Abstract
Summary: A 35-year-old Korean woman had Hashimoto encephalopathy of varying MR imaging appearance over 5 years that ranged from that of transient subcortical ischemia to that of gradually evolving multifocal signal intensity change accompanied by unilateral cerebellar atrophy. Thus, the MR imaging appearance of Hashimoto encephalopathy may simulate an ischemic stroke, multiple tumors or granulomas, or even a degenerative process.
Clinical presentations of Hashimoto encephalopathy (HE) are protean and nonspecific. Subacute encephalopathy with seizures or movement disorder, sometimes mimicking a prion disease, has frequently been reported (1). Despite a suggestion of cortical and subcortical system involvement in these cases, MR images have usually been unremarkable. A subgroup of patients presenting with clinical signs and symptoms referable to brain stem or cerebellum may demonstrate a transient focal abnormality consistent with an underlying pathophysiologic mechanism of vasculitis (2). Persistent focal lesions, atypical for vasculitis, may suggest another diagnosis. We report a case of HE and its MR imaging appearance, which included a transient subcortical ischemia to evolving multifocal signal intensity changes accompanied by unilateral cerebellar atrophy occurring over 5 years.
Case Report
Five years before admission, this 35-year-old woman experienced the onset of neurologic illness starting with a partial seizure disorder. An electroencephalogram at that time showed a left temporal lobe epileptiform discharge, and the MR image showed a nonenhancing left hippocampal mass and a right medullary lesion, best seen on fluid-attenuated inversion recovery (FLAIR) and T2-weighted images. (Fig 1A and D). Neurologic examination was normal, and she was treated with carbamazepine. A year later, she had a transient confusion accompanied by weakness of the left arm. Two years after the initial onset of symptoms, she developed gait unsteadiness and left-sided clumsiness. Follow-up MR imaging at that time showed resolution of the right basal ganglia ischemic lesion that was detected a year earlier (not shown).
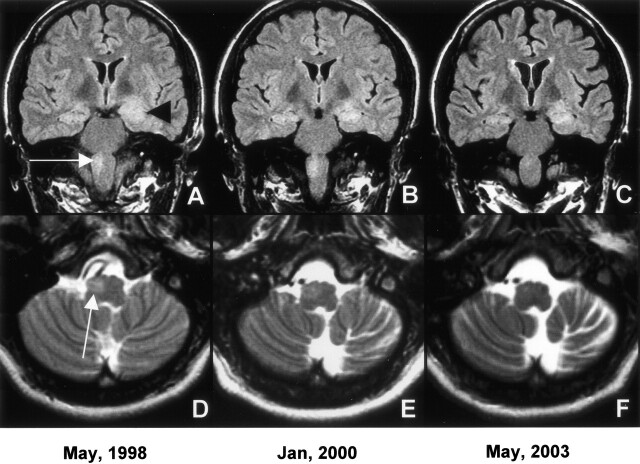
Fig 1.
Coronal FLAIR images (A–C) document decreasing left hippocampal mass effect and signal intensity over 5-year period (black arrowhead). In panel C, early cortical atrophy is evident. Evolving right medullary lesion (white arrow) and progressive contralateral cerebellar atrophy is seen on axial T2-weighted images (D–F).
In addition, the left hippocampal abnormality decreased in size, the right medullary lesion became less hyperintense, and early left cerebellar atrophy was seen (Fig1B and E). By the 4th year of her illness, the gait unsteadiness and tremor deteriorated to the point that she became wheelchair bound. Examination at that time showed bilateral Parkinsonian features with increased tone, marked limb unsteadiness, and intermittent upper extremity tremor without a palatal or ocular myoclonus. Extensive laboratory evaluation disclosed hypothyroidism with decreased T4 of 1 ∝g/dL (4.7–12.5∝g/dL) and T3 of 1 ng/dL (76–190 ng/dL), but a normal thyroid stimulating hormone level of 0.34 ∝lU (0.30–5.02 ∝lU). Elevated thyroid antibodies consisting of anti-thyroglobulin antibody at 117 U/mL (0–100U/mL) and antimicrosomal antibody of 1105 U/mL (0–100 U/mL) confirmed Hashimoto thryoiditis.
Markers for other collagen vascular diseases were negative, including normal antinuclear antibody, complement, and rheumatoid factor levels. The most recent MR imaging showed marked left cerebellar atrophy and near-complete resolution of the right medullary signal intensity but persistent left hippocampal signal intensity abnormality (Fig 1C and F). She was started on a thyroid supplement and a high-dose steroid. Over the next several weeks, she improved markedly and is now able to ambulate without assistance.
