogenic edema. A mass effect is produced on the ipsilateral ventricle, as well as on the gyri. B, Axial T1-weighted brain MR image obtained before the 10th cycle, in April 2002. The tumoral nodule has completely receded, and presence of left frontal encephalomalacia and a vacuum effect on the left frontal horn translate the decrease in mass effect.
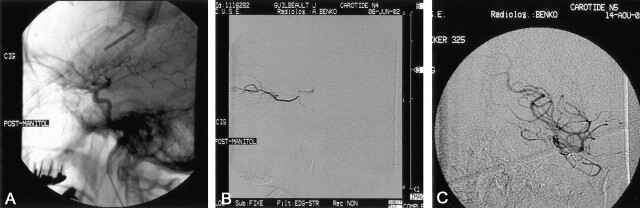
Fig 5.
The modified BBBD technique applied in patient 2. The patient had just undergone mannitol infusion. A, The catheter has been changed for a 3.5 microcatheter tracker and a first infusion is accomplished. B, The catheter is moved caudally until the identification of the origin of the ophthalmic artery. C, The catheter is positioned just above the origin of the ophthalmic artery to bypass it. Chemotherapy is infused in a supraophthalmic setting after the BBB has been disrupted by using the standard infraophthalmic carotid mannitol infusion.
Patient 3
A 59-year-old female patient, known for a bronchogenic adenocarcinoma, presented with gradual onset of right hemiparesis and hemihypesthesia. Investigation demonstrated the presence of a unique left parietal metastasis. She refused surgery and was enrolled and began treatment with intra-arterial carboplatin and BBBD in May 2002. Two days after the second cycle, she developed orbital swelling and pain. Decadron dosage was increased for 2 weeks, and we observed complete resolution of the symptomatology.
By the same technique of supraophthalmic catheterization post BBBD, the 3rd–5th cycles were performed without recurrence of the orbital myositis. Two days after her 6th cycle, however, signs of ocular toxicity recurred despite the supraophthalmic injection of carboplatin. The position of the catheter was borderline, and it is presumed that the catheter slipped back below the origin of the ophthalmic artery, although this was not controlled. Treatments were discontinued. The patient presented in the following months a progression of her primary disease and died of respiratory insufficiency in July 2002.
Discussion
Orbital pseudotumor is an inflammatory condition of one or more extraocular muscles and produces limitation of ocular motility. Many etiologies are responsible for orbital myositis, but rarely has it been associated with chemotherapy. The rapid response of orbital myositis to corticosteroids is almost diagnostic (4). The three patients reported in our series demonstrated significant improvement in the first few days on corticosteroids and almost complete resolution of there symptomatology after 4–9 weeks of treatment.
Intracarotid platinum compounds are widely used to treat a variety of malignant tumors. Numerous studies have established the activity of these molecules in the treatment of malignant brain tumors (12–18). The antineoplastic effects of platinum compounds are exerted through induction of DNA and RNA alkylation and cytoplasmic proteins modifications.
Intra-arterial administration following BBBD improves delivery of antitumoral drugs to the tumor and the hemisphere involved and allows higher efficiency with less systemic toxicity. Intracarotid administration, however, might be associated with higher ocular and neural toxicity because of the infusion of highly concentrated drugs. Ocular, retinal, and neural toxicity have been described with cisplatin administration and, less commonly, with carboplatin. Supraselective catheterization distal to the ophthalmic artery has been shown to reduce retinal and ocular toxicity associated with cisplatin infusion. Kupersmith et al (6) demonstrated a decrease in ocular toxicity associated with cisplatin or carmustine with supraophthalmic infusion; however, reports of severe ocular toxicity ipsilateral to a supraophthalmic infusion of cisplatin with or without nimustine have been outlined by several authors (7, 8).
To conserve its theoretical advantage, the BBBD procedure should be accomplished in a parent vessel, not in a supraselective way. Malignant gliomas are infiltrative lesions, and tumor cells have been identified at a distance from the radiologic abnormalities on MR images and isolated from areas appearing normal at pathologic examination in brain tumor patients (25). As for metastatic disease, the presence of multiple lesions is not unusual. Thus, the procedure should cover the greatest vascular distribution feasible. The orbital pseudotumor syndrome has never been observed in patients treated with BBBD and intra-arterial methotrexate infusion (26). We therefore concluded that the intra-arterial infusion of mannitol was not directly responsible for the syndrome and that repeated mannitol infusion could safely be accomplished even in patients who presented with the condition. After disruption, the position of a catheter just above the origin of the ophthalmic artery required to change the 5F catheter for a 3.5 tracker, a catheter that would be inappropriate for the infusion of high-flow mannitol, but that is ideal for slow delivery of chemotherapy. Thus, it appeared logical to accomplish disruption and change the catheter for the chemotherapy infusion in a supraophthalmic fashion. The theoretical risk of using a smaller catheter to infuse chemotherapy involves the possibility of increasing streaming that could, in turn, increase neurotoxicity (22). Nonetheless, by modifying the rate of administration, this risk can be minimized (22).
Patient 3 presented a relapse in her condition after uneventfully having undergone three additional cycles with supraophthalmic infusion. Although this is pure speculation, we believe that the catheter, having been placed just above the ophthalmic artery and twice slipping back below before chemotherapy infusion, moved and again fell below during the infusion. The catheter was checked just before the initiation of chemotherapy infusion, but, unfortunately, not thereafter.
