Abstract
Summary: Matched mask bone elimination (MMBE) is a technique for the automatic removal of bone pixels from CT angiography data sets. We describe the use of this technique in two patients with the clinical suspicion of dural sinus thrombosis. We conclude that multisection CT venography with MMBE is a useful adjunct for the visualization of the intracranial venous circulation by removing bone from the image. In contrast to techniques described previously, MMBE is fully automated and operator independent.
CT angiography (CTA) is widely used for imaging intracranial arteries and veins. One disadvantage of conventional CTA is that time-consuming and operator-dependent editing is needed to remove overprojecting bone for the angiographic display of intracranial vessels. Recently a new, fully automatic masking technique called matched mask bone elimination (MMBE) was developed to remove bone pixels from CTA data sets (1). In this report, we describe the use of MMBE for the angiographic display of multisection CTA data sets of the intracranial venous circulation in two patients with the clinical suspicion of dural sinus thrombosis.
Case Reports
Technique
In each of the two patients, two spiral CT scans of the head were made by using a CT scanner with a quadruple detector array (Mx8000; Philips Medical Systems, Best, the Netherlands). First, before the injection of contrast agent, an unenhanced spiral CT scan was made. After that, the CT venography examination was performed. For the CT venography examination, a spiral CT scan was made with a field of view of 210 mm, collimation of 4 × 1 mm, effective section thickness of 1.3 mm, increment of 0.5 mm, scan time of 0.75 seconds per 360° rotation, tube voltage of 90 kV, 360 mAs/section, and a table of feed of 4.7 mm/s (pitch 0.875). Nonionic contrast material (Omnipaque [Iohexol] containing 300 mg of iodine per milliliter; Nycomed, Amersham Health, Cork, Ireland) was injected in a cubital vein at 4 mL/s for a total of 120 mL. Scanning was started after a delay time that was measured with a delay test. The gantry was angulated to exclude the orbits from the acquired volume. A cephalic-to-caudal scanning progression was used. The unenhanced CT scan was made with a 100 mAs/section; ie, approximately one-quarter of the mAs value of the CT venography examination. All other parameters were the same as those for the contrast-enhanced CT scan. The duration of one helical CT scan was 30 seconds. The total effective dose was 1.4 mSv (including 0.3 mSv for the unenhanced scan and 1.1 mSv for the enhanced scan) as compared with an effective dose for conventional CT of the head of 1.2mSv (120 kV, 250 mAs, 4 × 5 mm collimation). Reconstructions were made with a voxel size of 0.41 × 0.41 × 0.50 mm3, 180° interpolation, and reconstruction kernel B (normal filter between smooth and medium sharp). All scans were processed off-line on a personal computer, equipped with a 650-MHz Pentium Pro processor (Intel, Santa Clara, CA).
The principle of the MMBE method is that the bone voxels are identified in the unenhanced data set and that the corresponding voxels in the CTA data set are given an arbitrarily low value (1). A key step in the MMBE procedure is the compensation for movements of the patient in between the two scans. Even minimal movements lead to serious artifacts in the processed images if not compensated. This compensation can be achieved by 3D registration of the unenhanced CT scan with the contrast-enhanced CT scan. For this purpose, a fully automatic 3D registration method was used. The registration procedure starts with an initialization step using chamfer matching, which has a capture range in the order of a few centimeters, to detect even large displacements of the patient automatically (2, 3). Next, the mean of the squared differences between the pixel values of each contrast-enhanced image and the corresponding pixel values in a cross-section of the 3D unenhanced data set were minimized, by using the downhill simplex method (4). To achieve an optimal match between the bony structures in both images, only pixels were used with CT values between 600 and 800 HU, which represent the edges of the bone. After registration, a threshold of 200 HU was applied to identify bone pixels. This threshold gave slightly better results than the threshold of 150 HU used by Venema et al (1) because of the lower tube voltage used in the present study (90 kV instead of 120 kV). The mask was slightly widened by means of 1 pixel to allow for partial volume effects and slight amounts of mismatch. The processing for the complete MMBE procedure took slightly more than 15 minutes. Finally, multiple intensity projection (MIP) images free from overprojecting bone were made in the conventional way on a graphical workstation (MxView; Philips Medical Systems). Limited manual editing was performed to remove some overprojecting arteries on the MIP data set.
