Abstract
BACKGROUND AND PURPOSE: The MR anatomy of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation, which traverse the temporal stem, is not well known. The purpose of this investigation was to study these structures in the anterior temporal lobe and the external and extreme capsules and to correlate the dissected anatomy with the cross-sectional MR anatomy.
METHODS: Progressive dissection was guided by three-dimensional MR renderings and cross-sectional images. Dissected segments of the tracts and the temporal stem were traced and projected onto reformatted images. The method of dissection tractography is detailed in a companion article.
RESULTS: The temporal stem extends posteriorly from the level of the amygdala to the level of the lateral geniculate body. The uncinate and inferior occipitofrontal fasciculi pass from the temporal lobe into the extreme and external capsules via the temporal stem. Meyer’s loop extends to the level of the amygdala, adjacent to the uncinate fasciculus and anterior commissure. These anatomic features were demonstrated on correlative cross-sectional MR images and compared with clinical examples.
CONCLUSION: This study clarified the MR anatomy of the uncinate and inferior occipitofrontal fasciculi and Meyer’s loop in the temporal stem and in the external and extreme capsules, helping to explain patterns of tumor spread. The inferior occipitofrontal fasciculus is an important yet previously neglected tract. These results provide a solid anatomic foundation for diffusion tractography of the normal temporal stem and its tracts, as well as their abnormalities in brain disorders such as epilepsy, postoperative complications, trauma, schizophrenia, and Alzheimer disease.
The stem of the temporal lobe (temporal stem) forms a white matter bridge between the temporal and frontal lobes, and as such, it plays an important role in a number of disorders. It is a route of tumor, infection, and seizure spread. It is also a critical landmark in surgery of the temporal lobe because of its proximity to the insula, the basal ganglia, and the external and extreme capsules. Transection of the temporal stem during tumor and epilepsy surgery has the potential to result in quadrantanopia or hemianopia. The temporal stem also has a role in cerebral disorders resulting from congenital, traumatic, surgical, and degenerative disconnection of the temporal and frontal lobes.
The surgical and cross-sectional MR anatomies of the temporal stem are not well known. This is because of the lack of distinguishing landmarks of its components that include the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation.
The goal of this study was to delineate the white matter tracts passing through the stem of the temporal lobe with anatomic dissection tractography. This method used MR-assisted dissection and coregistration of MR images of the dissected and intact brain specimens to demonstrate the dissected anatomy on cross-sectional MR images. We also sought to facilitate understanding of patterns of disease spread via these tracts by first defining their anatomic and MR locations and then analyzing correlating clinical examples.
Methods
Delineation of the Temporal Stem
The landmarks used by Ebeling and von Cramon (1) for the temporal stem were applied in this investigation. A color-coded straight line, representing the temporal stem, was traced between the white matter directly inferior to the circular sulcus of the insula and the lateral superior margin of the temporal horn. The temporal stem was traced in a series of sequential coronal images extending from the level of the amygdala anteriorly to the level of the lateral geniculate body posteriorly. At the level of the amygdala, anterior to the temporal horn, the lateral margin of the amygdala was used as a substitute landmark instead of the lateral superior margin of the temporal horn. The temporal stem line thickness was widened to 5 mm for visualization. The entire stack of sequential tracings of the temporal stem in the coronal plane were combined and reformatted into a single structure in the axial and sagittal planes (Fig 1).
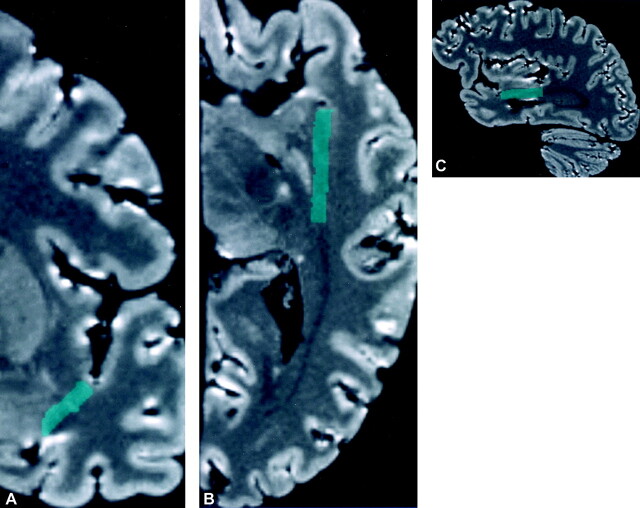
Fig 1.
MR localization of the temporal stem on multiplanar reformatted images of the specimen.
A, The temporal stem (green) is traced on a coronal image of the undissected specimen.
B and C, The entire stack of the sequential tracings of the temporal stem in the coronal plane was then combined and reformatted into a single structure in the axial (B) and sagittal planes (C).
Dissection and MR Imaging of Tracts
The method for dissection of the white matter tracts of the temporal stem and for delineation of these tracts on cross-sectional MR images is described in detail in a companion article on anatomic dissection tractography (2). Briefly, formalin-treated brain specimens were imaged at 1.5 T in the sagittal plane by using a 3D T1-weighted spoiled gradient-echo sequence before and after dissection. Progressive dissection of the white matter tracts in the anterior temporal lobe and insula was guided by 3D MR rendering and reformatted cross-sectional images of the specimens. Because of the close relationship of the uncinate fasciculus, the inferior occipitofrontal fasciculus, and Meyer’s loop in the temporal stem, specimens of the uncinate fasciculus were further dissected to demonstrate the three tracts in the same specimen. The lateral surfaces of the central segments of the uncinate fasciculus, the inferior occipitofrontal fasciculus, and Meyer’s loop were traced on 3D renderings of the MR images of the dissected specimen. The MR location of a tract was then determined by coregistering the MR images before and after dissection and by projecting the tracing of the tract onto reformatted images.
To visualize the uncinate fasciculus, the superior and middle temporal gyri and temporal pole were partially dissected away. The insular branches of the middle cerebral artery; the cortex of the insula; and segments of the extreme capsule, claustrum, external capsule, and orbital gyri were removed by dissection.
The inferior occipitofrontal fasciculus was identified by further dissection of the superior, middle, and inferior temporal lobe gyri and by dissection of the external and extreme capsule of the insula, superior to the uncinate fasciculus
To locate Meyer’s loop, the inferior occipitofrontal fasciculus and uncinate fasciculus were partially removed, and the middle and inferior temporal gyri were further dissected. The dissection did not extend all the way to the lateral geniculate body because removal of the entire temporal lobe segment of the inferior occipitofrontal fasciculus would have been required.
Cross-sectional MR images were created in multiple planes, with the white matter tracts color coded. Axial reformatted images were generated in two planes. One plane was created parallel to the bicommissural plane, passing through the center of the anterior commissure (3). This plane is minimally different from the Talairach method in which the bicommissural plane passes at the upper margin of the anterior commissure (4). The other plane was an oblique axial plane +17° to the bicommissural plane. The oblique axial plane was used to better demonstrate the relationship of the traced dissected tracts to the anterior commissure and to depict the length of the inferior occipitofrontal fasciculus. Coronal and sagittal reformatted images were generated in planes perpendicular to the bicommissural plane, and the Talairach coordinate for each image reformatted in the Talairach plane was determined. Images generated oblique to the Talairach vectors, therefore, did not have an associated Talairach coordinate.
Correlative clinical cases were collected to illustrate how pathologic involvement of the tracts contributed to the spread of disease or symptoms.
Results
Temporal Stem
Figure 1 shows the location and the anteroposterior extent of the temporal stem, which extends from the level of the amygdala to the level of the lateral geniculate body.
Uncinate Fasciculus
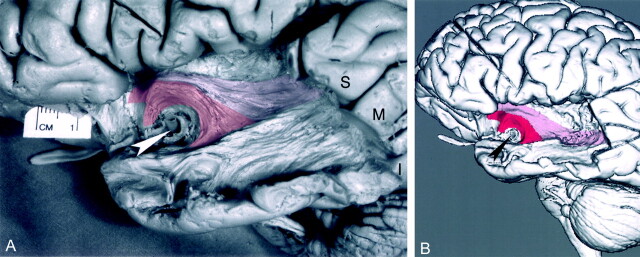
Dissection demonstrated the uncinate fasciculus in the white matter of the temporal lobe lateral to the amygdala and hippocampus. It curved upward, passing behind and above the trunk of the middle cerebral artery (M1 segment) into the extreme and external capsule medial to the insular cortex. From there, it continued into the posterior orbital gyrus. These features were well visualized on the anatomic dissection, the 3D MR rendering of the dissection, and a correlative parasagittal image from a patient with tumor involvement of this tract (Figs 2–4).
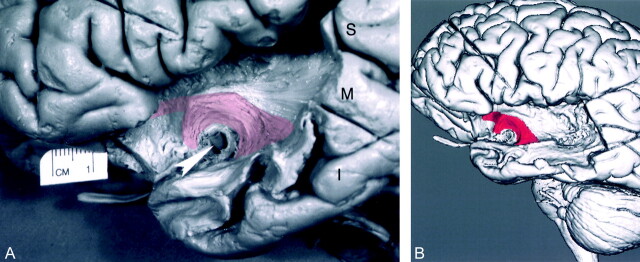
Fig 2.
Dissected uncinate fasciculus.
