Abstract
Summary: Delayed spinal cord injury following high-voltage electrical injury is a rare but well-documented phenomenon. The MR imaging features of this entity, however, have not been well documented. We report the MR imaging findings in a case of delayed sensory and motor deficits following a lightning strike. MR imaging revealed hyperintense signal within the cord on T2-weighted and STIR images extending from C1 to C3. Axial images localized the hyperintense signal to the posterolateral region of the spinal cord bilaterally. Follow-up MR imaging 6 weeks later demonstrated resolution of abnormal cord signal intensity.
The incidence of spinal cord injury following electrical trauma ranges between 2% and 5% (1, 2). Electrical injury may produce an immediate or delayed myelopathy (3). Immediate injury typically produces decreased levels of consciousness, paresthesias, and weakness. Significant or complete recovery is frequently observed (2). Delayed spinal cord injury is usually incomplete and progressive, and improvement is less common (4).
Case Report
While sleeping in a tent on a camping trip, a 58-year-old man was struck by lightning. After short-term loss of consciousness, he was unable to move his arms and legs. Over the next few hours, he gradually noticed some return of movement and feeling in the fingers, arms, and legs. Upon presentation to the emergency department, physical examination revealed second-degree burns in the right occiput and upper cervical skin, representing an entry point. Exit wounds were noted on the inferior aspect of the left chest wall. Two days after admission, the patient was discharged with no persistent sensory loss or paresthesias. The only remaining deficit was some difficulty walking.
Approximately 6 weeks after the initial electrical injury, the patient began to notice numbness, tingling, and dysthesia in both hands. Over the next week, the paresthesia gradually migrated into his forearms to the elbows and became noticeable in his feet. He also complained of weakness in his hands and legs. Finally, the patient described severe shocks going through his arms, trunk, and feet elicited by minor neck flexion.
Physical examination revealed mild weakness of the intrinsic muscles of the hands and finger extensors bilaterally. Lower extremity strength was normal. Sensory examination demonstrated impaired vibration perception in the fingertips bilaterally and in the toes of the right foot. There was mild impairment of pin prick and pain perception in the right foot. Right ankle jerk was absent. There was mild unsteadiness with Romberg testing and mild impairment of finger to nose test. Marked L’hermitte phenomenon was elicited.
Laboratory investigations were noncontributory except for CSF analysis, which revealed a moderate elevation in protein (0.89 g/L) with normal cell counts. Nerve conduction studies were normal except for the incidental documentation of right median nerve entrapment in the carpal tunnel. Specifically, compound sensory nerve action potential amplitudes were normal and there was no evidence of motor or sensory conduction slowing. Needle electromyeography findings were also normal. Medical history included a left shoulder dislocation, hypercholesterolemia, and chronic obstructive pulmonary disease.
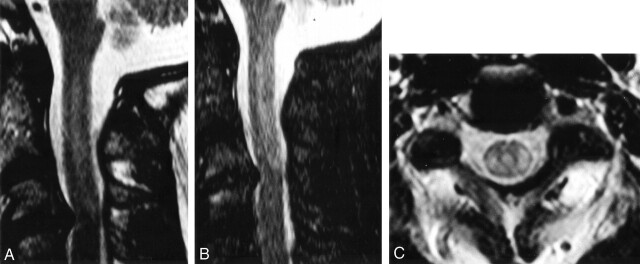
MR imaging revealed hyperintense signal within the cord on T2-weighted and short tau inversion recovery images extending from C1 to C3 (Fig 1). Axial images confirmed that the hyperintense signal was located within the posterolateral region of the spinal cord bilaterally. Of note, this portion of the cervical spinal cord was in the path of the entry and exit burn wounds. There was no cord expansion. The anterior third of the cord was normal. Degenerative changes at C3–C4 were most likely unrelated to the abnormal cord signal intensity. Radiologic diagnosis of cervical spinal cord lesion resulting from electrical injury was made.
Fig 1.
Initial MR imaging findings.
