Abstract
Summary: We describe hamartomas of possible thalamic origin. CT revealed marked calcification in the mass lesion, and MR imaging revealed contrast enhancement. Histologically, outgrowth of the glia was observed, but no neoplastic component was confirmed. Immunohistochemical staining was positive for the cell-adhesion factor N-CAM and negative for polysialic acid.
Neuronal hamartomas typically arise from the region of the hypothalamus and often present with precocious puberty, mass effect, or gelastic seizures. In this report, we describe the characteristics of a patient with a neuronal hamartoma that we hypothesize originated from the thalamus owing to embryologic and histopathologic characteristics and discuss the pathogenetic process of hamartomas, referring to previous reports.
Case Report
An 8-year-old boy had a history of left arm shaking and a decrease of grip strength for 6 months before presenting to our hospital. Abnormal hearing in his left ear had been observed 2 years earlier but had temporarily improved. Audiometry performed 2 months before admission, however, revealed decreased hearing in his left ear. His medical history was complicated by a ventricular septal defect (VSD) observed immediately after his birth, for which he has been receiving periodic treatment. He had no cognitive deficits and had been noted to develop normally.
Radiologic Findings
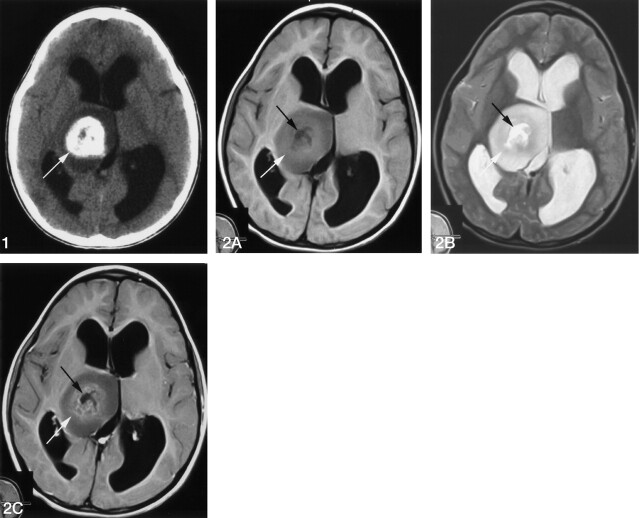
Noncontrast CT (Fig 1) performed to evaluate the patient’s hearing loss revealed a hypoattenuated mass lesion relative to gray matter in the right thalamus. Marked calcifications were noted in the center of the mass lesion. MR imaging (Figs 2 and 3) of the mass demonstrated low signal intensity on T1-weighted images and high signal intensity on T2-weighted images relative to gray matter. The region with calcium enhanced on postcontrast T1-weighted MR images, but surrounding regions did not appear to enhance. The center of the lesion demonstrated signal intensity characteristics similar to those of CSF. The mass lesion protruded into the third ventricle and was surrounded by normal-appearing brain parenchymal tissue in a beaklike fashion. The internal capsule and remaining thalamus were compressed by the mass lesion. There was no evidence of extension of the mass into the hypothalamic region. The mass significantly compressed the tectum mesencephali, which was the likely cause for the patient’s hydrocephalus.
Fig 1.
Noncontrast CT scan shows a low-attenuation mass in the right thalamus. Marked calcifications are seen in the center of the mass lesion (arrow).
Fig 2.
Axial MR images. All images are obtained at same level.
A, T1-weighted image (500/20 [TR/TE]). B, T2-weighted image (5000/88 [TR/TE]). C, T1-weighted (500/20 [TR/TE]) image after gadolinium-DTPA administration.
The mass lesion shows lower signal intensity on the T1-weighted image (A) and higher signal intensity on the T2-weighted MR image (B) relative to gray matter. Calcified regions show slightly greater signal intensity on the T1-weighted image (A) and are enhanced after administration of gadolinium-DTPA (C, light arrows). CSF-like signal intensity exists in the core of the lesion (dark arrows).