A, Photograph of the lateral aspect of a dissected cerebral hemisphere shows the segment of the uncinate fasciculus connecting the temporal and frontal lobes. To visualize the uncinate fasciculus, the anterior segment of the superior temporal gyrus (S), a portion of the middle temporal gyrus (M), and the temporal pole were partially removed by dissection. The insular (M2) branches of the middle cerebral artery were also removed by dissection. The horizontal segment (M1) of the middle cerebral artery was left in place as a landmark (arrowhead). The cortex of the insula and parts of the extreme capsule, the claustrum, the external capsule, and the orbital gyri were dissected away. The fiber bundles of the uncinate fasciculus (transparent red) originate in the white matter of the temporal lobe, course around the M1 segment, enter the extreme and external capsules, and continue into the orbital gyri. To visualize the underlying dissected uncinate fasciculus, we used a 10% transparent red that results in a pink appearance. I signifies the inferior temporal gyrus. To better demonstrate the dissected structures, the brain is tilted 30°, with the temporal lobe closer to the camera.
B, 3D MR rendering demonstrates the lateral surface of the dissected segment of the uncinate fasciculus (red). The uncinate fasciculus courses around the M1 segment of the middle cerebral artery and connects the temporal and frontal lobes.
The relationship of the lateral surface of the uncinate fasciculus to the adjacent structures was well delineated on reformatted cross-sectional MR images of the coregistered specimens (Fig 3). Coronal MR images showed the uncinate fasciculus coursing from the temporal lobe lateral to the amygdala, through the temporal stem, and into the lower segment of the extreme capsule lateral to the claustrum (Fig 3A). Axial reformatted images showed that the uncinate fasciculus passed from the temporal lobe through the temporal stem and into the lower region of the frontal lobe, just above the sylvian fissure, at the level of the anterior commissure (Fig 3B and C). At its most superior level, the uncinate fasciculus was below the level of the frontal horns of the lateral ventricles (Fig 3D). The 3D MR rendering and the reformatted cross-sectional MR images of the specimen were correlated with clinical examples demonstrating the spread of disease between temporal and frontal lobes via the uncinate fasciculus (Fig 4).
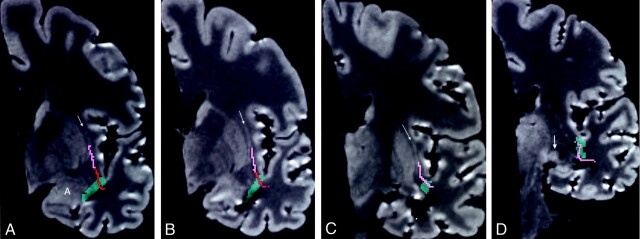
Fig 3.
Cross-sectional MR images of the uncinate fasciculus.
A, Coronal reformatted image of the specimen shows the uncinate fasciculus (red) passing from the temporal lobe lateral to the amygdala (A), through the temporal stem (green), and into the lower segment of the extreme capsule lateral to the claustrum (arrow). Coronal Talairach coordinate = −3.
B, Axial reformatted image shows segments of the uncinate fasciculus in the inferior frontal (F) and temporal (T) lobes at the level of the sylvian fissure pedicle (arrowhead). The temporal lobe segment is passing through the temporal stem. Axial Talairach coordinate = −9.
C, Oblique (+17°) axial reformatted image superior to the sylvian fissure pedicle shows the uncinate fasciculus passing from the temporal lobe through the temporal stem and into the lower region of the frontal lobe. This image is at the level of the inferior edge of the claustrum (thin arrow), putamen (P), head of the caudate nucleus (C), and anterior commissure (thick arrow). The oblique axial plane was generated at +17° to the bicommissural plane and used to better demonstrate the relationship of the dissected tracts to the anterior commissure. This oblique axial image is not in Talairach coordinates.
D, Axial image shows the most superior level at which the uncinate fasciculus (red) is present in the extreme capsule lateral to the claustrum (arrow). At its most superior level, the uncinate fasciculus is below the level of the frontal horns of the lateral ventricles. Axial Talairach coordinate = −1.
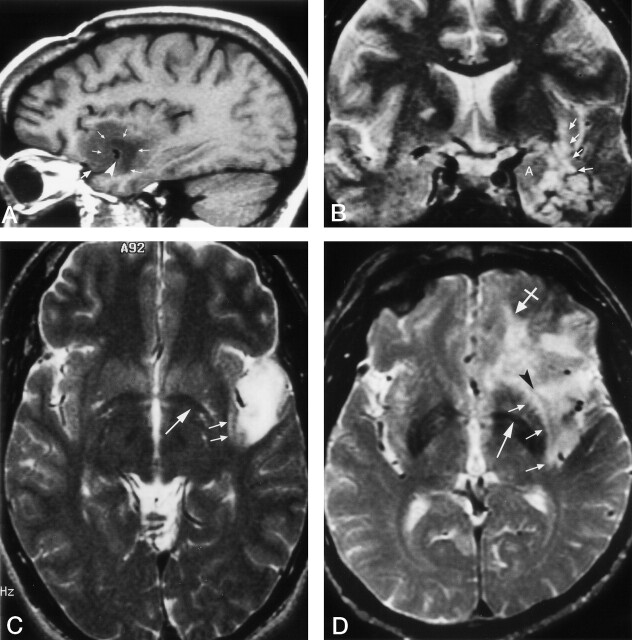
Fig 4.
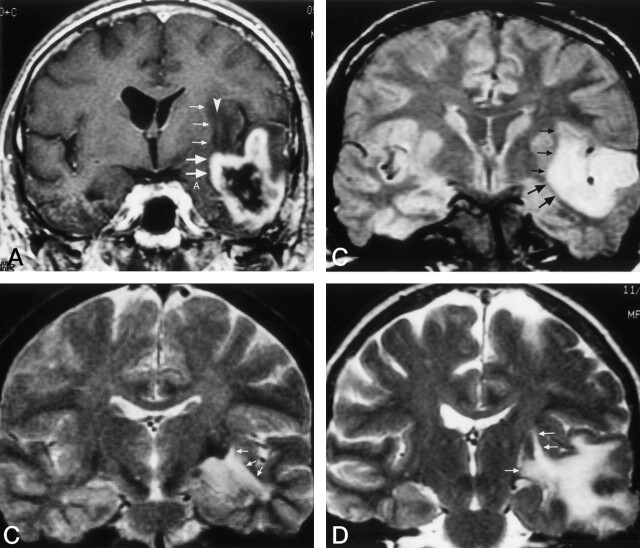
Clinical correlation of uncinate fasciculus involvement on cross-sectional MR imaging.
A, Nonenhanced parasagittal T1-weighted image in a 36-year-old woman with a temporal lobe astrocytoma extending into the frontal lobe. The region of the abnormal uncinate fasciculus (small arrows) can be identified within the low signal intensity of the tumor. It passes around the middle cerebral artery (arrowhead) into the frontal lobe, including the posterior orbital gyrus (large arrow). The relationship of the uncinate fasciculus to the middle cerebral artery is similar to that shown in the dissected specimen in Figure 2.
B, T2-weighted coronal image in a 44-year-old man with a temporal lobe astrocytoma. The tumor and edema are located adjacent to the amygdala (A). The location of the uncinate fasciculus on the coronal MR study in Figure 3A suggests the tumor is spreading out of the temporal lobe into the extreme and external capsules (arrows) via the region of the uncinate fasciculus.
C, T2-weighted axial image in a 42-year-old man with a temporal-lobe oligodendroglioma that is just beginning to spread into the extreme capsules (small arrows) via the region of the uncinate fasciculus at the level of the anterior commissure (large arrow).
D, T2-weighted axial image in a 60-year-old man with an oligodendroglioma. The tumor (small arrows), shown at the level of the anterior commissure (large arrow), has spread into the extreme and external capsules on either side of the claustrum (arrowhead) with further involvement of the orbital gyri (crossed arrow). Comparison of the images in C and D with Figure 3C illustrates the spread of the tumor from temporal to frontal lobe via the uncinate fasciculus.
Inferior Occipitofrontal Fasciculus
Dissection demonstrated that the inferior occipitofrontal fasciculus passed from the temporal lobe into the extreme and external capsules with the uncinate fasciculus (Fig 5). In the extreme and external capsules, it was superior to the uncinate fasciculus.
Fig 5.
Dissected inferior occipitofrontal fasciculus shown along with the uncinate fasciculus.
A, Photograph of the lateral aspect of a dissected cerebral hemisphere shows the inferior occipitofrontal fasciculus and uncinate fasciculus bridging the temporal and frontal lobes. This figure demonstrates additional dissection of the specimen in Figure 1. Further dissection of the superior (S), middle (M), inferior (I) temporal-lobe gyri and of the external and extreme capsule identified the inferior occipitofrontal fasciculus. The uncinate fasciculus (red) originates in the white matter of the temporal lobe and curves around the M1 segment of the middle cerebral artery (arrowhead), entering the extreme and external capsules. The inferior occipitofrontal fasciculus (pink), which connects the occipital and frontal lobes via the temporal lobe, passes into the extreme and external capsules adjacent and superior to the uncinate fasciculus. The inferior occipitofrontal fasciculus is a tract larger and longer than the uncinate fasciculus. In its course from the occipital to the frontal lobe, it traverses almost the entire length of the temporal lobe. To demonstrate the underlying dissected tracts, we used a 10% transparent red for the uncinate fasciculus and 10% transparent pink for the inferior occipitofrontal fasciculus. To better visualize the dissected structures, the brain is tilted 30°, with the temporal lobe closer to the camera.
B, 3D MR rendering demonstrates the surfaces of the dissected inferior occipitofrontal fasciculus (pink) and uncinate fasciculus (red). Arrowhead indicates the M1 segment of the middle cerebral artery.
