Abstract
BACKGROUND AND PURPOSE: Pyogenic ventriculitis is an uncommon manifestation of severe intracranial infection that might be clinically obscure. We hypothesized that determining characteristic imaging features of pyogenic ventriculitis in patients with appropriate risk factors might improve recognition of this severe infection.
METHODS: Review of the medical records from 1990 to 2000 revealed 17 cases (12 men, five women) that satisfied inclusion criteria of abscess (n = 3) and/or positive cultures or increased white cells and protein in ventricular (n = 12) or cisternal (n = 1) cerebrospinal fluid. In one case, the diagnosis of ventriculitis was based on the combination of bacterial growth in lumbar cerebrospinal fluid and follow-up imaging. Staphylococcus species and Enterobacter species were the most common organisms. Two neuroradiologists independently evaluated imaging studies for hydrocephalus, ventricular debris, periventricular attenuation or signal abnormality, ependymal enhancement, and signs of meningitis or abscess. Sixteen studies in 11 patients were performed after the intravenous administration of contrast material.
RESULTS: Ventricular debris was detected in 16 (94%) of 17 cases and was irregular in 13 (81%) of 16 cases. Hydrocephalus was present in 13 (76%) of 17 cases. Periventricular hyperintense signal was present in most (seven [78%] of nine) cases with MR imaging and was most conspicuous on fluid-attenuated inversion recovery sequences. Ependymal enhancement was detected in seven (64%) of 11 cases in which contrast material was administered. Signs of meningitis (eg, pial or duraarachnoid signal abnormality or enhancement) were present in 13 (76%) of 17 cases. Three cases had imaging signs of abscess.
CONCLUSION: Ventricular debris was the most frequent sign of ventriculitis in this series. An irregular level was characteristic of debris in ventriculitis. Hydrocephalus and ependymal enhancement were less frequent signs. Detection of ventricular debris might facilitate diagnosis of pyogenic ventriculitis, a potentially fatal infection, and thus permit appropriate therapy.
Pyogenic ventriculitis is an uncommon complication of intracranial infection in adults that has been variably referred to as ependymitis, ventricular empyema, pyocephalus, and ventriculitis (1–5). However, the imaging features of only a few cases of ventriculitis have been described in adults. Because ventriculitis is a frequent complication of meningitis in infants (6), it has been well described at sonography and CT in infants (7). Ventricular uptake in radionuclide brain scintigraphy using technetium-99m pertechnetate has been reported in children (8) and adults (4, 9). Although CT and MR imaging are the mainstays of neuroimaging in cases of adult meningitis, very few reports in sporadic cases have documented CT and MR imaging findings of ventriculitis (1–3, 10).
Because pyogenic ventriculitis is often clinically indolent and avoiding a fatal outcome relies on early treatment, we sought to improve the diagnosis of this entity. Our purpose was to characterize the CT and MR imaging features of pyogenic ventriculitis to hasten recognition of this grave, adult intracranial infection and, thus, to permit prompt, appropriate therapy.