A-C, Sagittal T2-weighted (A), sagittal short inversion time inversion-recovery (B), and axial T2-weighted (C) images of the cervical spine demonstrate hyperintense signal in the posterolateral aspect of the spinal cord from C1 to C3.
Clinically, symptoms were consistent with a diagnosis of delayed myelopathy secondary to electric injury with predominant dorsal column involvement. Significant sensory deficits in the setting of normal sensory potentials on electrodiagnostic testing suggested a preganglionic lesion (proximal to the dorsal root ganglion). The presence of L’hermitte phenomenon clinically suggested involvement of the cervical cord. The patient was treated with aspirin combined with dipyridamole and neurontin.
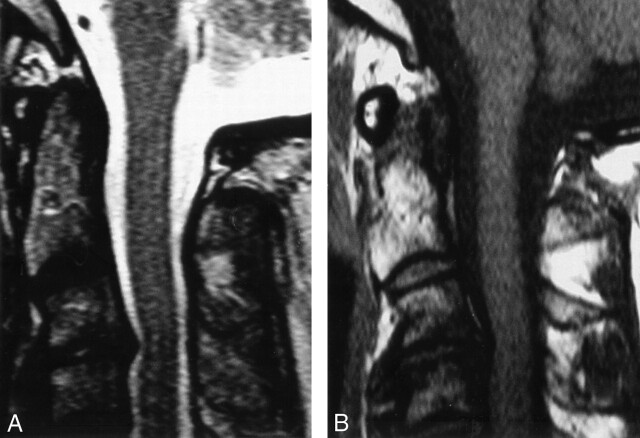
The patient was seen for follow-up 6 weeks later, at which time he described some improvement. He had less stiffness in his hands, improved sensation, and improved grip. L’hermitte phenomenon was still present. Follow-up MR imaging at this time demonstrated resolution of abnormal spinal cord signal intensity (Fig 2).
Fig 2.
MR imaging findings at 6-week follow-up.
A-C, Sagittal T2-weighted (A) and T1-weighted (B) images of the cervical spine demonstrate resolution of abnormal signal intensity.
Discussion
Numerous cases of myelopathy following electrical injury have been reported. To our knowledge, however, MR imaging findings have not been fully documented. Ghosh et al (5) described a case of cervical myelopathy with late-onset progressive motor neuron disease following electrical injury. The patient was examined 12 years after the injury, at which time MR imaging demonstrated cervical cord atrophy. No signal intensity abnormalities were described. Arevalo et al (6) reported two cases of neurologic symptoms immediately following electrical injury in which CT and MR imaging were both normal.
Clinical findings in delayed neurologic injuries are variable in location (CNS or peripheral), and hence, diagnostic imaging can be very useful. Positive MR imaging findings were extremely valuable in our case, because the clinical findings were somewhat confusing. The delayed neurologic symptoms typically appear within a variable period of days or months after the injury. Spinal cord lesions usually manifest as motor atrophy symptoms with a slowly progressive character (2). Arevalo et al (6) have suggested that lack of sensitivity of MR imaging in early spinal cord injuries may be due to the fact that myelomalacia or micromyelomalacia is not fully established at the time that the imaging is performed or because structural abnormalities are so small that they are not detectable. It is possible that in our case the 6-week interval was sufficient time for the damage to evolve to a state in which MR imaging findings were evident.
The exact mechanism of spinal cord damage following electrical injury remains unclear. Most lesions in this setting are incomplete, and most victims survive the accident, resulting in few autopsy specimens. The few postmortem examinations available show a prominent role of vascular lesions in the pathogenesis of this entity. The limited findings available include petechial hemorrhage, chromatolysis of the pyramidal and anterior horn cells, cavitation, swelling, and softening of the cord (2, 7, 8).
Tissue damage after electrical injury is mediated either thermally or electrically (9). The direct action of electrical fields can cause tissue damage by the production of heat through the Joule effect. In this model, the tissue temperature rises because of the passage of current through a resistive material. This leads to cord necrosis, which is compatible with a transverse lesion. The greatest resistance encountered by the flow is across the skin. The most significant thermal injuries are therefore usually seen in the skin at points of electrical entry and exit (10). Vascular damage is another mechanism of neurologic damage. In this model, direct injury to nutrient blood vessels can lead to ischemic changes within the spinal cord (5).