Dissected anatomic evaluations, as well as crosssectional images, demonstrated that the inferior occipitofrontal fasciculus had a long, anteroposterior course in the temporal lobe, unlike the uncinate fasciculus, which was situated in the anterior part of the temporal lobe (Figs 6 and 7A). On coronal images (Fig 6), the dissected inferior occipitofrontal fasciculus extended from the level of the amygdala to the level of the posterior thalamus and the lateral geniculate body. After exiting the temporal lobe, the inferior occipitofrontal fasciculus passed into the extreme and external capsules, where it was located superior to the uncinate fasciculus. Axial images demonstrated that the most superior region of the inferior occipitofrontal fasciculus was at the level of the frontal horn of the lateral ventricle (Fig 7A), whereas the most superior extent of the uncinate fasciculus was below the level of the frontal horn of the lateral ventricle (Fig 3D).
Fig 6.
Cross-sectional anatomic relationships of the inferior occipitofrontal fasciculus in sequential coronal MR planes.
A, Level of the frontal horn of the lateral ventricle and the anterior amygdala (A). This image shows the inferior occipitofrontal fasciculus (pink) in the external capsule, adjacent to the claustrum (arrow), and above the uncinate fasciculus (red); the last crosses the temporal stem (green). Coronal Talairach coordinate = −5.
B, Image at the level of the posterior amygdala and hippocampal head shows only the uncinate fasciculus in the temporal stem. The inferior occipitofrontal fasciculus is in the lower segment of the extreme capsule, lateral to the claustrum (arrow). Coronal Talairach coordinate = −7.
C, Level of the body of the lateral ventricle and the hippocampus. The inferior occipitofrontal fasciculus passes from the temporal lobe, through the temporal stem, and into the lower segment of the extreme capsule lateral to the claustrum (arrow). This level is posterior to the uncinate fasciculus. Coronal Talairach coordinate = −13.
D, The inferior occipitofrontal fasciculus crosses the temporal stem into the white matter lateral to the thalamus and lateral geniculate body (arrow). Coronal Talairach coordinate = −22.
Fig 7.
Images of the inferior occipitofrontal fasciculus.
A, Oblique (+17°) axial image shows the inferior occipitofrontal fasciculus coursingfrom the temporal lobe through the extreme and external capsules. The most superior region of the inferior occipitofrontal fasciculus (pink) is at the level of the frontal horn (arrow) of the lateral ventricle. This image and the series of coronal images in Figure 6 demonstrate the long anteroposterior extent of the dissected segment of the inferior occipitofrontal fasciculus. The oblique axial plane was used to better demonstrate the length of the inferior occipitofrontal fasciculus.
B, Oblique (+17°) axial image. Of note is the proximity of the inferior occipitofrontal fasciculus to the anterior commissure (arrow). The anterior commissure is best demonstrated in the oblique plane. On this image, only a small segment of the inferior occipitofrontal fasciculus is adjacent to the anterior commissure.
C, Axial image shows the inferior occipitofrontal fasciculus passing from the temporal lobe into the extreme capsule via the temporal stem (green). The images in B and C show that, at the level of the anterior commissure (arrow), both the uncinate fasciculus and the inferior occipitofrontal fasciculus are present in the extreme capsule. Axial Talairach coordinate = −5. Comparison of the images in B and C demonstrates that the proximity and relationship of the uncinate fasciculus and inferior occipitofrontal fasciculus to the anterior commissure depends on the obliquity of the axial plane.
The anatomic information provided by the correlative anatomic and MR study demonstrated the role of the inferior occipitofrontal fasciculus in tumor spread beyond the temporal lobe (Figs 8–10).
Fig 8.
Clinical correlation of the inferior occipitofrontal fasciculus in the sagittal plane. Parasagittal T2-weighted image in a 70-year-old man with a temporal-lobe glioblastoma multiforme. The tracts shown in the anatomic dissection (Fig 5A), the 3D MR rendering (Fig 5B), and the size of the lesion above the middle cerebral artery void (arrowhead) suggest that the tumor and edema has spread into the frontal lobe via both the uncinate fasciculus and the inferior occipitofrontal fasciculus.
Fig 9.
Clinical examples show involvement of the inferior occipitofrontal fasciculus at coronal MR planes corresponding to those in Figure 6.
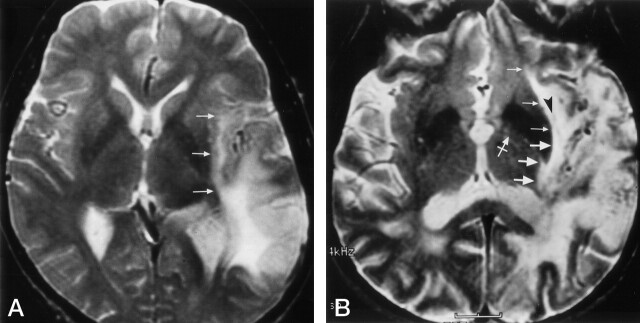
A, Enhanced T1-weighted image in a 58-year old man with a recurrent glioblastoma multiforme. This image is at the level of the frontal horn of the lateral ventricle, and the amygdala (A). Comparison with Figure 6A demonstrates that the enhanced tumor is spreading out of the temporal lobe via the region of the uncinate fasciculus (large arrows). The nonenhanced edema and tumor in the extreme and external capsules, around the claustrum (arrowhead), involve the region of the inferior occipitofrontal fasciculus (small arrows). This combined involvement of the uncinate fasciculus and the inferior occipitofrontal fasciculus may vary depending on the exact location of the upper margin of the uncinate fasciculus and the lower margin of the inferior occipitofrontal fasciculus and the angulation of the section.
B, Proton density–weighted image in a 52-year-old man with a temporal lobe astrocytoma spreading into the extreme and external capsules at the posterior amygdala/hippocampal head level. Comparison with Figure 6B shows that the tumor and edema is spreading out of the temporal lobe via the region of both the uncinate fasciculus (large arrows) and the inferior occipitofrontal fasciculus (small arrows).
C, T2-weighted image in a 36-year-old woman with a temporal lobe astrocytoma spreading into the extreme and external capsules. The tumor is at the level of the body of the lateral ventricle and hippocampus. Comparison with Figure 6C, obtained at a similar coronal plane, demonstrates that the tumor and edema are spreading out of the temporal lobe via the region of the inferior occipitofrontal fasciculus (arrows).
D, T2-weighted image in a 68-year-old woman with a temporal lobe anaplastic oligodendroglioma spreading into the deep white matter lateral to the thalamus. Comparison with Figure 6D demonstrates that the inferior occipitofrontal fasciculus (arrows) is involved in the tumor and edema spread out of the temporal lobe.
Fig 10.
Corresponding clinical examples involving the inferior occipital fasciculus in the axial plane.
A, In this 41-year-old woman, the tumor has spread to the extreme capsule. Comparison with Figure 7A shows that, at the level of the lateral ventricle, the tumor and edema is spreading out of the temporal lobe via the region of the inferior occipitofrontal fasciculus (arrows).
B, In this 61-year-old man, the tumor has spread into the extreme and external capsules around the claustrum (arrowhead). This image is at the level of the third ventricle and the anterior commissure (crossed arrow). Locations of the inferior occipitofrontal fasciculus and the uncinate fasciculus in Figure 7B and C suggest that the tumor and edema has spread out of the temporal lobe via the region of both the inferior occipitofrontal fasciculus (small arrows) and the uncinate fasciculus (large arrows). Depending on the angulation of the axial plane, or a few millimeters change in section selection, tumor spread at the anterior commissure level may involve the inferior occipitofrontal fasciculus and the uncinate fasciculus to a variable degree.
Meyer’s (Temporal) Loop of the Optic Radiation
Dissection demonstrated that the fibers of the optic radiation pathway passed laterally from the region of the lateral geniculate body. The fiber bundles then curved posteriorly forming Meyer’s loop (Fig 11A). The dissected surface of Meyer’s loop is shown on the sagittal 3D MR rendering of the dissected specimen (Figs 11B and C).
Fig 11.
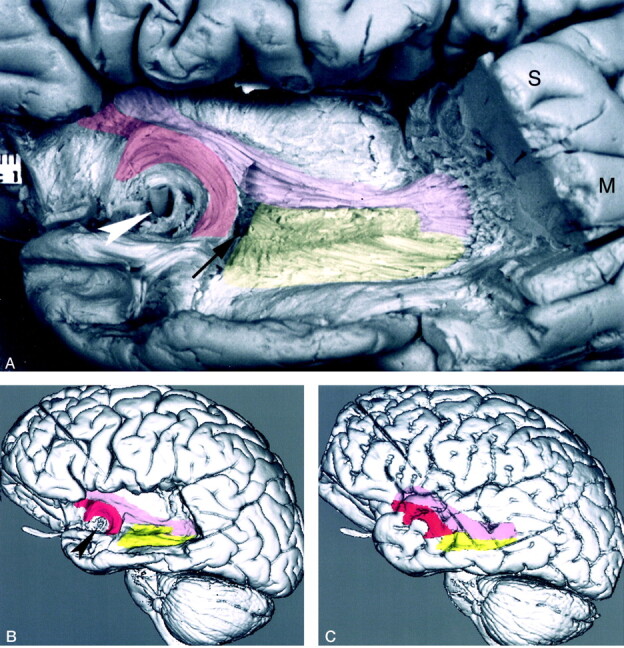
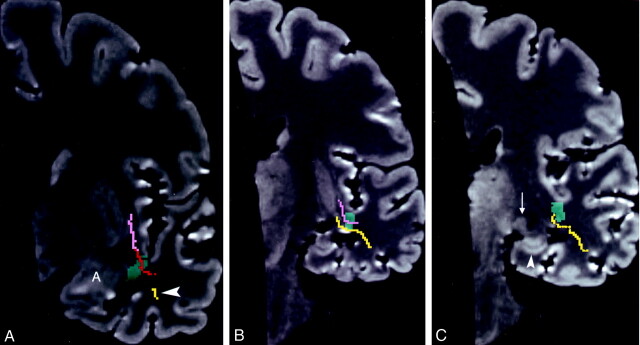
Dissected Meyer’s loop with the inferior occipitofrontal fasciculus and the uncinate fasciculus.
