Abstract
BACKGROUND AND PURPOSE: Papaverine is the primary intra-arterial (IA) treatment for vasospasm after aneurysmal subarachnoid hemorrhage (SAH); however, is it limited in effect and by adverse effects. We prospectively studied the use of IA nicardipine as a treatment for vasospasm.
METHODS: Over 12 months, all patients with SAH who required interventional treatment for vasospasm were given IA nicardipine with or without angioplasty. Vasospasm was determined by serial clinical assessments and/or daily transcranial Doppler (TCD) imaging and confirmed by angiography. Doses of IA nicardipine per vessel were 0.5–6 mg. All patients were monitored for increased intracranial pressure (ICP) and change in cardiovascular or neurologic status.
RESULTS: Forty-four vessels in 18 patients with vasospasm were treated with IA nicardipine alone. TCD data for 38 vessels (15 cases) were available. All vessels demonstrated immediate angiographic dilatation after IA nicardipine. No sustained cardiovascular changes were after treatment. ICP was transiently elevated in five patients and persistently elevated in one. Mean peak systolic velocities at TCD imaging were significantly reduced from pretreatment values in all treated vessels for 4 days after infusion (268.9 ± 77.8 vs 197.6 ± 74.1 cm/s, P < .001). Neurologic improvement after IA nicardipine occurred in eight (42.1%) patients. No clinical deterioration was noted.
CONCLUSION: As shown by TCD imaging, IA nicardipine has an immediate and sustained effect on vasospasm. It does not appear to have sustained effect on ICP or cardiovascular status. This treatment warrants further study to determine its safety and efficacy.
Vasospasm is one of the leading causes of mortality and morbidity in patients who survive the initial 24 hours after aneurysmal subarachnoid hemorrhage (SAH) (1). An estimated two-thirds of patients undergoing angiography between days 4 and 14 after hemorrhage have some degree of vasospasm. Of these patients, approximately one-third develop symptoms related to vasospasm (1), primarily through arterial narrowing leading to severe restriction of cerebral blood flow and multifocal infarctions of the brain. Approximately 5–10% of hospitalized SAH patients die from vasospasm (1). Current treatment of patients with SAH is therefore primarily focused on the prevention and treatment of vasospasm. The most widely used approaches include oral nimodipine therapy; induced hypertension, hemodilution, and hyperdynamic (triple-H) therapy; intra-arterial (IA) papaverine treatment; and balloon angioplasty.
In 1984, Zubkov et al (2) first described the use of balloon angioplasty as a treatment for cerebral vasospasm after SAH, and subsequent studies have validated this technique as an effective treatment of proximal vessel vasospasm (3–6) However, significant risks are associated with angioplasty, and it is therefore not indicated in every case (7–10). Moreover, certain patients have delayed cerebral ischemia due to distal vessel vasospasm for which mechanical treatment is not feasible. In these cases, IA infusions with numerous pharmacologic agents have been studied, with variable effectiveness.
Of these agents, IA papaverine has been most widely studied (11–16). Papaverine is an alkaloid derivative of an opioid that has been shown to result in vasodilatation of cerebral arteries through what is believed to be direct inhibitory effect on smooth muscle contraction (16). Experimental and clinical trials have shown that the effect of papaverine is short-lived (17–19) and that it has notable adverse effects, including hemodynamic compromise, increased intracranial pressure (ICP) (20, 21), seizures (22), and even paradoxical worsening of vasospasm (19). Kaku et al (12) reported that an IA infusion of papaverine resulted in a sustained treatment of vasospasm; they believed that the early timing of treatment was crucial in achieving optimal effectiveness. Results of subsequent experimental models have been consistent with this concept. However, each of the vessels in the study by Kaku et al was also treated with 0.5–1.0 mg of IA nicardipine. Although the authors noted no angiographic dilatation when 0.5 mg of nicardipine was administered alone, the effect of combined therapy on the vessels is not known.
Because of the lack of definitive effect of IA papaverine reported in the literature, we decided to use high-dose IA nicardipine as a treatment for vasospasm after SAH. Our hypothesis was that the administration of IA nicardipine at a dose higher than that previously reported results in angiographic dilatation and decreases transcranial Doppler (TCD) velocities without substantially increasing ICP or lowering blood pressure.