Nonthermal injuries can cause significant tissue damage. In a proposed mechanism known as “electroporation,” the direct action of electrical fields causes changes in the structure of cell membrane proteins. This leads to the formation of pores in the cell membrane (8). The cell membrane potential is disrupted, ultimately leading to cell rupture. The vulnerability of a cell to electroporation is proportional to the surface area of the cells. Cells with a large surface area, such as neurons, are most likely to be affected (6). Farrell and Starr (11) have shown that there is a latent period between injury and the onset of neurologic complications. Electroporation causes similar alterations in protein structure as those observed in nerve tissue after the passage of electrical current, which ultimately leads to cell death. If structural changes in membrane electrical breakdown immediately following electrocution are not sufficient to cause immediate cell death, they could cause delayed cell death and thus delayed neurologic symptoms.
The clinical, CSF, and MR imaging findings in this case would be more in keeping with a focal demyelinating cervical myelopathy in the general category of acute transverse myelitis (12). The clinical deficits suggested predominant involvement of posterior and lateral fasciculi and the relatively rapid recovery would be in keeping with demyelination rather than axonal degeneration. The raised CSF protein in this context may be attributed either to disruption of the blood-brain barrier or to the release of myelin proteins. The lack of CSF pleocytosis in the acute phase may argue against a prominent local inflammatory cellular response. One may propose that the main cellular target was the oligodendrocyte and that electroporation caused a reversible glial injury as opposed to the chronic progressive anterior horn cell degeneration noted in more delayed cases of electrical injury. It is also possible that electroporation causes physical disruption of myelin antigens, leading to a limited immune-mediated demyelination.
References
- 1.Varghese G, Mani MM, Redford JB. Spinal cord injuries following electrical accidents. Paraplejia 1986;24:159–166 [DOI] [PubMed] [Google Scholar]
- 2.Levine NS, Atkins A, McKeel DW, et al. Spinal cord injury following electrical accidents: case reports. J Trauma 1975;15:459–463 [DOI] [PubMed] [Google Scholar]
- 3.Petty PG, Parkin G. Electrical injury to the central nervous system. Neurosurgery 1986;19:282–284 [DOI] [PubMed] [Google Scholar]
- 4.John AD. Principles and Practice of Burns Management. New York: Churchill Livingstone;1996
- 5.Ghosh D, Gupta A, Kohli A. Electrical injury: cervical myelopathy with late onset progressive motor neuron disease. Aust NZ J Med 1995;25:263–264 [DOI] [PubMed] [Google Scholar]
- 6.Arevalo JM, Lorente JA, Balseiro-Gomez J. Spinal cord injury after electrical trauma treated in a burn unit. Burns 1999;25:449–452 [DOI] [PubMed] [Google Scholar]
- 7.Christensen JA. Delayed neurologic injury secondary to high voltage current with recovery. J Trauma 1980;20:166–168 [DOI] [PubMed] [Google Scholar]
- 8.Hunt JL, Mason AD, Masterson TS, Pruitt BA. The pathophysiology of acute electrical injuries. J Trauma 1976;16:335–340 [DOI] [PubMed] [Google Scholar]
- 9.Lee RC, Aarsvold JN, Chen W, et al. Biophysical mechanisms of cell membrane damage in electrical shock. Semin Neurol 1995;15:367–374 [DOI] [PubMed] [Google Scholar]
- 10.Lee RC. Injury by electrical forces: pathophysiology, manifestations, and therapy. Curr Probl Surg 1997;34:677–764 [DOI] [PubMed] [Google Scholar]
- 11.Farrell DF, Starr A. Delayed neurological sequelae of electrical injuries. Neurology 1968;18:601–606 [DOI] [PubMed] [Google Scholar]
- 12.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis [Review]. Neurology 2002;59:499–505 [DOI] [PubMed] [Google Scholar]