A, Photograph of the lateral aspect of a dissected cerebral hemisphere shows Meyer’s loop (yellow), the inferior occipitofrontal fasciculus (pink), and the uncinate fasciculus (red). This figure demonstrates additional dissection of the specimen in Figure 5A. Further dissection of the superior (S), middle (M), inferior temporal lobe gyri; the inferior occipitofrontal fasciculus; and the white matter of the temporal lobe was performed. To visualize the fiber bundles of the optic radiation, the fibers of the inferior part of the occipitofrontal fasciculus and the posterior part of the uncinate fasciculus were partially removed by dissection. The dissection demonstrates the multiple fibers of the optic radiation, which pass laterally and bend posteriorly to form the curving fiber bundles of Meyer’s loop. Of note is the proximity of the anterior commissure (arrow) to the anterior fiber bundle of Meyer’s loop, to the inferior occipitofrontal fasciculus, and to the uncinate fasciculus; the last curves around the horizontal segment of the middle cerebral artery (arrowhead). To demonstrate the underlying tracts, we used a 10% transparent red for the uncinate fasciculus, 10% pink for the inferior occipitofrontal fasciculus, and 10% transparent yellow for Meyer’s loop. To better visualize the dissected structures, the brain is tilted and angled, with the temporal lobe closer to the camera.
B, 3D MR rendering demonstrates the surfaces of the dissected Meyer’s loop, inferior occipitofrontal fasciculus and uncinate fasciculus. The inferior section of the inferior occipitofrontal fasciculus and the posterior section of the uncinate fasciculus shown in Figure 5B are not present here because they had to be removed by dissection to visualize the surface of Meyer’s loop. Arrowhead indicates the middle cerebral artery trunk.
C, 3D MR rendering of the undissected brain with the underlying dissected white matter tracts project to the cortical surface. The entire dissected surfaces of the uncinate fasciculus and inferior occipitofrontal fasciculus are shown in this image. The upper section of Meyer’s loop is not present on this image, as it is deep to the inferior occipitofrontal fasciculus.
The color tracing of Meyer’s loop shown in the multiplanar reformatted images demonstrates the location of segments of Meyer’s loop included in each MR sectional image (Figs 12 and 13). The most anterior extent of Meyer’s loop was at the level of the amygdala but did not reach the level of the tip of the temporal horn (Figs 12A and13A). Meyer’s loop extended over the roof of the temporal horn, passed through the temporal stem, and traveled along the lateral wall of the temporal horn (Fig 12A and B). Meyer’s loop was located deep to the inferior occipitofrontal fasciculus throughout much of its course. This relationship is shown in Figure 12B. In Figure 12C, the inferior occipitofrontal fasciculus is not included in the reformatting.
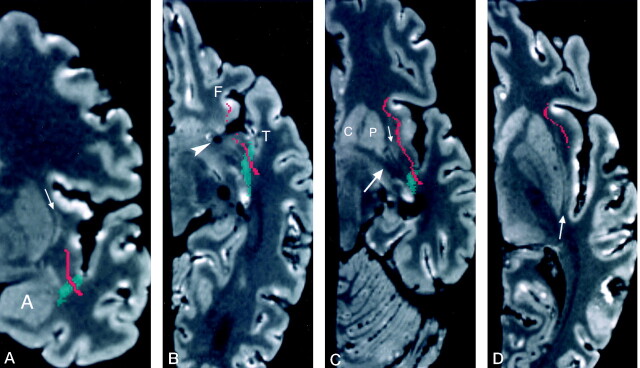
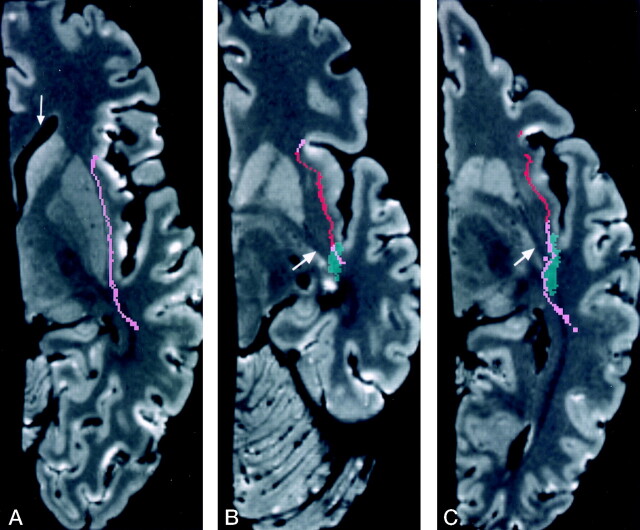
Fig 12.
Coronal images through Meyer’s loop.
A, Image at the level of the amygdala (A) shows the most anterior extent of Meyer’s loop (arrowhead) and its relationship to the dissected uncinate fasciculus (red) and the inferior occipitalfrontal fasciculus (pink). Coronal Talairach coordinate = −7.
B, Image at the level of the hippocampus (just anterior to the lateral geniculate body) shows Meyer’s loop superior and lateral to the temporal horn, crossing the temporal stem (green). Meyer’s loop (yellow) is located deep to the inferior occipitofrontal fasciculus (pink). This relationship is maintained throughout most of the course of these structures. Coronal Talairach coordinate = −18.
C, Image at the level of the lateral geniculate body (arrow) shows Meyer’s loop located above and lateral to the temporal horn. The inferior occipitofrontal fasciculus is not included in this image. Arrowhead indicates the hippocampus. Coronal Talairach coordinate = −23.
Fig 13.
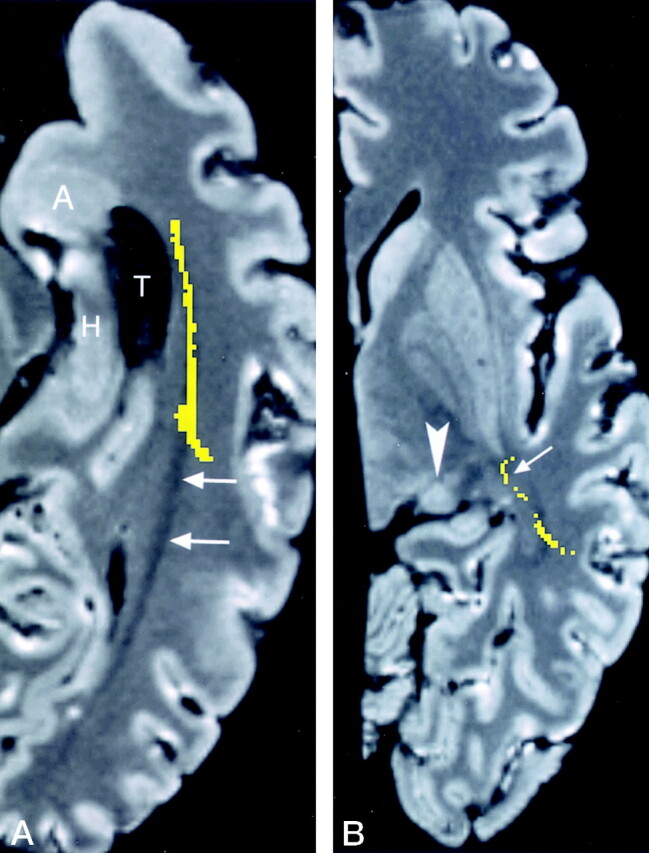
Axial images through Meyer’s loop.
A, Reformatted image obtained in an axial plane parallel to floor of the temporal lobe is advantageous for showing Meyer’s loop (yellow) coursing along the lateral wall of the temporal horn (T). Of note is the most anterior extent of Meyer’s loop, which is at the level of the amygdala (A) but does not reach the level of the tip of the temporal horn (compare with Fig 12A). The undissected optic radiation (arrows) is passing toward the occipital lobe. H indicates the hippocampus.
B, Oblique (+17°) axial image at the level of the lateral geniculate body (arrowhead), shows Meyer’s loop (arrow) crossing over the region of the roof of the temporal horn, which is below this image level. This relationship is more obvious on the coronal images in Figure 12B and C.
The anatomic information provided by the correlative anatomic and MR study of Meyer’s loop helped explain its involvement with lesions of the temporal lobe (Figs 14 and 15).