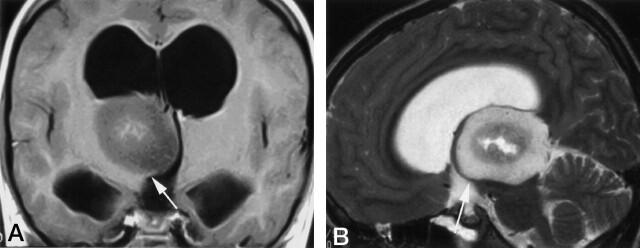
Fig 3.
A, Coronal image after administration of gadolinium-DTPA (500/20 [TR/TE]). B, Sagittal T2-weighted image (4000/88.9 [TR/TE]). The margin of the mass lesion is covered with thalamus extending in a beaklike shape (arrows).
Surgery and Pathology
On the basis of imaging findings, a glioma arising from the thalamus was suspected and craniotomy was performed. Intraoperative frozen sections did not reveal a neoplastic component, so a more extensive biopsy of the calcified region was performed. Histopathologic study of these biopsies confirmed the presence of a typical neuronal hamartoma.
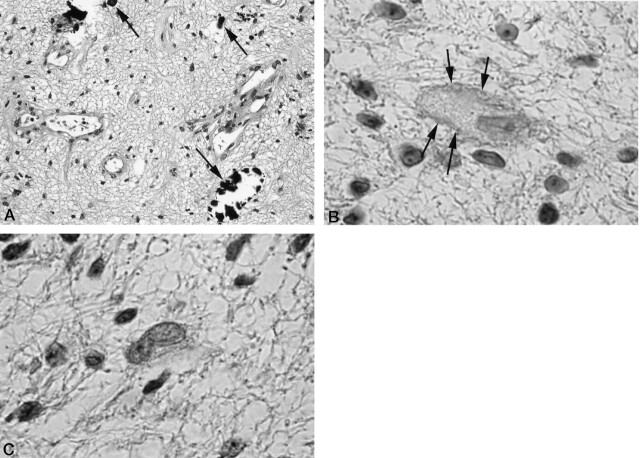
Histopathologic study revealed a proliferation of vessels as well as minute calcifications. Gliosis owing to proliferation of astrocytes was observed, but there were no atypical cells or mitotic nucleus. Immunohistochemical staining was positive for the cell-adhesion factor N-CAM and negative for polysialic acid (Fig 4). MR imaging performed 6 months after the operation revealed no increase in the size of the mass lesion.
Fig 4.
A, Hematoxylin and eosin stain (magnification ×100). B, N-CAM stain (magnification ×200). C, Polysialic acid stain (magnification ×200).
Proliferation of vessels and minute calcified corpuscles (dark arrows) are observed. There are no atypical cells or abnormal division of nucleus. Immunohistochemical staining is positive for N-CAM (dark arrows) and negative for polysialic acid.
Discussion
Neuronal hamartoma is a rare entity arising from the hypothalamus region and typically projects into the interpeduncular fossa, between the pituitary infundibulum and the mamillary bodies (1). Patients, often younger than 3 years, tend to present with early onset of precocious puberty. Male sex predominance has been noted (2, 3). Hypothalamic hamartomas are regarded as a heterotopia and are composed of well-differentiated neuroglial tissue (1, 2). On MR images, the lesions are isointense to gray matter and do not typically demonstrate contrast enhancement on T1-weighted images. They are typically hyperintense on T2-weighted images (1, 2). Cyst formations and calcifications have been rarely reported (1, 2, 4).
In our case, two atypical imaging findings were demonstrated: the hamartoma originated in the thalamus, and contrast enhancement was observed in the area of the calcified portion. Hamartomas, though most often associated with the hypothalamus, have been reported as arising in the thalamus (5), as in our case. Embryologically, the thalamus and hypothalamus are differentiated from the diencephalon, which develops in the 5th gestational week, and are anatomically separated by the hypothalamic sulcus in the 7th gestational week (6). Therefore, in our case, hamartoma was thought to occur in the diencephalon before the thalamus and hypothalamus were differentiated. Because the thalamus and hypothalamus could have migrated owing to the hamartoma in the diencephalon, our case could be explained as an embryonic phenomenon. Sasaki et al reported on a hamartoma occurring in the lateral ventricle (7). In their case, it was possible that hamartoma developed in the roof plate of the diencephalon because of migration into the lateral ventricle.