Methods
Subjects
During the 12 months from January 2002 to January 2003, all patients aged 18 years or older with SAH-induced vasospasm were considered for IA nicardipine therapy. As part of standard practice at our institution, endovascular treatment of vasospasm is reserved for patients who either do not show improvement or who worsen neurologically despite optimal medical care. We define optimal medical care as the implementation of triple-H therapy, in which patients undergo induced hypertension and volume resuscitation. Therefore, IA nicardipine was considered only in medically refractory patients and did not serve as a substitute for our standard clinical care. This treatment was withheld from patients who had a history of allergic reaction to or an intolerance of calcium antagonist drugs.
Management of Vasospasm
In addition to close neurologic monitoring, all patients with SAH in our neurointensive care unit undergo bedside TCD sonography. We have established TCD criteria for vasospasm, which are as follows: Mild vasospasm is defined as time-averaged peak systolic velocities (PSVs) of 200–249 cm/s and/or a Lindegaard ratio (LR) of 3–3.9 (23). Moderate vasospasm is defined as PSV of 250–299 cm/s and/or an LR of 4.0–4.9. Severe vasospasm is defined as PSV of ≥300 cm/s and/or an LR ≥ 5. Intensive care of any patient who had either an onset of clinical symptoms or TCD velocities suggestive of vasospasm included triple-H therapy. The decision to perform angiography was based on criteria just noted. Once in the angiography suite, patients underwent four-vessel angiography, during which a neurointerventionalist (J.A.D. or J.C.P.) evaluated vasospasm. The degree of mild (<40%), moderate (40%–75%), or severe (>75%) vasospasm was determined by evaluating the lumen of the vessel in comparison with more normal segments of artery proximal and distal to the narrowing or with estimated normal tapering of branches versus the cavernous carotid artery. Angiograms or CT angiograms obtained at the time of initial presentation were used as baselines in comparing relative vascular size. Also, normal variation was taken into account; that is, in the A1 segments where one side may be dominant compared with the contralateral side. The final element was the delay in the relative transit time. Proximal vasospasm was treated with IA nicardipine and/or transluminal angioplasty, while distal vessel vasospasm was treated with IA nicardipine alone.
Administration of IA Nicardipine
In one subject (case 1), nicardipine (Cardene IV; ESP Pharma, Inc; Edison, NJ) was diluted in normal sodium chloride solution (0.9% NaCl) to a concentration of 0.1 mg/mL and administered in 1-mL aliquots through the microcatheter. In all the subsequent administrations, nicardipine was diluted in 0.9% NaCl to a concentration of 0.1 mg/mL and administered in 1-mL aliquots through a microcatheter to a maximal dose of 5 mg per vessel. This dosage was derived from the accepted intravenous bolus-dosing schedule for the treatment of hypertension (24). Dose per vessel was also based on the angiographic effect observed. Administration was believed to be adequate when vasospasm was no longer appreciable in the vessel receiving nicardipine. All patients undergoing angiography had a ventriculostomy in place. Their ICP was carefully monitored during the administration of IA nicardipine. If ICP increased substantially (>20 mm Hg), the ventriculostomy was opened and allowed to drain to lower the ICP. ICP was recorded at the beginning of angiography and at the end of the procedure.
Transluminal Angioplasty
Transluminal angioplasty was performed with the use of a angioplasty balloon 3.5 × 10 mm (Commodore [Cordis, Miami, FL], 1.2-mL volume), which was prepared with half-strength contrast material (Berlex, Wayne, NJ) and advanced through a guide catheter over the microwire (Agility 10; Cordis). The angioplasty catheter was then advanced into the proximal vascular segment, where dilations were performed in succession, moving distally in the vessel. Control angiograms were done through the guide catheter after this procedure. Following IA nicardipine and/or transluminal angioplasty treatments, patients were monitored. The decision to perform angioplasty was based on the ability to perform this procedure without substantial risk of dissection or perforation. The final decision of feasibility and safety of balloon angioplasty was at the discretion of the treating interventional neuroradiologist.
Post-Treatment Neurologic Examinations
Each patient underwent neurologic assessment by one of the members of the neurointensive care service (N.B., G.R.) before and after treatment for vasospasm. Given the lack of an applicable standardized examination score for patients with SAH, post-treatment examinations were simply determined to be improved, unchanged, or worse. For each patient, neurologic examination findings were noted after each interventional treatment. As a result, in patients undergoing interventional treatments on multiple days, more than one post-treatment neurologic examination was recorded.