Case 1
A 45-year-old woman presented with headache and clinical signs of intracranial hypertension. To exclude dural sinus thrombosis, CT venography was performed. After the MMBE procedure, no bone remnants were present in the CTA data set. The axial sections and the MIP images demonstrated patent dural sinuses (Fig 1). No thrombus was seen. The dural sinuses, cortical veins, and deep venous system were demonstrated with exquisite detail.
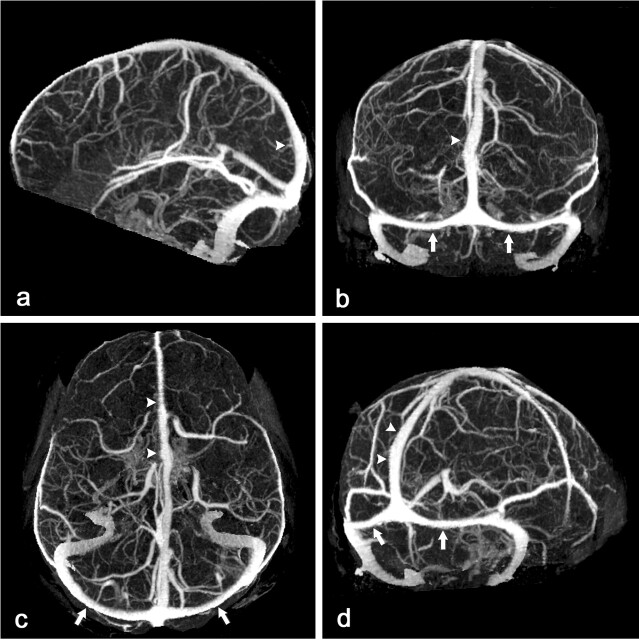
Fig 1.
CT scan of a 45-year-old woman with clinical suspicion of dural sinus thrombosis.
Lateral (A), anteroposterior (B), caudocranial (C), and oblique sagittal (D) MIP of CTA data set after MMBE. The projections demonstrate normal appearance of the superior sagittal sinus (arrowheads), the transverse sinuses (arrows), the deep venous system, and the superficial cortical veins without any overlying bone structures.
Case 2
A 27-year-old man presented with fever, headache, and some weakness of the right arm. Neurologic examination revealed bilateral papilledema. To exclude dural sinus thrombosis, CT venography was performed. After the MMBE procedure, no bone remnants were present on the MIP images obtained from the CTA data set. The axial sections and the MIP images of the CT venography data set demonstrated a filling defect in the superior sagittal and bilateral transverse dural sinuses, consistent with thrombosis (Fig 2). Some enhancement of the dural sinus wall was present. The superficial cortical veins and deep venous system were patent. The subependymal and transmedullary veins were dilated.
Fig 2.
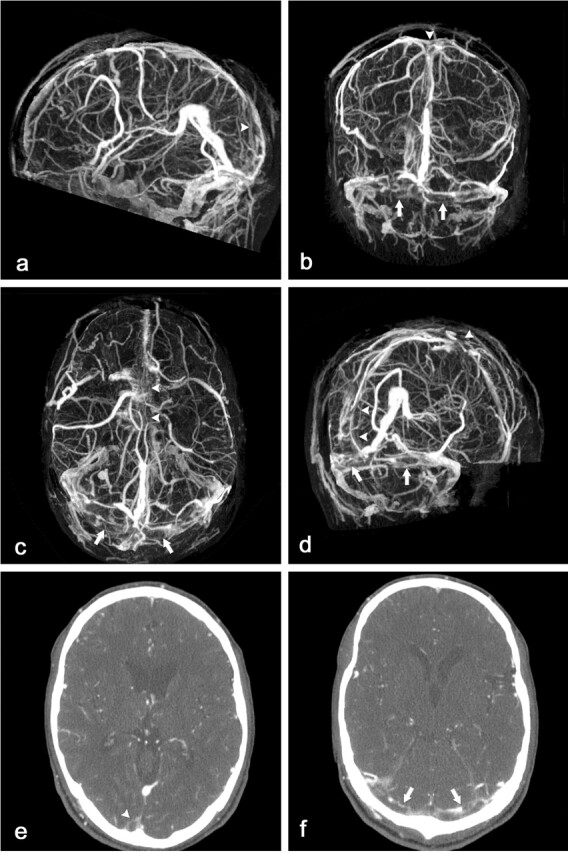
CT scan of a 27-year-old man with clinical suspicion of dural sinus thrombosis.