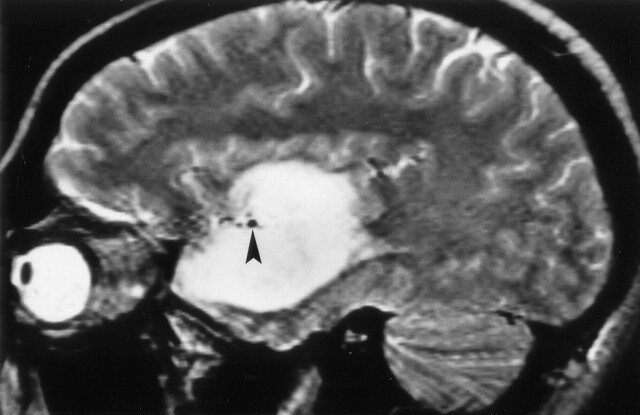
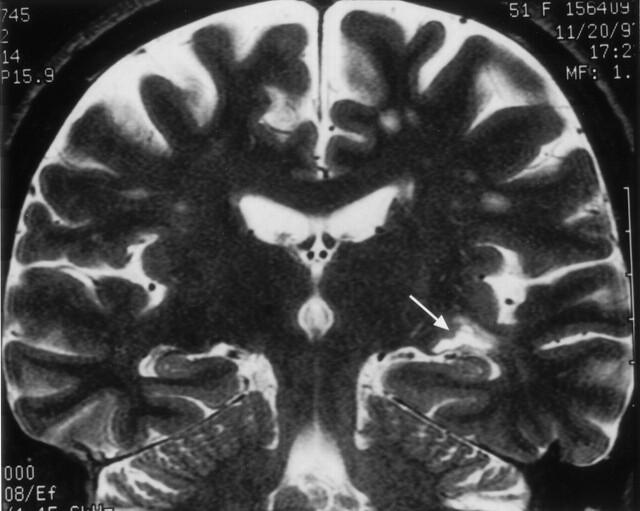
Fig 14.
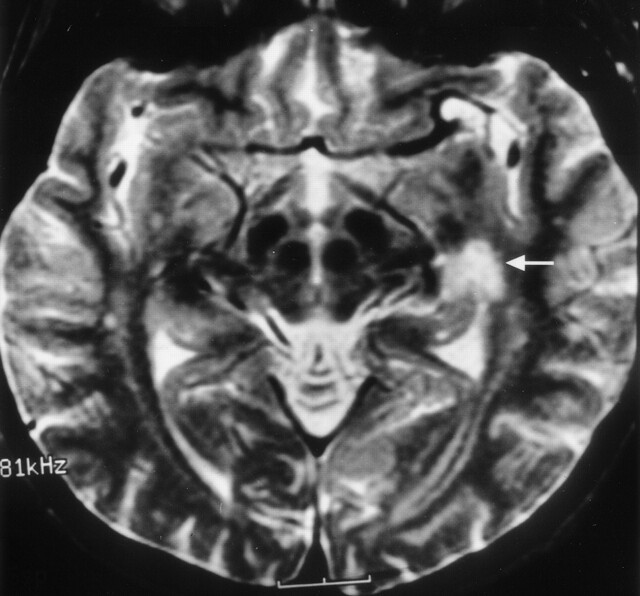
Clinical correlation of Meyer’s loop in the coronal plane. In this 51-year-old woman with a recent stroke and quadrantanopia, an ischemic lesion is present in the region of Meyer’s loop (arrow), superior to the hippocampus and temporal horn. Compare with Figure 12B
Fig 15.
Clinical correlation of Meyer’s loop in the axial plane. In this 51-year-old woman with a recent stroke and quadrantanopia (same patient as in Fig 14), an ischemic lesion is present in the region of Meyer’s loop (arrow). Compare with Figure 13B.
Discussion
The Temporal Stem
Anatomy.—
The stem of the temporal lobe (temporal stem) forms a bridge between the temporal lobe and other regions of the brain. It is important to establish its cross-sectional anatomy because of its role as a reciprocal route of tumor, infection, and seizure spread and also its functional significance. The term temporal stem seems to have resulted from the pictorial appearance of the structure on coronal anatomic sections of the brain and was apparently initiated by Horel (5). In an earlier publication, Horel and Misantone (6) referred to it as the albal stalk, “a thin band of white matter … that forms a bridge between the medullary core of the temporal lobe and the capsules of the brain stem.” The term albal stalks was also used in reference to the temporal lobe gyri of the macaque brain in an earlier publication (7). In the article published in 1978, Horel used temporal stem and albal stalk interchangeably and labeled the temporal stem on a coronal diagram of the right hemisphere of the brain (5). He stated that it contains connections of afferent and efferent fibers of the temporal cortex and amygdala but that it carried no connections of the hippocampus. Referring to Horel’s article, Cirillo et al (8) stated that the “medullary core of the temporal lobe in coronal section in humans has the form of a thick stem with its base pointed medially and with branches extending into the gyri of the temporal lobe.” They continued, “it is reasonable to assume that this white matter represents the connections between … the temporal cortex/amygdala and the orbital frontal cortex, the striatum and the thalamus.” Horel and Misantone (6) also described impaired visual discrimination resulting from transection of the temporal stem in monkeys. Since Horel’s articles, the term temporal stem has been frequently used in the literature, but rarely in the radiologic literature (9, 10)
Ebeling and Cramon’s (1) detailed article describes the temporal stem as forming a “narrow gate between the lower circular sulcus of the insula and the roof of the temporal horn in the deep white matter of the second temporal gyrus.” They describe the following fiber tracts passing through the temporal stem: anterior commissure, uncinate fasciculus, inferior occipitofrontal fasciculus, Meyer’s loop of the optic radiation, and inferior thalamic fibers.
Duvernoy (11) uses a slightly different landmark in describing the temporal stem as the narrow lamina of white matter between the temporal horn and the fundus of the superior temporal sulcus. We chose to use Ebeling and Cramon’s (1) landmarks because drawing the temporal stem line toward the superior temporal sulcus places the superior temporal gyrus outside the temporal lobe. Additionally, in many of the specimen photographs in Duvernoy’s books (3, 11, 12), the indicated location of the temporal stem appears to be closer to the circular sulcus of the insula than to the superior temporal sulcus.
Duvernoy’s (3, 12) specimen photographs indicate the region of the temporal stem extending from the level of the amygdala anteriorly to the level of the lateral geniculate body posteriorly. Therefore, in our investigation, we used these same anterior and posterior limits. Similar anteroposterior landmarks were used for transection of the temporal stem in monkeys studied for amnesia (13).
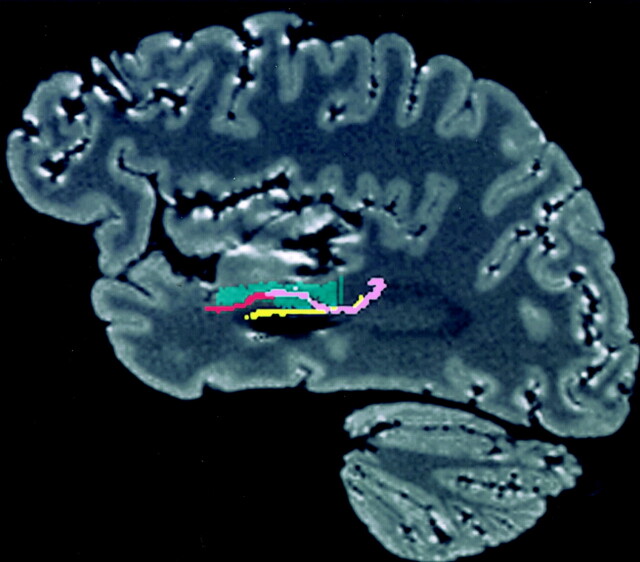
Our investigation shows that, at its anterior extent, the temporal stem is crossed by the uncinate fasciculus, while the inferior occipitofrontal fasciculus and Meyer’s loop cross its posterior extent (Figs 3, 6, 12, 16). The temporal stem appears to be supplied by the anterior choroidal artery (14).
Fig 16.
Relationship between the temporal stem and tracts on a reformatted sagittal MR image. The uncinate fasciculus (red) traverses the anterior part of the temporal stem (green), while the inferior occipitofrontal fasciculus (pink) and Meyer’s loop (yellow) pass through the posterior part. The inferior occipitofrontal fasciculus and Meyer’s loop pass through the temporal stem almost throughout its entire length.
Functional Significance.—
The temporal stem plays an important role in a number of disorders, including amnesia, Klüver-Bucy syndrome, traumatic brain injury, temporal lobe epilepsy, and Alzheimer disease.
Horel, in his publication introducing the term temporal stem, suggested that the amnesic syndrome that results from medial temporal removals was due to cutting some of the fiber connections that are contained within the stem rather than to hippocampal damage (5). Ebeling and Reulen (15) advised avoiding damaging the temporal stem during temporal lobe surgery. Surgical lesions of the temporal stem may result in abnormalities of learning, spatial, visual, and verbal functions. Additionally, the efferent fiber systems of the temporal allocortex, neocortex, and amygdala are severed, and the temporal association areas become isolated. Horel’s challenge to the hippocampal memory hypothesis has remained controversial and has generated a large number of experimental studies in monkeys and clinical studies in patients. Multiple investigations have dealt with this controversy as it specifically relates to the temporal stem (5, 6, 8, 13, 14, 16–25).
Studies in monkeys suggest that the Klüver-Bucy syndrome occurs when damage to the temporal stem and fornix substantially reduces information flow between the occipital, temporal, and frontal lobes (16, 23). Reduction in the volume of the temporal stem region has been shown to result from traumatic brain injury (10). As axons are the most susceptible brain structures to shear injury and its atrophic sequelae, the reduction in the volume of the temporal stem relates to it being the area in which both efferent and afferent white matter tracts converge as they enter and exit the temporal lobe.