Data Analysis
To understand a true effect of IA nicardipine, only data from patients receiving IA nicardipine alone were analyzed. Mann-Whitney U and paired-sample t tests were used to compare baseline, before and after treatment PSV and mean velocities (Vmean) values, and to compare PSV and Vmean changes after treatment (before − after). Daily TCD PSV changes were evaluated with an analysis of variance (ANOVA) for repeated measures or ANOVA with the Friedman method, followed by Wilcoxon or paired t tests, as appropriate. Significance was set at P < .05. All calculations were performed by using SPSS version 8.02 (SPSS, Chicago, IL).
Results
For 48 vessels in 24 cases, IA nicardipine was used as treatment for vasospasm; IA nicardipine was used as monotherapy in only 18 cases (38 vessels). In five cases, angioplasty was used in addition to IA nicardipine, and in one case, IA papaverine was used a day before IA nicardipine and angioplasty. Because modalities other than or in addition to IA nicardipine were used in these cases, they were excluded from subsequent analysis. Thirteen (72.2%) of 18 patients in the IA-nicardipine only group were female, with a mean age of 49.8 ± 9.5 years. Table 1 presents the clinical characteristics of the patients, including size and location of aneurysm, Hunt-Hess classification, and Fisher group. All vessels that were treated with IA nicardipine demonstrated angiographic improvement in the degree of vasospasm. Vasospasm treatment occurred 4–14 days after SAH (Table 2).
TABLE 1:
Clinical characteristics of IA nicardipine patients
| Case/Age (y)/Sex | Medical History | Hunt-Hess Grade | Fisher Group | Aneurysm Location | Size (mm) |
|---|---|---|---|---|---|
| 2/60/M | Hypertension | 2 | 2 | R Pcomm | 10 |
| R ICA, unruptured | 7 | ||||
| 4/42/F | Hypertension | 4 | 3 | L ICA | 20 |
| Migraines | |||||
| 5/50/F | None | 3 | 3 | L Pcomm | 6 |
| 6/50/F | Hypertension | 3 | 3 | BA | 6 |
| 7/45/F | Migraines | 4 | 3 | BA | 9 |
| Tobacco abuse | L ICA, unruptured | 3 | |||
| R PICA, unruptured | 6–7 | ||||
| 9/49/F | Hypercholesterolemia | 2 | 3 | L ICA | 8 |
| 10/40/F | Alcohol abuse | 4 | 3 | L Pcomm | 8 |
| 11/41/F | Hypertension | 3 | 3 | L MCA | 6 |
| 12/67/F | 4 | 3 | R MCA | 5 | |
| 13/49/M | Hypertension, hypercholesterolemia | 5 | 3 | L Pcomm | 3 |
| 14/59/M | Tobacco abuse, CAD, hypertension | 4 | 3 | L PICA | 8 |
| 15/65/F | None | 4 | 3 | Acomm | 5 |
| 19/55/F | Polysubstance abuse, depression, migraine | 1 | 2 | R ICA | 8 |
| 20/53/F | None | 4 | 3 | L ICA | 8 |
| 21/44/M | Tobacco abuse | 4 | 3 | BA | 10 |
| 22/42/M | Tobacco abuse, hypertension | 3 | 3 | Acomm | 4 |
| 23/28/F | None | 4 | 3 | L MCA | 6 |
| 24/64/F | Hypertension, TIA, hypercholesterolemia | 1 | 2 | L Pcomm | 3 |
Note.—Acomm = anterior communicating artery, BA = basilar artery, CAD = coronary artery disease, ICA = internal carotid artery, MCA = middle cerebral artery, Pcomm = posterior communicating artery, PICA = posterior inferior cerebellar artery, TIA = transient ischemic attack.