Hypothalamic hamartoma is the primary feature of Pallister-Hall syndrome. Other major manifestations of the syndrome include polydactyly, imperforate anus, and hypopituitarism (8). Reported congenital heart defects include patent ductus arteriosus and VSD (9). Our case was complicated with VSD. The membranous part of the interventricular septum is formed during the 6th–7th gestational week (10). Therefore, if hamartoma occurred in the diencephalons, as we suspect, this defect of the interventricular septum may have developed at almost the same time as the hamartoma, and our patient had one of subtypes of Pallister-Hall syndrome.
Because cystic change, calcification, and contrast enhancement at MR imaging can be observed in hamartoma, Prasad et al (4) reported that cystic changes in hamartoma are caused by hemorrhage or liquefactive necrosis resulting from ischemic change in it. In addition, they thought that cystic changes seen in their case might be attributed to ischemic necrosis, in view of the size of the lesions. It was thought that ischemic change due to the increase of hamartoma caused hemorrhage and then degeneration, and calcifications occurred in our case. In addition, enhancement in hamartoma after administration of contrast medium was thought to be caused by vascular hyperplasia.
Although our patient had the mass lesion that was large enough to cause hydrocephalus, he had only symptoms related to compression of the corticospinal tract. When hamartoma occurs in the regions that do not cause severe disorders, including hypothalamic disorders, no symptoms might be observed in some cases.
Immunohistochemical staining was positive for N-CAM and negative for polysialic acid. N-CAM is a cell-adhesion factor, and polysialic acid exists in N-CAM and prevents cell adhesion. Polysialic acid is present in ganglion migration in the embryonic brain and, with some exceptions, is not observed in the adult brain (11). Polysialic acid was negative in our case, which strongly suggested that the hamartoma was composed of the differentiated tissues. Thus, our results positively supported the principle of performing follow-up observations for patients confirmed to have hamartoma.
References
- 1.Osborn AG. Diagnostic Neuroradiology. St. Louis, Mo: Mosby;1994;481 [Google Scholar]
- 2.Orrison WW Jr. Neuroimaging. Philadelphia: WB Saunders;1998;1594–1596
- 3.Barkovich AJ. Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott-Raven;1995;518–519
- 4.Prasad S, Shah J, Patkar D, Gala B, Patankar T. Giant hypothalamic hamartoma with cystic change: report of two cases and review of the literature. Neuroradiology 2000;42:648–650 [DOI] [PubMed] [Google Scholar]
- 5.Rossiter JP, Khalifa MM, Nag S. Diencephalic neuronal hamartoma associated with congenital obstructive hydrocephalus, anophthalmia, cleft lip and palate and severe mental retardation: a possible new syndrome. Acta Neuropathol 2000;99:685–690 [DOI] [PubMed] [Google Scholar]
- 6.Sadler TW. Langman’s Medical Embryology. 8th ed. Philadelphia: Lippincott-Raven;2000;432–433
- 7.Sasaki T, Matsuno A, Inoh Y, Asai A, Kirino T. A rare case of hamartoma in the lateral ventricle: case report. Surg Neurol 1997;47:23–27 [DOI] [PubMed] [Google Scholar]
- 8.Gorlin RJ, Cohen MM, Jr, Levin LS. Syndromes of the Head and Neck. 3rd ed. New York: Oxford University Press;1990;903–905
- 9.Biesecker LG, Graham JM Jr. Pallister-Hall syndrome. J Med Genet 1996;33:585–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadler TW. Langman’s Medical Embryology. 8th ed. Philadelphia: Lippincott-Raven;2000;230–232
- 11.Nakayama J, Angata K, Ong E, et al. Polysialic acid, a unique glycan that is developmentally regulated by two polysialyltransferases, PST and STX, in the central nervous system: from biosynthesis to function. Pathol Int 1998;48:665–677 [DOI] [PubMed] [Google Scholar]