Lateral (A), anteroposterior (B), caudocranial (C), and oblique sagittal (D) MIP of CTA data set after MMBE demonstrates complete bone removal and occlusion of the superior sagittal sinus (arrowheads) and both transverse sinuses (arrows). Note some overprojecting enhancement of the transverse sinus walls. The superficial cortical veins and deep venous system are patent.
Axial contrast-enhanced 1-mm sections demonstrate filling defects in the superior sagittal sinus (E, arrowhead) and both transverse sinuses (F, arrows).
Discussion
Previous reports have noted that CT venography has a high sensitivity for depicting the intracerebral venous circulation as compared with digital subtraction angiography (5). CT venography is superior to MR venography in the identification of cerebral veins and dural sinuses and is at least equivalent in establishing the diagnosis of dural sinus thrombosis (6). Advantages of CT venography as compared with MR angiography are that it is less expensive and that the time to diagnosis in the initial workup of a patient is shorter (5). It can be instantly performed as an adjunct to an unenhanced CT scan in patients undergoing the initial workup. Because the procedure duration is less than 1 minute, the image quality is hardly impaired by patient motion, and patient monitoring is easier in critically ill patients as compared with MR imaging (5). Although the diagnosis of dural sinus thrombosis can be made by evaluation of the axial thin-section contrast-enhanced source images of a helical CT scan, 3D MIP images free from overprojecting bone clearly have additional value. These reconstructions are valuable as a communication tool with clinicians, have the ability to be viewed in a limitless number of views, and reduce the likelihood that normal anatomic variations, such as high splitting of the superior sagittal sinus, will be mistaken for an empty delta sign (7).
An essential step in CT venography is removal of bone from the images. Bone removal by using subtraction of an enhanced from an unenhanced CT scan has been described elsewhere (8). This technique has several drawbacks. First, subtraction always adds noise to the CTA images (1). Second, subtraction without matching, as used by Imakita et al (8), requires that the patient remain completely still, which is often difficult to achieve. CT venography has been performed by using a technique developed by Casey et al (7), which they term a “graded subtraction” technique, but which actually is a masking technique for removal of the bone from the CT images. In their method, no precontrast scan is made. An initial mask is generated by thresholding followed by widening of the mask by one or more dilation steps. Because the thresholding has to be performed with the contrast-enhanced images, a suitable threshold has to be chosen carefully by a qualified operator to avoid the elimination of the contrast as well. Thresholding images that contain the contrast-enhanced arteries and venous structures implies the use of a rather high threshold, as much as 400 HU (7) or even slightly more than 500 HU (5). Therefore, the quality of the initial mask can be rather poor, and this quality has to be improved by using one or several dilation steps, at the discretion of the operator. A drawback is therefore that this technique is not automatic and that the results obtained will be operator dependent. Moreover, several dilation steps may have to be used to remove the bone sufficiently, but that may also affect the contrast-enhanced structures. Even then the quality of the bone removal may be mediocre, especially in regions with thin bone structures that have not been included in the initial mask. This method is best performed by a radiologist with detailed knowledge of the cerebrovascular anatomy, to avoid accidental elimination of dural sinuses and cortical veins during the procedure (7). Wetzel et al (5) reported that removal of the thin bone layers at the skull base was not possible without a cutoff of sinus structures because of an overlap in attenuation values. As a consequence, in their study the cavernous sinus was seen on only 62% of the MIP images.