TABLE 2:
Physiologic response to vasospasm treatment
| Case | Postbleeding Day | Vessel | Nicardipine (mg) | ICP (cm H2O) |
Mean Arterial Pressure (mm Hg) |
||
|---|---|---|---|---|---|---|---|
| Pretreatment | Post-Treatment | Pretreatment | Post-Treatment | ||||
| 2 | 7 | R MCA | 3 | 20 | 26* | 131 | 132 |
| R ACA | 2 | ||||||
| 4 | 7 | BA | 5 | 15 | 8 | 109 | 107 |
| 5 | 4 | R ICA | 5 | 7 | 7† | 136 | 129 |
| L ICA | 5 | ||||||
| L Vert | 5 | ||||||
| 6 | 7 | L ICA | 5 | 8 | 8† | 111 | 108 |
| 7 | 6 | R ICA | 5 | 14 | 13 | 110 | 117 |
| BA | 3 | ||||||
| 9 | 12 | R ACA | 5 | 14 | 11 | 113 | 111 |
| L ACA | 5 | ||||||
| 10 | 4 | L ICA | 5 | 14 | 14 | 105 | 105 |
| 11 | 6 | L MCA | 5 | NA | NA | 130 | 136 |
| 12 | 6 | R ICA | 2 | 11 | 11† | 93 | 88 |
| L ICA | 3 | ||||||
| 13 | 13 | R ICA | 5 | ||||
| L ICA | 2.5 | 12 | 10 | 95 | 100 | ||
| 14 | 11 | L Vert | 5 | 18 | 13 | 95 | 100 |
| 15 | 14 | L ICA | 2.5 | 15 | 12 | 110 | 115 |
| 19 | 6 | R ICA | 2 | 18 | 18† | 118 | 118 |
| 20 | 10 | L ACA | 4 | 7 | 7 | 120 | 116 |
| L MCA | 4 | ||||||
| R MCA | 4 | ||||||
| 21 | 4 | R ACA | 4 | 15 | 15† | 120 | 123 |
| L MCA | 3 | ||||||
| L ACA | 3 | ||||||
| 6 | R ICA | 4 | 13 | 11 | 110 | 112 | |
| 22 | 8 | L ICA | 2 | ||||
| L PCA | 1 | 12 | 10 | 96 | 112 | ||
| 23 | 9 | R ACA | 3 | 14 | 14 | 100 | 105 |
| 24 | 7 | L ICA | 4 | 20 | 15 | 95 | 95 |
Note.—ACA = anterior cerebral artery, BA = basilar artery, MCA = middle cerebral artery, NA = not applicable, PCA = posterior cerebral artery, Vert = vertebral artery.
Treatment was aborted as a result of sustained elevated ICP.
ICP was transiently elevated but returned to baseline with opening of the ventriculostomy drain.
Hemodynamic Response to IA Nicardipine
In six patients, ICP increased in relation to the administration of IA nicardipine. In five patients, transiently increased ICP quickly returned to baseline on opening of the ventriculostomy drain, and ICP remained at baseline at the end of the treatment. In one patient (case 2), angiography had to be aborted because of a sustained rise in ICP that did not respond to ventricular drainage; however, a postprocedural CT scan demonstrated an intracerebral hemorrhage along the tract of the ventriculostomy that was placed during the procedure. There were no notable changes in the mean arterial pressure or an increase in the vasopressor requirements to maintain hypertensive therapy in the cases treated with IA nicardipine alone. None of the patients showed signs or symptoms consistent with pulmonary edema, and no laboratory findings consistent with azotemia were noted after treatment.
TCD Data
Although 44 vessels in 18 cases were treated with IA nicardipine alone, baseline and follow-up TCD data were available for only 15 cases. In cases with IA nicardipine administered into the internal carotid artery, we analyzed TCD data of the ipsilateral middle cerebral artery and anterior cerebral artery. In two cases (4 and 5), pretreatment TCD data were not available for the vertebrobasilar circulation. At our institution, when the ruptured aneurysm is in the anterior circulation and when the predominant collection of subarachnoid blood is away from the brain stem cisterns, the posterior circulation is not routinely assessed with TCD. In case 19, a single vessel (right internal carotid artery) was treated at the time of diagnostic angiography before any TCD data could be obtained. None of the four vessels (right middle cerebral artery and anterior cerebral artery for case 19) developed TCD evidence of vasospasm rebound during post-treatment follow-up. In case 20, no ultrasonographic bone windows could be found to insonate the intracranial arteries, two of which later underwent severe vasospasm. Subsequent analyses included only 38 vessels with both baseline and follow-up data. The LR was not included in follow-up TCD analysis because, at our institution, the LR is used as an additional measure in assessing the severity of vasospasm when the PSV is 200 cm/s or greater. Because post-treatment PSV was 200 cm/s or less in most cases, the LR was not available for analysis.