Methods
Patient Cohort
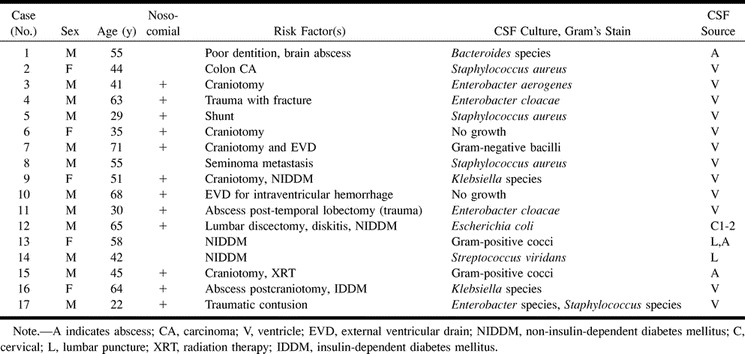
We reviewed the electronic medical records from January 1990 to March 2000 and discovered 17 cases (12 men, five women) that met our criteria for the diagnosis of pyogenic ventriculitis. Our diagnostic standards included intracranial abscess (n = 3) and/or positive cultures or increased white cells in ventricular (n = 12) or cisternal (n = 1) cerebrospinal fluid (CSF). One case demonstrated both brain abscess and bacterial growth on lumbar CSF culture. One case (Case 14) demonstrated bacterial growth on lumbar tap and progressive imaging signs on follow-up images. In two cases (Cases 6 and 10; Table), organisms were not demonstrated in ventricular CSF. In these cases, there were increased white blood cells (with increased polymorphonuclear cells) and elevated protein in the ventricular CSF. These CSF data were considered to be compatible with ventricular pus. The patients ranged in age from 22 to 71 years (mean age, 49 years). Organisms found on culture were Enterobacter species (n = 4), Staphylococcus species (n = 4), Klebsiella species (n = 2), Streptococcus species (n = 1), Escherichia coli (n = 1), and Bacteroides species (n = 1). One case (Case 17) demonstrated both Enterobacter and Staphylococcus species on culture. Two cases demonstrated gram-positive cocci, and one case demonstrated gram-negative bacilli on microscopy.
Image Acquisition
CT
Fifteen patients underwent CT scanning at 3-mm collimation with 5-mm interval in the posterior fossa and at 10-mm collimation and interval above the tentorium cerebelli. Five patients also underwent scanning after the intravenous administration of iothalamate meglumine (150 mL).
MR Imaging
All nine patients who underwent MR imaging were studied with a 1.5-T imager using standard T1-weighted (600/8/2 [TR/TE/excitations]), fast spin-echo T2-weighted (4000/102/2–4), and proton density–weighted (2400/16/2) sequences, in addition to gradient-echo sequences (350/11/2–3; flip angle, 20 degrees). Eight patients underwent T1-weighted imaging after intravenous administration of gadoteridol, 0.1 mmol/kg. Fast fluid-attenuated inversion recovery (FLAIR) imaging (10002/145eff/1; flip angle, 90 degrees) was performed for seven cases, and diffusion-weighted imaging (b = 1000; TR/TE, 10,000/minimum) and apparent diffusion coefficient maps were available for two cases.
Sixteen imaging studies (CT, seven; MR, nine) in 11 patients were performed after the intravenous administration of contrast material.
Image Interpretation
Two neuroradiologists independently evaluated 30 imaging studies (CT, 20; MR, 10) of the 17 patients for hydrocephalus, ventricular debris, periventricular attenuation or signal abnormality, ependymal enhancement, and signs of meningitis (eg, pial/duraarachnoid enhancement or subarachnoid signal abnormality) or abscess (enhancement and signal abnormality). Ventricular debris was further characterized as having a regular (parallel to scanning table) or irregular level. Criteria for periventricular attenuation or signal abnormality related to ventriculitis included focal, asymmetrical, or polar. Differences between readers were adjudicated by consensus between the two readers. The criterion for major disagreement was presence or absence of a finding.
Results
Risk Factors
Several patients had more than one predisposing condition for ventriculitis (Table). Risk factors for ventriculitis in this series included craniotomy (n = 7), diabetes (n = 5), neurosurgical device (n = 3), head injury (n = 3), and poor dentition (n = 1). Preexisting infections consisted of intracranial abscess (n = 3) and diskitis (n = 1). Six patients had multiple risk factors.
Imaging Findings
Ventricular debris was detected in 16 (94%) of 17 cases and was irregular in 13 (81%) of those 16 (Figs 1–5). The debris was hyperintense to CSF on T1-weighted images and hypointense to CSF on T2-weighted images. Hydrocephalus was present in 13 (76%) of 17 cases. Periventricular hyperintense signal was present in most (seven [78%] of nine) cases with MR imaging and was most conspicuous on FLAIR images (Figs 1 and 5). Ependymal enhancement was detected in seven (64%) of 11 cases in which contrast material was administered (Figs 2 and 5). Signs of meningitis were present in 13 (76%) of 17 cases (Fig 3). Three cases had imaging signs of abscess (Figs 2 and 4). Septation of the ventricles in one case was noted as a late finding (at 6 weeks) (Fig 5).