An MR diffusion study in patients who underwent temporal lobe surgery for epilepsy showed elevated water diffusivity in the temporal stem on the side of the operation compared with the temporal stem of the contralateral side as well as with values in healthy subjects (26). The study also showed similar findings in the temporal stem of patients with mesial temporal sclerosis. The authors postulated that the increased water diffusivity in the temporal stem resulted from gliosis in the postoperative patients and from wallerian degeneration in the patients with mesial temporal sclerosis.
Increased water diffusion in the temporal stem has been described in MR diffusion studies of patients with Alzheimer disease. It has been postulated that the increased water diffusion results from disruption and loss of axonal membranes and the myelin in the fiber tracts of the temporal stem (9, 27).
Uncinate Fasciculus
Anatomy.—
The uncinate fasciculus of this investigation should be distinguished from the uncinate fasciculus of the cerebellum, which is also known as the uncinate fasciculus of Russell (28). The uncinate (hooklike) fasciculus we studied is also named the temporofrontal (frontotemporal) fasciculus.
The uncinate fasciculus can be divided into temporal, insular, and frontal segments (1). The temporal segment of the uncinate fasciculus originates from cortical nuclei of the amygdala (areas 28, 34, and 36) and the anterior three temporal convolutions (areas 20 and 38), in front of the temporal horn. All the fibers come together as a solid tract in the anterior temporal stem in the white matter of the middle temporal gyrus, anterior to the temporal horn. The uncinate fasciculus passes upward over the lateral nucleus of the amygdala toward the limen insula. The insular segment of the uncinate fasciculus, in the limen insulae, is situated below the putamen and the claustrum. The limen insulae is the threshold of the island of Reil and a band of transition between the anterior portion of the gray matter of the insula and the anterior perforated substance (28). The insular segment of the uncinate fasciculus measures 2–5 mm in height and 3–7 mm in width (1). The inner fibers of the uncinate fasciculus pass through the external capsule and the outer fibers pass through the extreme capsule (1, 29, 30). Therefore, a part of the claustrum is imbedded in the uncinate fasciculus. In the region of the extreme and external capsules, the uncinate fasciculus is inferior to the inferior occipitofrontal fasciculus and there is some merging of these two fiber bundles (1, 31). The frontal segment of the uncinate fasciculus has a fanlike shape in the frontal-orbital white matter and is oriented in a horizontal plane. Its frontal fibers are adjacent to the lateral border of the inferior occipitofrontal fasciculus without a distinct separation between these two fiber tracts. Near the frontal pole of the hemisphere the uncinate fasciculus and inferior occipitofrontal fasciculus fibers are greatly intermingled (32).
The fibers of the uncinate fasciculus connect the cortical nuclei of the amygdala and the uncus with the subcallosal region. The tips of the superior, medial, and inferior temporal gyri are connected, via the uncinate fasciculus, with the gyrus rectus and medial and lateral orbital gyri as well as the orbital segment of the inferior frontal gyrus. It consists of both afferent and efferent fibers to both the temporal and frontal areas (32). The uncinate fasciculus has a ventral part that connects the orbital cortex with the amygdala and hippocampal gyrus, and a dorsal section that interconnects the temporal pole cortex with the rostral end of the middle frontal gyrus (33, 34).
Functional Significance.—
Several experimental studies in monkeys have shown that the uncinate fasciculus is a monosynaptic corticocortical route of interaction between the temporal and frontal lobes. Review of many experimental studies supports the role of the uncinate fasciculus as one of several connections whose disruption results in severe memory impairment (25). The disruption in connectivity between the temporal and frontal lobes via the uncinate fasciculus is postulated as a possible cause of posttraumatic retrograde amnesia (35). In addition, patients who undergo anterior temporal lobectomy have object and action naming deficits resulting from the disruption of frontotemporal connections mediated by the uncinate fasciculus (36). However, it appears that the uncinate fasciculus does not play a role in visual memory (25, 37, 38).
Results of diffusion-tensor imaging studies seem to support the long-standing theory that disruption in connectivity between the temporal and frontal lobes via the uncinate fasciculus may partly explain some of the symptoms of schizophrenia (39, 40). Patients with schizophrenia lack the normal left-greater-than-right asymmetry in the anisotropy in the regions of the uncinate fasciculus. These findings support the theory that patients with schizophrenia have abnormalities of myelin and reduced neuronal integrity in the uncinate fasciculus. The lack of asymmetry in the size of the uncinate fasciculus in patients with schizophrenia has also been studied pathologically (41).
Inferior Occipitofrontal Fasciculus
Anatomy.—
Although the inferior occipitofrontal fasciculus is a prominent component of the temporal stem, its anatomy is not widely known. It is rarely mentioned in the current literature (1, 27, 42–44). The inferior occipitofrontal fasciculus is often confused with the uncinate fasciculus (45). The inferior occipitofrontal fasciculus is also referred to as the inferior fronto-occipital fasciculus (33, 42, 46). Curran (47) provided the most detailed and classic description of the dissected inferior occipitofrontal fasciculus with photographs of an actual dissection. Several other publications also include actual dissections of the uncinate fasciculus and inferior occipitofrontal fasciculus (34, 48–52).
Davis (53) confirmed Curran’s description of the inferior occipitofrontal fasciculus. In his detailed and informative study, Davis reviewed the controversial and confusing historical description and nomenclature of the tracts of the occipital and temporal lobes. He challenged the classic description and illustration of the inferior longitudinal fasciculus of a tract extending from the occipital to the temporal poles. This description is still used in the current literature. Davis stated, “the fibers in question can be definitely shown to continue in unbroken fashion from the occipital to the frontal lobes. In fact, no bundle of fibers can be found by dissection, which fits the description accepted by textbook authors. I believe that the more definite name, fasciculus occipitofrontal inferior, should be used to designate this bundle.”
According to Curran (47), the inferior occipitofrontal fasciculus is a large association bundle of fibers connecting the occipital and frontal lobes. It also contains fibers that connect the frontal lobe with the posterior part of the parietal and temporal lobes. From the lateral aspect of the frontal lobe, including the Broca region, the fibers of this fasciculus converge into a single bundle that passes along the lateral inferior aspect of the lentiform nucleus at the inferior aspect of the claustrum and superior to the uncinate fasciculus. The inferior occipitofrontal fasciculus proceeds posteriorly as a distinct, almost round, bundle and again spreads out to a fan shape as it approaches and enters the occipital lobe. As the inferior occipitofrontal fasciculus proceeds posteriorly, its inferior fibers pass along the temporal and occipital horns of the lateral ventricle. The optic radiation and the tapetum of the corpus callosum lie between the inferior occipitofrontal fasciculus and the ependyma of the lateral ventricle. The relationship of these structures is maintained along the entire length of the temporal horns. However, as these tracts proceed posteriorly along the occipital horn, there is frequent intercrossing of the fibers of these tracts.
In the temporal lobe, the inferior occipitofrontal fasciculus is associated with the inferior longitudinal fasciculus which interconnects the occipital cortex (lingula, cuneus, and lateral surface) with the cortex of the superior, middle and inferior temporal (and possibly the hippocampal) gyri (31, 47). The inferior occipitofrontal fasciculus is also described as connecting more superiorly located areas in the frontal lobe with more posterior areas in the temporal lobe than those connected by the uncinate fasciculus. It thus appears to include fibers connecting the auditory (area 22), and visual (areas 20 and 21) association cortex in the temporal lobe with the prefrontal cortex (54, 55).
The anatomic relationship of the inferior occipitofrontal and uncinate fasciculi in the insular region has been described in a number of ways. Curran (47) describes the inferior occipitofrontal fasciculus as swinging “to the lower external side of the lenticular nucleus and the external capsule.” Although he does not specifically state it, his description implies that the inferior occipitofrontal fasciculus is situated in the extreme capsule. Riley (30) shows the inferior occipitofrontal fasciculus only within the extreme capsule. However, Ebeling and Cramon (1) state that the inferior occipitofrontal fasciculus is located both in the extreme and external capsules. According to Klingler and Gloor (56) the uncinate fasciculus and the inferior occipitofrontal fasciculus also pass together between the two plates of the claustral gray matter. Our investigation showed that the uncinate fasciculus and the inferior occipitofrontal fasciculus to be present both in the extreme and external capsules.
Curran (47) briefly mentions that he had to remove the upper part of the uncinate fasciculus to demonstrate the lower segment of the inferior occipitofrontal fasciculus. Although Ebeling and Cramon (1) briefly state that the uncinate fasciculus is lateral to the inferior occipitofrontal fasciculus, their line drawing shows the inferior occipitofrontal fasciculus superior to the uncinate fasciculus and this relationship is stated in the legend of the figure. Riley’s (30) histologic atlas shows the inferior occipitofrontal fasciculus adjacent and superior to the uncinate fasciculus. This relationship is also diagrammed by other authors (57).
The relationship of the uncinate fasciculus and inferior occipitofrontal fasciculus to the anterior commissure in the axial plane depends on the angulation of the axial plane, and a minimal change in the axial level of the section. This can be seen by comparing Figures 3C and 7B and C. The aforementioned relationships are explained by the close relationship of these structures, as shown in Riley’s atlas (30). Curran stated, “In the region where the anterior commissure enters the temporal lobe and where its fibers spread out and mingle with the temporal part of the corona radiata … the corona and the anterior commissure, together with a part of the uncinate fasciculus … form the bed on which this part of the inferior occipitofrontal fasciculus rests.” Therefore, depending on the angulation of the axial plane and minimal change in the level of the section, tumor spread at the level of the anterior commissure level may involve the uncinate fasciculus, the inferior occipitofrontal fasciculus, or both.