Discussion
The patient had documented evolving MR imaging changes spanning 5 years of a transient ischemia, left hippocampal and right medullary lesions, and a unilateral cerebellar atrophy illustrating a wide range of MR imaging appearances that can be seen in a single case of HE. Despite the advanced unilateral cerebellar atrophy, marked improvement with steroids was seen, consistent with clinico-radiologic dissociation that is often seen in HE. Among HE cases presenting with seizures, bitemporal symmetrical hippocampal lesions with focal edema have been reported (3). Our case differs in its unilaterality and delayed gradual resolution of the edematous lesion when followed with sequential MR imaging. In our patient, a separate medullary lesion was initially present, which helped exclude a tumor or an infection; however, in cases with a single mass, a biopsy may have to be considered.
Slowly evolving signal intensity change was also seen in the right medulla involving the inferior olivary nucleus. Subsequent progressive contralateral cerebellar atrophy is consistent with the known pathway of inferior olivary to contralateral dentate nucleus as part of the Guillain-Mollaret triangle. An unusual movement disorder, known as myorhythemia-palatal myoclonus from involvement of Guillain-Mollaret triangle, has been emphasized as a manifestation of HE (4), although both reported patients had normal MR imaging results. In our case, it was difficult to identify myorhythemia clearly in the presence of marked ataxia and Parkinsonism, because a distinctive slow-resting tremor was not observed. Selim and Drachman (5) were able to study five women and one man, all clinically euthyroid, from one institution during a 12-year period. All presented with progressive ataxia of months to years in duration. MR images showed bilateral midline cerebellar atrophy, and an additional two patients had pontine atrophy, simulating olivopontocerebellar degeneration.
Pathologic studies have been rare. Single case reports document changes typical of vasculitis with perivascular lymphocytic infiltration primarily involving leptomeninges and brain stem venules on autopsy (6) and both arterioles and venules from a cerebral biopsy specimen (7). Whether chronic vasculitis is responsible for slowly evolving lesions seen at MR imaging is presently an unresolved issue. Similarly, the role of antithyroid antibodies that are intrathecally produced in HE is unclear (8).
Our case demonstrates that focal lesions in HE may simulate cerebral tumor, granuloma, infection, ischemic stroke, or even a degenerative process. Others have reported diffuse white matter involvement simulating leukodystrophy only to normalize following treatment 4 months later (9). Evolving multifocal high signal intensity lesions on T2-weighted and FLAIR images and unilateral cerebellar atrophy, as seen here, expand on the spectrum of MR imaging manifestations in HE.
Footnotes
The opinions or assertions contained herein are the private views of the author (G.Y.C) and are not to be construed as representing the views of the Department of Defense, the Department of the Army, or the Uniformed Services University of the Health Sciences.
References
- 1.Seipelt M, Zerr I, Nau R, et al. Hashimoto’s encephalitis as a differential diagnosis of Creutzfeldt–Jakob disease. J Neurol Neurosurg Psychiatry 1999;66:172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mouzak A, Agathos P, Vourdeli-Giannakoura E. Subacute cerebellar syndrome and Hashimoto’s thyroiditis: association or simple coincidence? Act Neurol Scand 2002;106:374–378 [DOI] [PubMed] [Google Scholar]
- 3.McCabe DJH, Burke T, Connolly S, Hutchinson M. Amnesic syndrome with bilateral mesial temporal lobe involvement in Hashimoto’s encephalopathy. Neurology 2000;54:737. [DOI] [PubMed] [Google Scholar]
- 4.Erickson JC, Carrasco H, Grimes JB, et al. Palatal tremor and myorhythemia in Hashimoto’s encephalopathy. Neurology 2002;58:504–505 [DOI] [PubMed] [Google Scholar]
- 5.Selim M, Drachman DA. Ataxia associated with Hashimoto’s disease: progressive non-familial adult onset cerebellar degeneration with autoimmune thyroiditis. J Neurol Neurosurg Psychiatry 2001;71:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolte KW, Unbehaun A, Sieker H, Kloss TM, Paulus W. Hashimoto encephalopathy: a brainstem vasculitis? Neurology 2000;54:769. [DOI] [PubMed] [Google Scholar]
- 7.Shibata N, Yamamoto Y, Sunami N, et al. Isolated angiitis of the CNS associated with Hashimoto’s disease. Rinsho Shinkeigaku 1992;32:191–198 [PubMed] [Google Scholar]
- 8.Ferracci F, Moretto G, Candeago RM, et al. Antithyroid antibodies in the CSF: their role in the pathogenesis of Hashimoto’s encephalopathy. Neurology 2003;60:712–714 [DOI] [PubMed] [Google Scholar]
- 9.Bohnen NILJ, Parnell KJ, Harper CM. Reversible MRI findings in a patient with Hashimoto’s encephalopathy. Neurology 1997;49:246–247 [DOI] [PubMed] [Google Scholar]