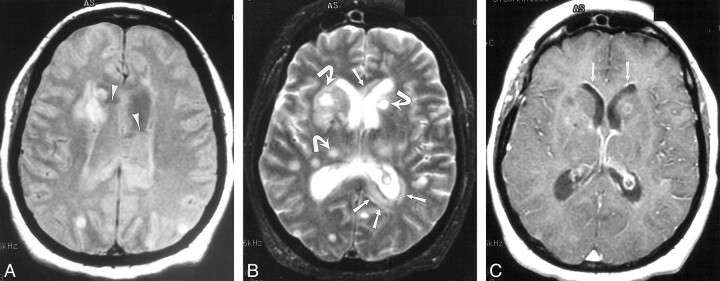
fig 1.
Case 3. 41-year-old patient who underwent routine postoperative MR imaging 4 days after resection of a posterior fossa ependymoma. Enterobacter aerogenes grew on culture of ventricular CSF.
A, Axial gradient-echo image (950/25/1 [TR/TE/excitation]; flip angle, 20 degrees) shows preexisting ventricular enlargement, related to the tumor, and straight fluid levels with susceptibility artifact within the occipital horns consistent with blood (arrows).
B, CT scan from postoperative day 10 also shows this material.
C–E, On postoperative day 15, the patient deteriorated. Repeat imaging demonstrates hydrocephalus and irregular debris within the ventricles on CT (C; arrows) and FLAIR images (10,002/145/1 [TR/TEeff/excitation]; inversion time, 2200) (D; open arrows) that does not contain blood products on the gradient-echo image (850/25/1 [TR/TE/excitation]; flip angle, 20 degrees) (E). There also is extensive periventricular hyperintensity (D; arrows), likely representing inflammatory change. Because there was no autopsy, we can only speculate that the hyperintensity in the globus pallidus (arrowheads) might be the result of cerebritis or infarction from vasculitis.
fig 2.
Case 13. 58-year-old patient with diabetes presented with altered mental status and multiple abscesses resulting from microaerophilic streptococcus. Gram-positive cocci were found on lumbar CSF analysis as well. Signal abnormality in the periventricular white matter and numerous abscesses (arrows) distributed throughout the deep and subcortical white matter are seen on proton density–weighted (2000/19/1 [TR/TEeff/excitation]) (A) and T2-weighted images (2800/256/1) (B). The irregular ventricular debris (arrowheads) is more clearly demonstrated on the proton density–weighted image (A). T1-weighted image (600/31/1) (C) obtained after intravenous administration of gadopentetate dimeglumine show minimal ependymal enhancement (arrows)
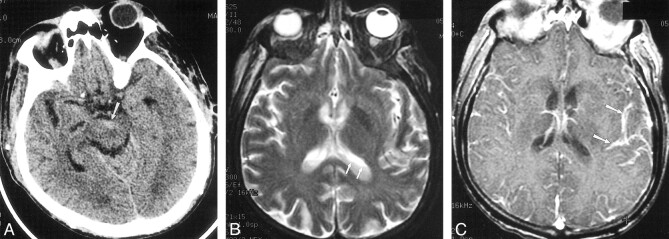
fig 3.
Case 12. 66-year-old patient with diabetes and secondary biliary cirrhosis died following infection with E coli meningitis, diagnosed by C1-C2 puncture. Delayed diagnosis occurred as a result of mild signs and symptoms of infection.
A, Initial CT scan shows debris and slight hyperattenuation (arrow) compared with CSF, producing an irregular level in the interpeduncular cistern.
B, MR imaging was deferred for 2 days because of minor central nervous system symptoms. T2-weighted image (2300/96/2 [TR/TE/excitations]) shows irregular debris (arrows) layering in the lateral ventricles.
C, T1-weighted contrast-enhanced image (500/26/1) shows striking pial subarachnoid space enhancement (arrows), but no ependymal enhancement.