Functional Significance.—
Although the temporal stem and the uncinate fasciculus are frequently described routes of lesion spread between the temporal and frontal lobes, the role of the inferior occipitofrontal fasciculus is rarely mentioned in the literature (42). Usually, its involvement is incorrectly attributed to the uncinate fasciculus alone. In the neuroradiologic literature, Cowley (42) examined the role of the inferior occipitofrontal fasciculus in the spread of edema in an experimental monkey model and in CT studies of patients with vasogenic edema. In addition, both the uncinate fasciculus and the inferior occipitofrontal fasciculus have a role in extratemporal lesions triggering temporal-lobe syndromes, such as visual hallucinations (31–33). In some patients, lesions of the inferior occipitofrontal fasciculus in association with several other tracts contribute to produce global aphasia (34).
Review of the literature for experimental studies of amnesia and clinical disorders (eg, postoperative cognitive impairment, posttraumatic brain syndromes, schizophrenia, Alzheimer disease) revealed that the inferior occipitofrontal fasciculus is an unrecognized component of the temporal stem region involved in these abnormalities. The results of this investigation accentuate the major role that the previously neglected inferior occipitofrontal fasciculus is a route of temporal tumor spread and also a potentially important component of disorders in the region of the temporal stem. Additionally, because of the long anteroposterior extent of the inferior occipitofrontal fasciculus in the temporal lobe and temporal stem, it may play a larger role than the uncinate fasciculus in the spread of lesions beyond the temporal lobe.
Meyer’s Loop of the Optic Radiation
Anatomy.—
The MR anatomy of Meyer’s loop of the optic radiation pathway is not well known. Although diagrammatic representations of Meyer’s loop are common in the literature, photographs of actual dissections are rare (48–50, 58). The fibers of the optic radiation pass from the lateral geniculate body to the visual region in the occipital cortex. After leaving the cells of the lateral geniculate body, the optic radiation divides into three bundles (15). The fibers of the anterior bundle course laterally and anteriorly over the roof of the temporal horn, and then curve backward, forming Meyer’s loop, also called the Meyer-Archambault loop (28). The initial terms used by investigators in the late 19th and early 20th centuries were the temporal knee and the temporal loop (59). According to Elze (60), Flechsig first described the temporal knee in the fetal brain, where it is apparently easier to see.
After curving posteriorly, the fibers of Meyer’s loop course along the inferolateral aspect of the temporal horn (15, 59). Our study demonstrated the close relationship of the optic radiation and the inferior occipitofrontal fasciculus along the wall of the temporal horn (Fig 12B). This relationship continues along the lateral wall of the occipital horn (47). As the fibers course posteriorly, along the trigone and the temporal horn, they pass under the lateral ventricle and enter the lower lip of the calcarine fissure. The central bundle passes directly laterally, crosses the roof of the temporal horn and then courses along the lateral wall and roof of the trigone and occipital horn to the occipital pole. The posterior bundle courses directly posteriorly, over the roof of the trigone and occipital horn, to the upper lip of the calcarine fissure. Thus, the lateral wall and the roof of the temporal and occipital horns are formed by the three bundles of the optic radiation.
According to Rasmussen (58) the optic radiation fibers are separated from the ependyma of the temporal horn by a thin layer of corpus callosum fibers—the tapetum. The fibers of the corpus callosum are more prominent posteriorly. Anteriorly, the intervening tapetum forms a thinner and thinner layer and ultimately disappears completely. Rasmussen noted that some of the looping fibers are difficult to display because of their small size and the great admixture with other fiber tracts.
Functional Significance.—
The dissections in the present investigation demonstrated the arrangement of Meyer’s loop in bundles (Fig 11A). These loops are arranged in an anterior to posterior direction and represent specific and precisely ordered sectors in the superior quadrant of the visual field (61–63).
The anterior bundle, Meyer’s loop, has a variable and controversial anterior course before passing backwards. The exact position of Meyer’s loop is important for surgery of the anterior temporal lobe, as it can be damaged during surgery resulting in a homonymous upper quadrantanopia (quadrantic hemianopia). The exact relationship of the most anterior fibers of Meyer’s loop to the tip of the temporal horn is also controversial (15, 64) and is based on clinical observations of the presence or absence of visual field defects following temporal lobe surgery. In his dissection of Meyer’s loop, Rasmussen (58) noted that some of the fibers come close to the fibers of the anterior commissure and that they can be demonstrated by dissection as far forward as the tip of the temporal horn and the lateral surface of the amygdaloid nucleus. Van Buren and Baldwin (61) noted that the tip of the temporal horn is not capped by optic radiation fibers. This fact was also observed in our investigation (Fig 13A).
According to Ebeling and Reulen (15) the distance between the tip of the temporal lobe and the anterior edge of Meyer’s loop is 27 ± 3.5 mm, with a range of 22–37 mm. The authors state that the average location of the anterior edge of Meyer’s loop is 5 ± 3.9 mm in front of the tip of the temporal horn; the most anterior location is 10 mm, and the most posterior is 5 mm behind the tip of the temporal horn. The anterior limit of Meyer’s loop as manifested by visual field deficit in patients who underwent temporal lobe resections for epilepsy has been investigated (64, 65). The high percentage of superior homonymous field cuts contralateral to the resection encountered in their patients indicated that the anterior extent of Meyer’s loop runs more rostral than the tip of the temporal horn and may lie 20 mm from the tip of the temporal lobe tip. Therefore, limiting the resection to 20 mm from the temporal tip could preserve the optic radiation.
Implications for Diffusion Imaging
This investigation provides anatomic and MR data applicable to in vivo white matter tractography. Diffusion-tensor imaging has been successful in the study of large white matter tracts such as the corpus callosum and the corticospinal tracts, which consist of numerous, tightly packed fiber bundles coursing in the same direction. These tracts are clearly identified on MR studies and easily correlated with their depiction in classic anatomic texts. However, several brain white matter tracts cannot be identified on MR studies because they are indistinguishable from the surrounding white matter. Fibers of the uncinate fasciculus, the inferior occipitofrontal fasciculus, and similar white matter tracts are intermingled with other fibers that course in various directions (47). This may explain why they are not clearly visible on MR images. As a result, their depiction in current articles describing diffusion-tensor imaging is imprecise, as it is based on sketches of the tracts and not on precise data provided by dissected material. With the precise MR location of these tracts obtained by using our dissection tractography method, normal in vivo diffusion-tensor imaging of these tracts will gain in accuracy.
Additionally, the analysis of in vivo diffusion tractography of the temporal stem and its tracts, used in the investigation of brain disorders (eg, epilepsy, postoperative complications, craniocerebral trauma, schizophrenia, Alzheimer disease) can become more precise, as it will be based on a solid anatomic foundation.
Conclusions
This anatomic and cross-sectional MR investigation clarified the anatomy of the uncinate and inferior occipitofrontal fasciculi in the anterior temporal lobe and in the external and extreme capsules. The anatomy of Meyer’s loop and the temporal stem was also delineated. The inferior occipitofrontal fasciculus is a major, previously neglected component of the anterior temporal lobe, temporal stem, and external and extreme capsules. The inferior occipitofrontal fasciculus is an important route of tumor, infection, and seizure spread. Its role in other brain disorders resulting from disconnection of the temporal and frontal lobes has not been appreciated in the literature, and its involvement has been incorrectly attributed to the uncinate fasciculus alone. Patterns of lesionspread beyond the temporal lobe are best understood in terms of white matter tracts. Involvement of multiple white matter tracts—the uncinate and inferior occipitofrontal fasciculi frontally and the inferior occipitofrontal fasciculus and optic radiation occipitally—is common. This study also provided anatomic and MR data that will enable more precise in vivo diffusion-tensor imaging of the normal white matter tracts and their abnormalities.