Before this report, the occurrence of orbitalmyositis had always been considered an absolute contraindication to treatment continuation in centers performing intra-arterial chemotherapy and BBBD (20). We believe that this should no longer be considered the case and that the modification to the technique of BBBD described in this report should be considered in these circumstances.
References
- 1.Miller NR, Newman NJ. Walsh & Hoyst’s clinical neuro-ophthalmology: the essentials. 5th edition. Philadelphia: Lippincott, Williams and Wilkins;1998. :677–679
- 2.Lee MS, Lessell S. Orbital myositis posing as cluster headache. Arch Neurol 2002;59:635–636 [DOI] [PubMed] [Google Scholar]
- 3.Maurer I, Zierz SJ. Recurrent orbital myositis: report of a familial incidence. Arch Neurol 1999;56:1407–1409 [DOI] [PubMed] [Google Scholar]
- 4.Nabili S, McCarey DW, Browne B, Capell HA. A case of orbital myositis associated with rheumatoid arthritis. Ann Rheum Dis 2002;61:938–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollard Z. Acute rectus muscle palsy in children as a result of orbital myositis. J Pediatr 1996;128:230–233 [DOI] [PubMed] [Google Scholar]
- 6.Kupersmith MJ, Frohman LP, Choi IS, et al. Visual system toxicity following intra-arterial chemotherapy. Neurology 1988;38:284–289 [DOI] [PubMed] [Google Scholar]
- 7.Margo CE, Murtagh FR. Ocular and orbital toxicity after intracarotid cisplatin therapy. Am J Ophthalmol 1993;116:508–509 [DOI] [PubMed] [Google Scholar]
- 8.Wu HM, Lee AG, Lehane DE et al. Ocular and orbital complications of intraarterial cisplatin. J Neuroophthalmol 1997;17:195–198 [PubMed] [Google Scholar]
- 9.O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical pharmacokinetics and administration of established platinum drugs. Drugs 2000;59(Suppl)4:S19–S27 [DOI] [PubMed] [Google Scholar]
- 10.Selvaratnam G, Philips RH, Mohamed AK, Radzi A. Adverse effects of cytotoxics-platinum agents. Adverse Drug React Toxicol Rev 1997;16:171–197 [PubMed] [Google Scholar]
- 11.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol 1999;17:409–422 [DOI] [PubMed] [Google Scholar]
- 12.Yung WKA, Mechitler Laszlo, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a phase II study. J Clin Oncol 1991;9:860–864 [DOI] [PubMed] [Google Scholar]
- 13.Twelves CJ, Ash CM, Miles DW, et al. Activity and toxicity of carboplatin and iproplatin in relapsed high-grade glioma. Cancer Chemother Pharmacol 1991;27:481–483 [DOI] [PubMed] [Google Scholar]
- 14.Warnick RE, Prados MD, Mack EE, et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neuro-Oncol 1994;19:69–74 [DOI] [PubMed] [Google Scholar]
- 15.Lunardi P, Farah JO, Mastronardi L, et al. Intravenous administration of high doses of carboplatin in multimodal treatment of high grade gliomas: a phase II study. Acta Neurochirurgica 1996;138:215–220 [DOI] [PubMed] [Google Scholar]
- 16.Prados MD, Warnick RE, Mack EE, et al. Intravenous carboplatin for recurrent gliomas. Am J Clin Oncol 1996;19:609–612 [DOI] [PubMed] [Google Scholar]
- 17.Friedman HS, Shelley L, Rasheed K, Friedman AH. Treatment of adults with progressive oligodendroglioma with carboplatin (CBDCA): preliminary results. Med Pediatr Oncol 1998;31:16–18 [DOI] [PubMed] [Google Scholar]
- 18.Huncharedk M, Kupelnick B, Bishop D. Platinum analogues in the treatment of recurrent high grade astrocytoma. Cancer Treat Rev 1998;24:307–316 [DOI] [PubMed] [Google Scholar]
- 19.Williams PC, Henner WD, Roman-Goldstein S, et al. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery 1995;37:17–27 [DOI] [PubMed] [Google Scholar]
- 20.Doolittle ND, Miner ME, Hall WA, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000;88:637–647 [DOI] [PubMed] [Google Scholar]
- 21.Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery 1998;42:1083–1100 [DOI] [PubMed] [Google Scholar]
- 22.Fortin D, McAllister LD, Nesbit G, et al. Unusual cervical spine cord toxicity associated with intra-arterial carboplatin, intra-arterial or intravenous etoposide phosphate, and intravenous cyclophosphamide with osmotic blood brain-barrier disruption in the vertebral artery. AJNR Am J Neuroradiol 1999;20:1794–1802 [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins A, Boudrias M, Fortin D. Osmotic blood-brain barrier disruption to enhance chemotherapy delivery in the treatment of brain tumors: the Sherbrooke experience. A-07. Can J Neurol Sci 2002; (Suppl 1):S9. [DOI] [PubMed]
- 24.Fortin D, Neuwelt EA Therapeutic Manipulation of the Blood-Brain Barrier: Neurobase-Neurosurgery. 1st ed. San Diego: Medlink Neurology;2002
- 25.Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg 1997;86:525–531 [DOI] [PubMed] [Google Scholar]
- 26.McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 2000;46:51–61 [PubMed] [Google Scholar]