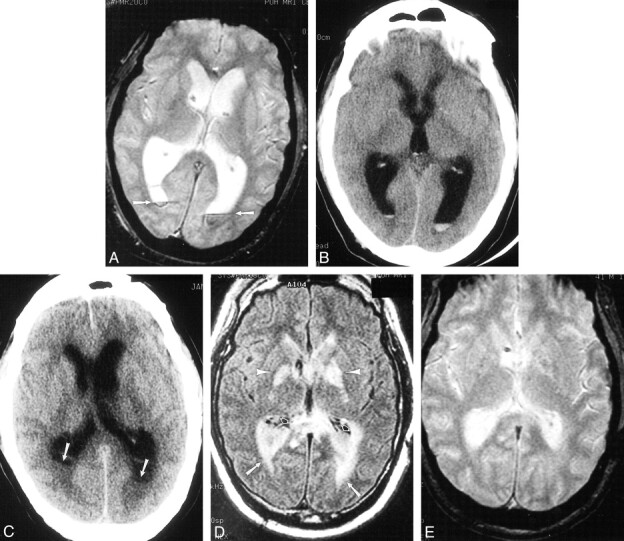
fig 5.
Case 14. 42-year-old patient with diabetes initially presented to an outside facility with meningitis and underwent serial imaging demonstrating the imaging course of ventriculitis during a 7-week period. Streptococcus viridans grew on CSF culture.
A, Initial contrast-enhanced CT scan shows irregular ventricular debris (arrowheads), hydrocephalus, and ependymal enhancement (arrows).
B–D, MR imaging performed 2 weeks later shows ventricular debris (short arrows) and periventricular signal abnormality (long arrows) on proton density–weighted (2000/15/1 [TR/TEeff/excitation]) (B) and FLAIR images (9002/147/1; inversion time, 2200) (C). T1-weighted image (800/20/1) obtained after gadopentetate dimeglumine administration shows extensive ependymal enhancement (arrows) and enhancement of a left posterior cerebral territory infarct.
E and F, There is progression of findings on MR imaging performed 7 weeks after initial presentation. FLAIR (10002/155/1; inversion time, 2200) (E) and T1-weighted images (800/8/1) (F) show loculation of ventricles (arrows) with persistent periventricular signal abnormality, but diminished ependymal enhancement.
Agreement between the two readers was excellent. Major disagreements occurred as follows: three (10%) of 30 imaging studies for hydrocephalus; three (10%) of 30 studies for subarachnoid space debris; two (6.7%) of 30 studies for ventricular debris; and one (6.3%) of 16 contrast-enhanced studies for pial enhancement. In one of the two cases in which there was disagreement regarding the presence of ventricular debris, there was disagreement for CT findings but agreement for MR imaging findings.
Discussion
Unsuspected ventriculitis might be a source of persistent infection and therapeutic failure in the management of meningitis (11,12). Gram-negative bacteria, in particular, might more often be resistant to standard antibiotics (13). Because fatal neurologic damage might occur, even in cases in which infection is ultimately eradicated, early treatment of gram-negative bacillary meningitis is crucial (14). Subsequent studies have shown that delayed CSF sterilization is directly related to neurologic deterioration in children (15). Because the presence or absence of ventriculitis might affect management decisions, its detection is critical to ensure early, adequate treatment of meningitis.
Delayed or inadequate treatment as a result of delayed recognition of relatively benign signs and symptoms, therefore, might account, at least in part, for the high mortality rates associated with meningitis (13). One series that examined reasons for delayed treatment of meningitis cited failure to consider the diagnosis as a significant cause (16). A noninvasive method for detection of ventriculitis would be highly desirable, since it could potentially avoid the morbidity of invasive methods of diagnosis. Recognition of relatively subtle imaging signs of ventriculitis, therefore, might have an impact on patient care. We are aware of no recent literature that documents the frequency with which ventriculitis complicates adult pyogenic meningitis; however, failure to recognize this entity might account for the scant literature. Furthermore, although not established in adults, the presence of ventriculitis in infants imposed greater morbidity and mortality rates (17).