References
- 1.Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148 [DOI] [PubMed] [Google Scholar]
- 2.Kier EL, Staib LH, Davis LM, Bronen RA. Anatomic dissection tractography: a new method for precise MR localization of white matter tracts. AJNR Am J Neuroradiol 2004. :25:000–000 [PMC free article] [PubMed] [Google Scholar]
- 3.Duvernoy HM. The Human Brain, Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. 2nd ed. Vienna: Springer-Verlag,1999;122–143
- 4.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme,1988. :5
- 5.Horel JA. The neuroanatomy of amnesia: a critique of the hippocampal memory hypothesis. Brain 1978;101(3):403–445 [DOI] [PubMed] [Google Scholar]
- 6.Horel JA, Misantone LJ. Visual discrimination impaired by cutting temporal lobe connections. Science 1976;193:336–338 [DOI] [PubMed] [Google Scholar]
- 7.Whitlock D, Nauta W. Subcortical projections from the temporal neocortex in macaca mulatta. J Comp Neurol 1956;106:183–212 [DOI] [PubMed] [Google Scholar]
- 8.Cirillo RA, Horel JA, George PJ. Lesions of the anterior temporal stem and the performance of delayed match-to-sample and visual discriminations in monkeys. Behav Brain Res 1989;34(1–2):55–69 [DOI] [PubMed] [Google Scholar]
- 9.Kantarci K, Jack CR, Jr, Xu YC, et al. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology 2001;219(1):101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigler ED, Anderson CV, Blatter DD, Andersob CV. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 2002;23:255–266 [PMC free article] [PubMed] [Google Scholar]
- 11.Duvernoy HM. The Human Hippocampus. 2nd ed. Berlin: Springer-Verlag,1998;39:115–31 [Google Scholar]
- 12.Duvernoy HM. The Human Brain Stem and Cerebellum: Surface, Structure, Vascularization, and Tri Dimensional Sectional Anatomy with MRI. Vienna: Springer-Verlag,1995;266–295
- 13.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: amygdala-hippocampus versus temporal stem. Science 1982;218(4579):1337–1339 [DOI] [PubMed] [Google Scholar]
- 14.Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang 1997;58:355–402 [DOI] [PubMed] [Google Scholar]
- 15.Ebeling U, Reulen HJ. Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir 1988;92:29–36 [DOI] [PubMed] [Google Scholar]
- 16.Horel JA, Misantone LJ. The Kluver-Bucy syndrome produced by partial isolation of the temporal lobe. Exp Neurol 1974;42(1):101–112 [DOI] [PubMed] [Google Scholar]
- 17.Squire LR. The neuropsychology of human memory. Ann Rev Neurosci 1982;5:241–273 [DOI] [PubMed] [Google Scholar]
- 18.Zola-Morgan S, Squire LR. Preserved learning in monkeys with medial temporal lesions: sparing of motor and cognitive skills. J Neurosci 1984;4(4):1072–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corkin S, Amaral DG, Gonzalez RG, et al. H. M.’s medial temporal lobe lesion: findings from magnetic resonance imaging. J Neurosci 1997;17(10):3964–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Easton A, Gaffan D. Crossed unilateral lesions of the medial forebrain bundle and either inferior temporal or frontal cortex impair object-reward association learning in Rhesus monkeys. Neuropsychologia 2001;39(1):71–82 [DOI] [PubMed] [Google Scholar]
- 21.Gaffan D. What is a memory system? Horel’s critique revisited. Behav Brain Res 2001;127(1–2):5–11 [DOI] [PubMed] [Google Scholar]
- 22.Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia 2001;39(1):51–70 [DOI] [PubMed] [Google Scholar]
- 23.Maclean CJ, Gaffan D, Baker HF, et al. Visual discrimination learning impairments produced by combined transections of the anterior temporal stem, amygdala and fornix in marmoset monkeys. Brain Res 2001;888(1):34–50 [DOI] [PubMed] [Google Scholar]
- 24.Easton A, Ridley RM, Baker HF, et al. Unilateral lesions of the cholinergic basal forebrain and fornix in one hemisphere and inferior temporal cortex in the opposite hemisphere produce severe learning impairments in rhesus monkeys. Cereb Cortex 2002;12(7):729–736 [DOI] [PubMed] [Google Scholar]
- 25.Gaffan D, Easton A, Parker A. Interaction of inferior temporal cortex with frontal cortex and basal forebrain: double dissociation in strategy implementation and associative learning. J Neurosci 2002;22(16):7288–7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kantarci K, Shin C, Britton JW, et al. Comparative diagnostic utility of 1H MRS and DWI in evaluation of temporal lobe epilepsy. Neurology 2002;58(12):1745–1753 [DOI] [PubMed] [Google Scholar]
- 27.Hanyu H, Sakurai H, Iwamoto T, et al. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer’s disease. J Neurol Sci 1998;156:195–200 [DOI] [PubMed] [Google Scholar]
- 28.Stedman’s Medical Dictionary. 25th ed. Baltimore: Williams & Wilkins,1990
- 29.Dejerine J. Anatomie Des Centres Nerveux. Vol 1. Paris: J Rueff,1895. :532–577
- 30.Riley HA. An Atlas of the Basal Ganglia, Brain Stem and Spinal Cord. Based on Myelin-Stained Material. Baltimore: Williams & Wilkins,1943;282–310, 576, 582–583
- 31.Crosby EC, Humphery T, Lauer EW. Correlative Anatomy of the Nervous System. New York: MacMiillan,1962;357:402–409 [Google Scholar]
- 32.Schneider RC, Crosby EC, Bacchi BK, et al. Temporal or occipital hallucinations triggered from frontal lobe lesions. Neurology 1961;11:172–179 [DOI] [PubMed] [Google Scholar]
- 33.Schneider RC, Crosby EC, Farhat SM. Extratemporal lesions triggering the temporal-lobe syndrome. J Neurosurg 1965;22:246–253 [DOI] [PubMed] [Google Scholar]
- 34.Schneider RC, Crosby EC, Calhoun HD. Surgery of convulsive seizures and allied disorders. In: Schneider RC, Kahn EA, Crosby EC, Taren JA, eds. Correlative Neurosurgery. Springfield: Thomas,1982;1:576–580 [Google Scholar]
- 35.Levine B, Black SE, Cabeza R, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain 1998;121(pt 10):1951–1973 [DOI] [PubMed] [Google Scholar]
- 36.Lu LH, Crosson B, Nadeau SE, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia 2002;40(9):1608–1621 [DOI] [PubMed] [Google Scholar]
- 37.Gaffan D, Eacott MJ. Visual learning for an auditory secondary reinforcer by macaques is intact after uncinate fascicle section: indirect evidence for the involvement of the corpus striatum. Eur J Neurosci 1995;7(9):1866–1871 [DOI] [PubMed] [Google Scholar]
- 38.Gaffan D, Eacott MJ. Uncinate fascicle section leaves delayed matching-to-sample intact, with both large and small stimulus sets. Exp Brain Res 1995;105(1):175–180 [DOI] [PubMed] [Google Scholar]
- 39.Kubicki M, Westin CF, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry 2002;159(5):813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns J, Job D, Bastin ME, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 2003;182:439–443 [PubMed] [Google Scholar]
- 41.Highley JR, Walker MA, Esiri MM, et al. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex 2002;12(11):1218–1224 [DOI] [PubMed] [Google Scholar]
- 42.Cowley AR. Influence of fiber tracts on the CT appearance of cerebral edema: anatomic-pathologic Correlation. AJNR Am J Neuroradiol 1983;4:915–925 [PMC free article] [PubMed] [Google Scholar]
- 43.Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002;17(1):77–94 [DOI] [PubMed] [Google Scholar]
- 44.Sindou M, Guenot M. Surgical anatomy of the temporal lobe for epilepsy surgery. Adv Tech Stand Neurosurg 2003;28:315–343 [DOI] [PubMed] [Google Scholar]
- 45.Sedat J, Duvernoy H. Anatomical study of the temporal lobe: correlations with nuclear magnetic resonance. J Neuroradiol 1990;17(1):26–49 [PubMed] [Google Scholar]
- 46.Duus P. Topical Diagnosis in Neurology. New York: Thieme,1989;258–260
- 47.Curran EJ. A new association fiber tract in the cerebrum. with remarks on the fiber tract dissection method of studying the brain. J Comp Neurol 1909;19:645–656 [Google Scholar]
- 48.Bassett DL. A Stereoscopic Atlas of Human Anatomy. Section I: The Central Nervous System. Portland: Sawyer’s,1952. :reels 13–5,13–6, 14–2, 14–3, 14–4
- 49.Ludwig E, Klingler J. Atlas Cerebri Humani. Boston: Little, Brown,1956;plates5–8
- 50.Gluhbegovic N, Williams TH. The Human Brain: A Photographic Guide. Philadelphia: Harper & Row,1980;127–129
- 51.Montemurro DG, Bruni JE. The Human Brain in Dissection. 2nd ed. New York: Oxford University Press,1988;46–56
- 52.Kiernan JA. Barr’s The Human Nervous System. 7th ed. Philadelphia: Lippincott-Raven,1998;298–312
- 53.Davis LE. An anatomic study of the inferior longitudinal faciculus. Arch Neurol Psychiatr 1921;5:370–381 [Google Scholar]
- 54.Gloor P. The Temporal lobe and Limbic System. New York: Oxford University Press,1997;239–241
- 55.Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol 1988;273(1):52–66 [DOI] [PubMed] [Google Scholar]
- 56.Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol 1960;115:333–369 [DOI] [PubMed] [Google Scholar]
- 57.Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System: a Synopsis and Atlas. 3rd ed. New York: Springer-Verlag,1988. :98 ,367 [Google Scholar]
- 58.Rasmussen AT. The extent of recurrent geniculo-calcarine fibers (loop of Archambault and Meyer) as demonstrated by gross brain dissection. Anat Rec 1943;85:277–284 [Google Scholar]
- 59.Polyak S. The Vertebrate Visual System. Chicago: University of Chicago Press,1957;405–409
- 60.Elze C. Einige fasersysteme des mensnschlichen grosshirnes mit der abfaserungsmethode untersuchts. Z Anat 1928;88:166–178 [Google Scholar]
- 61.Van Buren J, Baldwin M. The architecture of the optic radiation in the temporal lobe in man. Brain 1958;81:15–40 [DOI] [PubMed] [Google Scholar]
- 62.Miller NR. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. 4th ed. Baltimore: Williams & Wilkins,1982. :1:79–82 [Google Scholar]
- 63.Hughes TS, Abou-Khalil B, Lavin PJ, et al. Visual field defects after temporal lobe resection: a prospective quantitative analysis. Neurology 1999;53(1):167–172 [DOI] [PubMed] [Google Scholar]
- 64.Krolak-Salmon P, Guenot M, Tiliket C, et al. Anatomy of optic nerve radiations as assessed by static perimetry and MRI after tailored temporal lobectomy. Br J Ophthalmol 2000;84(8):884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guenot M, Krolak-Salmon P, Mertens P, et al. MRI assessment of the anatomy of optic radiations after temporal lobe epilepsy surgery. Stereotac Func Neurosurg 1999;73:84–87 [DOI] [PubMed] [Google Scholar]