The MMBE technique described in this communication does not suffer from these drawbacks. A relatively low, and constant, threshold of 200 HU can be used, because an unenhanced CT scan is used in the thresholding (1). Because the initial mask is already of good quality, only one dilation step has to be used. As a consequence of these features, the MMBE procedure is fully automatic, and the results obtained are completely independent of the operator and are therefore reproducible and of high quality.
The bone-elimination procedure features two points: 3D image matching and bone removal according to a matched mask. Image matching is important, because the head of the patient is prone to movement, and minimal movement leads to serious artifacts in the processed images. An advantage of the MMBE technique over subtraction is that low-dose unenhanced images can be used, because the noise of the unenhanced images is not added to that of the CTA images. Moreover, the unenhanced images can be stored for future use when repeat CTA examinations are indicated.
The MMBE method was originally developed for the processing of CTA examinations of the intracranial carotid arteries and the circle of Willis (1). By using this method Venema et al (1) have demonstrated that vessels at the region of the skull base, such as the petrous and cavernous segments of the internal carotid arteries, can be depicted without any overlapping bone. The present study demonstrates that the method can also be successfully applied in CT venography.
The registration method described in this article performed well in the compensation for patient motion between the two scans. Because of the high capture range of the initialization step of the registration procedure the method is very robust. If the patient moves during acquisition of one of the two scans, artifacts inevitably will be present in the processed images. These artifacts will be restricted to the section positions at which the movement occurred, because in our approach each contrast-enhanced image is registered separately with the unenhanced volume.
Previous studies used single-section helical CT to visualize the intracranial venous system with a scan duration of 60 seconds (5, 7). Multiple thin-section helical CT allows imaging of the entire brain within 30 seconds during peak venous enhancement. In dural sinus thrombosis, the wall of the thrombosed sinus may enhance, which may obscure the low attenuating voxels of the intraluminal thrombus on MIP images (7). Therefore, to exclude thrombosis, the CT venograms should always be interpreted in conjunction with the axial contrast-enhanced source images.
Conclusions
We conclude that MMBE is a useful adjunct for the angiographic display in CT venography, removing the bone effectively while retaining the quality of the source images with only a modest increase in radiation dose. This technique is fully automated, not operator dependent, and can be performed by a technician. Interactive viewing of the venous vasculature in any plane is possible without hindrance of overlying bone structures. This new development may further enhance the use of CT venography as the technique of choice for the evaluation of patients in whom abnormalities of the intracranial venous circulation are suspected. Larger studies are needed to evaluate whether this technique may supplant MR venography or intra-arterial digital subtraction angiography.
References
- 1.Venema HW, Hulsmans FJH, den Heeten GJ. CT angiography of the circle of Willis and the intracranial carotid arteries: maximum intensity projection with matched mask bone elimination. Radiology 2001;218:893–898 [DOI] [PubMed] [Google Scholar]
- 2.Van den Elsen PA, Pol EJD, Viergever MA. Medical image matching: a review with classification. IEEE Eng Med Biol 1993;12:26–39 [Google Scholar]
- 3.Van Herk M, Kooy HM. Automatic three-dimensional correlation of CT-CT, CT-MRI, and CT-SPECT using chamfer matching. Med Phys 1994;21:1163–1178 [DOI] [PubMed] [Google Scholar]
- 4.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C++: The Art of Scientific Computing. 2nd ed. Cambridge: Cambridge University Press;2002. :413–417
- 5.Wetzel SG, Kirsch E, Stock KW, et al. Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography. AJNR Am J Neuroradiol 1999;20:249–255 [PMC free article] [PubMed] [Google Scholar]
- 6.Ozsvath RR, Casey SO, Lustrin ES, et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol 1997;169:1699–1707 [DOI] [PubMed] [Google Scholar]
- 7.Casey SO, Alberico RA, Patel M, et al. Cerebral CT venography. Radiology 1996;198:163–170 [DOI] [PubMed] [Google Scholar]
- 8.Imakita S, Onishi Y, Hashimoto T, et al. Subtraction CT angiography with controlled-orbital helical scanning for detection of intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:291–295 [PMC free article] [PubMed] [Google Scholar]