The most frequent type of infection associated with pyogenic ventriculitis in this series was gram-negative meningitis (nine [60%] of 15 cases in which an organism was demonstrated by culture or Gram's stain) followed by Staphylococcus species. That most cases were gram-negative infections coincides with previous series that identified cases of ventriculitis (11, 14, 18). The mortality rate of gram-negative bacillary meningitis approaches 50% in large series (11–13, 18, 19). The mortality rate of meningitis caused by gram-positive organisms remains high (30%), as well, despite advances in antibiotic therapy (20).
The frequency of gram-negative bacillary meningitis has increased steadily during the past 30 years, which likely reflects an increase in nosocomial meningitis (18). Indeed, Durand et al (18) reported that 46% of recurrent nosocomial meningitis was gram-negative. In this series, seven (58%) of 12 cases of nosocomial ventriculitis were caused by gram-negative organisms. Meningitis caused by gram-negative bacilli presents a challenge in terms of management and detection because its course is often indolent (13) and it is prone to recurrence (18).
The relative lack of fever and severe presenting symptoms in these cases might reflect the predilection of meningitis for immunocompromised patients, including those with alcoholism, cirrhosis, or diabetes and patients recently having undergone surgery (14, 19). The predisposing factors for ventriculitis and gram-negative meningitis that we report are similar to those in other series (14, 19). Nosocomial meningitis often is attributable to recent neurosurgical procedure or to a neurosurgical device (18). Additional conditions that have been catalogued include CSF leak and head injury (18).
In our series, we sought to identify CT and MR imaging features of pyogenic ventriculitis in an effort to improve detection and effect prompt treatment. Ventricular debris was the most characteristic finding (Figs 1–5). This material might be irregular in contour because of the high protein content and, possibly, necrotic material. Elevated protein content in CSF might be related to a decrease in CSF production, as reported by Breeze et al (21) in studying a rabbit model of E coli ventriculitis. Similarly, the intermediate attenuation and signal intensity of the intraventricular material also might result from protein and necrotic material within the CSF (21). Breeze et al (21) found that the choroid plexuses of the animals were “covered by a sequestrum of debris, bacteria, leukocytes, and proteinaceous matrix.” In a study of infants, Berman and Banker (22) described tufts of glial tissue projecting through areas of denuded ependyma into the ventricular exudate that also might contribute to the intraventricular debris. They also attributed several cases of hydrocephalus to obstruction of the aqueduct of Sylvius or the foramina of Luschka and Magendie by bridging glial projections. These projections also might contribute to late septation of the ventricles (Fig 5). In our experience, an irregular level within the ventricle was quite specific for pus and was distinguished from either the straight level of acute blood or the casting configuration of clotted blood (Fig 1). Although debris in the ventricles has been reported in sporadic cases (1, 2, 10), to our knowledge, the irregular configuration of debris reported in this series has not been described previously. In the two cases for which diffusion-weighted imaging was available, the debris was more conspicuous because of its bright signal. Neither case demonstrated restricted diffusion (Fig 4), despite what others have observed in abscess (23). No conclusion can be drawn, however, from this limited experience with diffusion-weighted imaging in ventriculitis. Diffusion-weighted imaging might still prove an important sequence in diagnosing intracranial infection insofar as it might increase lesion conspicuity. The presence of ventricular debris was the most commonly observed (94%) imaging sign of ventriculitis.
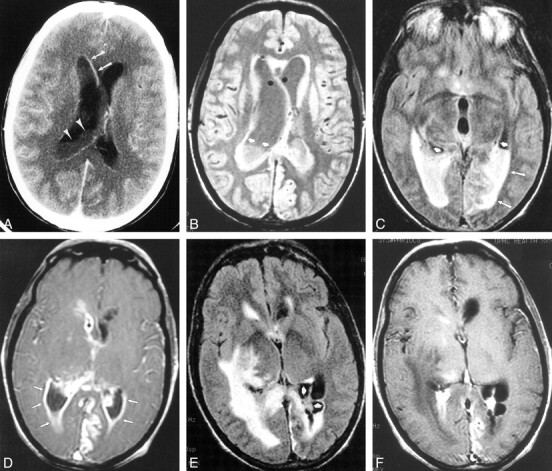
fig 4.