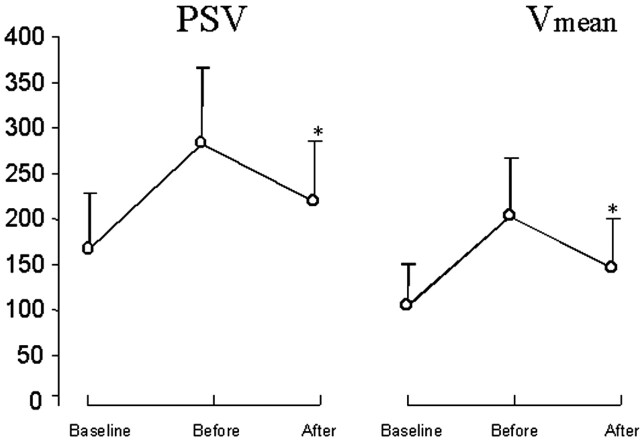
According to our institutional criteria, all of the vessels demonstrated a PSV increase from baseline to the day of treatment (day 0) with velocities consistent with moderate or severe vasospasm. Absolute TCD velocity changes after treatment (before − after) showed a significant tendency to be more prominent if transluminal angioplasty was performed in addition to IA nicardipine therapy. PSVs at baseline were 161.9 ± 61.9 cm/s and rose to 277.4 ± 80.8 cm/s on the day of treatment and subsequently dropped to 212.8 ± 65.7 cm/s on the day following treatment (P < .001). Similarly, baseline Vmean rose from 105.6 ± 43.9 cm/s to 201.3 ± 65.8 cm/s on the day of IA nicardipine treatment and subsequently dropped to 145.1 ± 54.1 cm/s the day afterward (P < .001) (Fig 1).
Fig 1.
Comparison of PSV and Vmean at baseline on the treatment day (Before) and on post-treatment day 1 (After). * indicates P < .001.
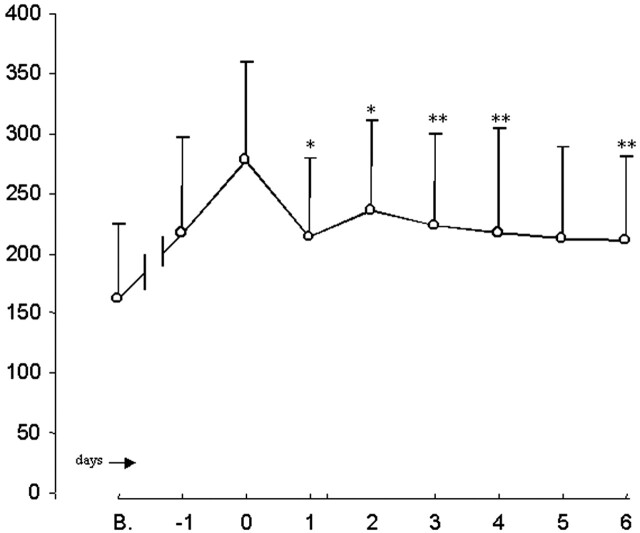
In all patients treated with IA nicardipine, post-treatment TCD velocities were significantly decreased on all subsequent days, except for post-treatment day 5, compared with highest values before treatment (Fig 2). The number of vessels reported decreased from 38 on day 0 to 22 on day 6. This decline was due to either a withdrawal of care that was unrelated to any effects of vasospasm treatment or complete resolution of symptoms and stable post-treatment TCD velocities. As a result, statistical analyses were performed in only 31 and 26 vessels from days 4 and 5, respectively.
Fig 2.
PSV in all vessels treated in relation to vasospasm on the treatment day (day 0). B indicates baseline. * indicates P < .001. ** indicates .001 ≤ P < .001.
Post-Treatment Neurologic Examinations
Of 18 patients, one underwent treatment on multiple days, which resulted in 19 post-treatment neurologic examinations. Seven examinations were performed in patients receiving IA nicardipine and angioplasty, and the remaining 23 were for patients receiving only IA nicardipine. Overall, eight (42.1%) of 19 examinations showed an improvement after treatment (Table 3).