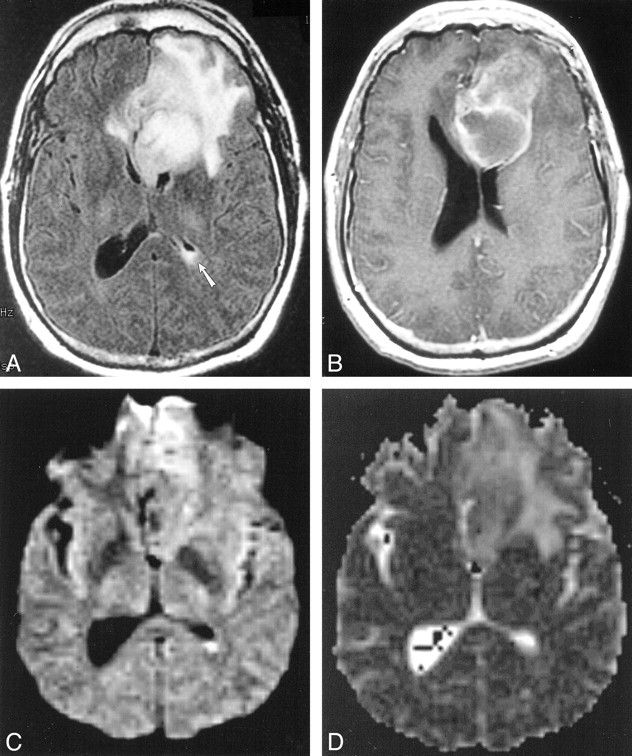
Case 15. Five months after left frontal craniotomy and resection of an oligodendroglioma, 45-year-old patient presented with headache. Postoperative therapy included adjuvant radiation therapy and steroids for headaches. This MR imaging study prompted a repeat operation in which two abscesses containing gram-positive cocci, one superficial and one deep, were discovered.
A, FLAIR image (9002/165/1 [TR/TEeff/excitation]; inversion time, 2200) shows a large left frontal mass with surrounding signal abnormality (abscess at operation) and debris in the left atrium.
B, Axial T1-weighted image (500/20/1 [TR/TE/excitation]) shows a cavitary left frontal lesion with peripheral enhancement. At second operation, this proved to be a deep abscess that was in continuity with the ventricle
C, Diffusion-weighted image (10,000/96.8/1000 [TR/TEeff/TI]) shows hyperintense signal in the left ventricular debris.
D, Apparent diffusion coefficient maps do not show restricted diffusion in the left ventricular pus.
Hydrocephalus was present in 76% of cases in this series and has been documented in some of the few cases reports in the literature (1, 2, 10). Periventricular signal abnormality, detected in 78% of cases with MR imaging, likely reflects the periventricular inflammatory change observed at pathology (9). Transependymal CSF was considered a less likely explanation for the periventricular signal abnormality because of the circumferential pattern observed in these cases. Other potential sources of periventricular signal abnormality include swollen subependymal astrocytes and perivascular infiltration with lymphocytes and plasma cells (24); chronic infection might result in subependymal astrocytic and microglial proliferation (22). Because the periventricular signal abnormality and ependymal enhancement occurred together in most cases (n = 5), this inflammation might explain the ependymal enhancement that we observed in most (64%) patients who also underwent imaging after contrast medium administration. Denuding of the ependyma, as described in infants with ventriculitis, could potentially be responsible for, or be coincident with, breakdown of the blood-brain barrier and, hence, enhancement (22). Ependymal enhancement also has been described in occasional case reports of ventriculitis (1, 2), however, it is not specific for infection.
The small sample size and retrospective design are limitations of this investigation. Nonetheless, the reported imaging findings of ventriculitis, correlated with the clinical data, represent the first step toward early diagnosis and appropriate treatment of gram-negative meningitis. A prospective study is needed to establish the true incidence of ventriculitis and its impact on outcome.