TABLE 3:
Neurologic response to IA nicardipine with or without angioplasty
| Case* | Postbleeding Day | Vessel | Nicardipine (mg) | Vasospasm | Post-Treatment Neurologic Examination |
|---|---|---|---|---|---|
| 2 | 7 | R MCA | 3 | Mild | Yes |
| R ACA | 2 | Mild | |||
| 4 | 7 | BA | 5 | Moderate | No |
| 5 | 4 | R ICA | 5 | Mild | Yes |
| L ICA | 5 | Moderate | |||
| L Vert | 5 | Moderate | |||
| 6 | 7 | L ICA | 5 | Severe | No |
| 7 | 6 | R ICA | 5 | Moderate | No |
| BA | 3 | Moderate | |||
| 9 | 12 | R ACA | 5 | Moderate | No |
| L ACA | 5 | Moderate | |||
| 10 | 4 | L ICA | 5 | Moderate | Yes |
| 11 | 6 | L MCA | Moderate | Yes | |
| 12 | 6 | R ICA | 2 | Moderate | No |
| L ICA | 3 | Moderate | |||
| 13 | 13 | R ICA | 5 | Moderate | |
| L ICA | 2.5 | Mild | |||
| 14 | 11 | L Vert | 5 | Mild | No |
| 15 | 14 | L ICA | 2.5 | Mild | No |
| 19 | 6 | R ICA | 2 | Mild | Yes |
| L ACA | 4 | Severe | |||
| 20 | 10 | L ACA | 4 | Severe | Yes |
| L MCA | 4 | Severe | |||
| R MCA | 4 | Severe | |||
| R ACA | 4 | Severe | |||
| 21 | 4 | L MCA | 3 | Mild | No |
| L MCA | 3 | Mild | |||
| L ACA | 3 | Mild | |||
| 6 | R ICA | 4 | Severe | No | |
| L ICA | 2 | Mild | |||
| 22 | 8 | L ICA | 2 | Mild | No |
| L PCA | 1 | Moderate | |||
| 23 | 9 | R ACA | 3 | Mild | Yes |
| 24 | 7 | L ICA | 4 | Mild | Yes |
Note.—ACA = anterior cerebral artery, BA = basilar artery, MCA = middle cerebral artery, PCA = posterior cerebral artery, Vert = vertebral artery.
Case 1 involved the use of papaverine and was not included.
Discussion
The pathway by which SAH-induced arterial spasm occurs is not yet fully understood; however, both in vitro and clinical evidence suggest the efficacy of calcium antagonists in treating vasospasm (25–28). The oral dihydropyridine calcium-antagonist nimodipine has been demonstrated to reduce the incidence of vasospasm-induced cerebral infarction by 34% and the rate of poor outcomes by 40% (29). This drug, however, has been limited by a lack of tolerance or effectiveness in certain patients (30–32).
Nicardipine is also a dihydropyridine calcium antagonist that, similar to nimodipine, has more selective effects on vascular smooth muscle than on cardiac muscle. Unlike nimodipine, nicardipine has the advantage of parenteral administration in North America. Initial dose-escalation studies demonstrated that intravenous nicardipine resulted in notable improvement in angiographic and symptomatic vasospasm at a dose of 0.15 mg/kg/h (33). A subsequent, prospective, multicenter, randomized, double-blind, placebo-controlled study was conducted to determine the efficacy of high-dose intravenous nicardipine in the prevention of vasospasm. Results from this and a second study with low-dose intravenous nicardipine (0.075 mg/kg/h) demonstrated that the nicardipine-treated groups had a significantly lower incidence of symptomatic, angiographic, and TCD vasospasm (34–36). However, each of the dosing schedules was limited in its effectiveness by prolonged hypotension, pulmonary edema, and renal dysfunction.
Kasuya et al (37) reported that angiographic vasospasm is prevented in arteries with the placement of nicardipine prolonged-release implants at the time of surgery. The effectiveness of this intrathecal administration is limited by rates of diffusion and appears to prevent vasospasm only in the arteries adjacent to the implants.
Improvement shown in 42.1% of post-treatment neurologic examinations is encouraging, but objective data to prove any long-term benefits are insufficient. Intermediate assessments by daily TCD demonstrate a potential for beneficial effects. Only one of the patients (5.6%) treated with IA nicardipine required multiple treatments. This sustained effect is greater than what has been previously reported for papaverine (15). Moreover, unlike in the intravenous nicardipine trial, our study of IA nicardipine was not associated with any hemodynamic effect, azotemia, or pulmonary edema. The main adverse effect was increased ICP in six patients. Although ICP quickly returned to baseline with opening of the ventriculostomy drain in five patients, one patient did not respond and had to be treated with mannitol. This patient had a notable intracranial hematoma and difficult-to-control ICPs before entering the angiography suite. We believe that the subsequent, sustained high ICP was the result of poor intracranial compliance that resulted from the hematoma and not a direct consequence of IA nicardipine infusion. A substantial rise in ICP has been one of the limiting consequences of IA papaverine therapy, and therefore, this potential adverse effect of IA nicardipine must be further evaluated in future studies.
Conclusions
Although this study included a small number of patients, we believe that this initial experience indicates a potentially effective pharmacologic treatment for vasospasm, especially in vessels not amenable to angioplasty. Future studies should continue focusing on safety and better define the optimal dosing and overall efficacy of this therapy.
References
- 1.Macdonald RL, Weir B. Epidemiology. In: Cerebral Vasospasm. San Diego, Ca: Academic Press;2004. :16–18
- 2.Zubkov YN, Nikiforov BM, Shustin VA. Balloon catheter technique for dilation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochirur 1984;70:65–69 [DOI] [PubMed] [Google Scholar]
- 3.Barnwell SL, Higashida RT, Halbach VV, Dowd CF, Wilson CB, Hieshima GB. Transluminal angioplasty of intracerebral vessels for cerebral arterial spasm: reversal of neurological deficits after delayed treatment. Neurosurgery 1989;24:424–429 [DOI] [PubMed] [Google Scholar]
- 4.Bejjani GK, Bank WO, Olan WJ, Sekhar L. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery 1998;42:979–986 [DOI] [PubMed] [Google Scholar]
- 5.Eskridge JM, Newell DW, Pendleton GA. Transluminal angioplasty for treatment of vasospasm. Neurosurg Clin N Am 1990;1:387–399 [PubMed] [Google Scholar]
- 6.Polin R, Coenen V, Hansen CA, et al. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 2000;92:284–290 [DOI] [PubMed] [Google Scholar]
- 7.The American Society of Interventional and Therapeutic Neuroradiology: Mechanical and pharmacologic treatment of vasospasm. AJNR Am J Neuroradiol 2001;22:S26–S27 [PMC free article] [PubMed] [Google Scholar]
- 8.Brothers MF, Hedlund LW, Friedman AH. Arterial rupture during distal vasospasm angioplasty [abstract] Neuroradiology 1997;33:S155 [Google Scholar]
- 9.Linskey ME, Horton JA, Rao GR, Yonas H. Fatal rupture of the intracranial carotid artery during transluminal angioplasty for vasospasm induced by subarachnoid hemorrhage. J Neurosurg 1991;74:985–990 [DOI] [PubMed] [Google Scholar]
- 10.Takis C, Kwan ES, Pessin MS, Jacobs DH, Caplan LR. Intracranial angioplasty: experience and complications. AJNR Am J Neuroradiol 1997;18:1661–1668 [PMC free article] [PubMed] [Google Scholar]
- 11.Fandino J, Kaku Y, Schuknecht B, Valavanis A, Yonekawa Y. Improvement of cerebral oxygenation patterns and metabolic validation of superselective intraarterial infusion of papaverine for the treatment of cerebral vasospasm. J Neurosurg 1998;89:93–100 [DOI] [PubMed] [Google Scholar]
- 12.Kaku Y, Yonekawa Y, Tetsuya T, Kiyoshi K. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg 1992;77:842–827 [DOI] [PubMed] [Google Scholar]
- 13.Kassell NF, Helm G, Simmons N, Philips CD, Cail WS. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg 1992;77:842–847 [DOI] [PubMed] [Google Scholar]
- 14.Kazan S. Effects of intra-arterial papaverine on the chronic period of cerebral arterial vasospasm in rats. Acta Neurol Scand 1998;98:354–359 [DOI] [PubMed] [Google Scholar]
- 15.Milburn JM, Moran CJ, Cross DT, Diringer MN, Pilgram TK, Dacey RG. Increase in diameters of vasospastic intracranial arteries by intraarterial papverine administration. J Neurosurg 1998;88:38–42 [DOI] [PubMed] [Google Scholar]
- 16.Vajkoczy P, Horn P, Bauhuf C, et al Effect of intra-arterial papaverine on regional cerebral blood flow in hemodynamically relevant cerebral vasospasm. Stroke 2001;32:498–505 [DOI] [PubMed] [Google Scholar]
- 17.Tsurushima H, Kamezaki T, Nagatomo Y, Hyodo A, Nose T. Complications associated with intraarterial administration of papaverine for vasospasm following subarachnoid hemorrhage–two case reports. Neurol Med Chir 2000;40:112–115 [DOI] [PubMed] [Google Scholar]
- 18.Numaguchi Y, Zoarski GH, Clouston JE, et al. Okawara SH. Repeat intra-arterial papaverine for recurrent cerebral vasospasm after subarachnoid haemorrhage. Neuroradiology 1997;39:751–759 [DOI] [PubMed] [Google Scholar]
- 19.Mathis JM, Jensen ME, Dion JE. Technical considerations on intra-arterial papaverine hydrochloride for cerebral vasospasm. Neuroradiology 1997;39:90–98 [DOI] [PubMed] [Google Scholar]
- 20.Giordano MJ, Pryor JC, Moran CJ, Cross DT, Diringer MN. Intraarterial papaverine may significantly increase intracranial pressure. Chicago: Congress of Neurologic Surgeons;1994
- 21.Cross DT III, Moran CJ, Angtuaco EE, Milburn JM, Diringer MN, Dacey RG Jr. Intracranial pressure monitoring during intraarterial papaverine infusion for cerebral vasospasm. AJNR Am J Neuroradiol 1998;19:1319–23 [PMC free article] [PubMed] [Google Scholar]
- 22.Carhuapoma JR, Qureshi AI, Tamargo RJ, Mathis JM, Hanley DF. Intra-arterial papaverine-induced seizures: case report and review of the literature. Surg Neurol 2001;56:159–163 [DOI] [PubMed] [Google Scholar]
- 23.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm after subarachnoid hemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochirurgica Suppl (Wein) 1988;42:81–84 [DOI] [PubMed] [Google Scholar]
- 24.Cardene Product Information. Physicians Desk Reference. Montvale, NJ: Thompson PDR;2000
- 25.Abe K, Iwanaga H, Inada E. Effect of nicardipine and diltiazem on internal carotid artery blood flow velocity and local cerebral blood flow during cerebral aneurysm surgery for subarachnoid hemorrhage. J Clin Anesthes 1994;6:99–105 [DOI] [PubMed] [Google Scholar]
- 26.Alabad JA, Salom JB, Torregrosa G, Miranda FJ, Jover T, Alborch E. Changes in the cerebrovascular effects of endothelin-1 and nicardipine after experimental subarachnoid hemorrhage. Neurosurgery 1993;33:707–715 [DOI] [PubMed] [Google Scholar]
- 27.Frazee J, Bevan JA, Bevan RD, Jones KR, Bivens LV. Early treatment with diltiazem reduces delayed cerebral vascular narrowing after subarachnoid hemorrhage. Neurosurgery 1988;23:611–615 [DOI] [PubMed] [Google Scholar]
- 28.Takayasu M, Suzuki Y, Shibuya M, et al. The effects of HA compound calcium antagonists on delayed cerebral vasospasm in dogs. J Neurosurg 1986;65:80–85 [DOI] [PubMed] [Google Scholar]
- 29.Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid hemorrhage: British aneurysm nimodipine trial. BMJ 1989;298:636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porchet F, Chiolero R, de Tribolet N. Hypotensive effect of nimodipine during treatment for aneurysmal subarachnoid haemorrhage. Acta Neurochirurgica 1995;137:62–69 [DOI] [PubMed] [Google Scholar]
- 31.Devlin JW, Coplin WM, Murry KR, Rengachary SS, Wilson RF. Nimodipine-induced acute hypoxemia: case report. Neurosurgery 2000;47:1243–1246, discussion 1246–1247 [DOI] [PubMed] [Google Scholar]
- 32.Fahy BG. Pseudoobstruction of the colon: early recognition and therapy. J Neurosurg Anesthesiol 1996;8:133–136 [DOI] [PubMed] [Google Scholar]
- 33.Flamm ES, Adams HP Jr, Beck DW, et al. Dose-escalation study of intravenous nicardipine in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 1988;68:393–400 [DOI] [PubMed] [Google Scholar]
- 34.Haley EC, Kassell NF, Torner JC, Truskowski LL, Germanson TP, et al. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage: a report of the Cooperative Aneurysm Study. J Neurosurg 1993;78:537–547 [DOI] [PubMed] [Google Scholar]
- 35.Haley EC, Kassell NF, Torner JC, Truskowski LL, Germanson TP, et al. A randomized nicardipine in subarachnoid hemorrhage: angiographic and transcranial Doppler ultrasound results: a report of the Cooperative Aneurysm Study. J Neurosurg 1993;78:548–553 [DOI] [PubMed] [Google Scholar]
- 36.Haley EC, Kassell NF, Torner JC, Truskowski LL, Germanson TP, et al. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage: a report of the Cooperative Aneurysm Study. J Neurosurg 1994;80:788–796 [DOI] [PubMed] [Google Scholar]
- 37.Kasuya H, Onsa H, Takeshita M, Okada Y, Hori T. Efficacy and safety of nicardipine prolonged release implants for preventing vasospasm in humans. Stroke 2002;33:1011–1015 [DOI] [PubMed] [Google Scholar]