Conclusion
Ventriculitis may be an indolent and lethal infection and is a potential source of persistent infection, even when meningitis is treated. Early diagnosis is essential for the appropriate treatment of ventriculitis. The finding of irregular ventricular debris is especially characteristic of ventriculitis and, in the appropriate clinical setting, should prompt aggressive therapy.
Pyogenic ventriculitis: clinical data
Acknowledgments
We thank Kay Watt for help with manuscript preparation.
Footnotes
Presented in part at the annual meeting of the American Society of Neuroradiology, San Diego,1999.
Address reprint requests to Melanie B. Fukui, MD, Division of Neuroradiology, Department of Radiology, Allegheny General Hospital, 320 East North Ave, Pittsburgh, PA 15212.
References
- 1.Bakshi R, Kinkel P, Mechtler L, et al. Cerebral ventricular empyema associated with severe adult pyogenic meningitis: computed tomography findings. Clin Neurol Neurosurg 1997;99:252-255 [DOI] [PubMed] [Google Scholar]
- 2.Barloon TJ, Yuh WT, Knepper LE, et al. Cerebral ventriculitis: MR findings. J Comput Assist Tomogr 1990;14:272-275 [DOI] [PubMed] [Google Scholar]
- 3.Bodino J, Lylyk P, Del Valle M, et al. Computed tomography in purulent meningitis. Am J Dis Child 1982;136:495-501 [DOI] [PubMed] [Google Scholar]
- 4.Wormser G, Strashun A. Ventriculitis complicating gram negative meninigitis in an adult: diagnosis by radioisotope brain scanning and computerized tomography. Mt Sinai J Med 1980;47:575-578 [PubMed] [Google Scholar]
- 5.Vachon L, Mikity V. Computed tomography and ultrasound in purulent ventriculitis. J Ultrasound Med 1987;6:269-271 [DOI] [PubMed] [Google Scholar]
- 6.Salmon J. Ventriculitis complicating meningitis. Am J Dis Child 1972;124:35-40 [DOI] [PubMed] [Google Scholar]
- 7.Sarwar M, Falkoff G, Naseem M. Radiologic techniques in the diagnosis of CNS infections. Neurol Clin 1986;4:41-68 [PubMed] [Google Scholar]
- 8.Fulmer L, Sfakianakis G. Cerebral ventricle visualization during brain scanning with 99m tc-pertechnetate. J Nucl Med 1974;15:202-204 [PubMed] [Google Scholar]
- 9.Lee H. Unilateral pyogenic ventriculitis. J Nucl Med 1977;18:403. [PubMed] [Google Scholar]
- 10.Zimmerman R, Patel S, Bilaniuk L. Demonstration of purulent bacterial intracranial infections by computed tomography. AJR Am J Roentgenol 1976;127:155-165 [DOI] [PubMed] [Google Scholar]
- 11.Kaiser A, McGee Z. Aminoglycoside therapy of gram negative bacillary meningitis. N Engl J Med 1975;293:1215-1220 [DOI] [PubMed] [Google Scholar]
- 12.Rahal JJ, Hyams P, Simberkoff M, et al. Combined intrathecal and intramuscular gentamicin for gram negative meningitis: pharmacologic study of 21 patients. N Engl J Med 1974;290:1394-1398 [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Chang WN, Chuang YC, et al. The prognostic factors of adult gram-negative bacillary meningitis. J Hosp Infect 1998;40:27-34 [DOI] [PubMed] [Google Scholar]
- 14.Mangi R, Holstein L, Andriole V. Treatment of gram negative bacillary meningitis with intrathecal gentamicin. Yale J Biol Med 1977;50:31-41 [PMC free article] [PubMed] [Google Scholar]
- 15.Schaad U, Suter S, Gianella-Borradori A, et al. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meninigitis in children. N Engl J Med 1990;322:141-147 [DOI] [PubMed] [Google Scholar]
- 16.Wilks D, Lever AM. Reasons for delay in administration of antibiotics to patients with meningitis and meningococcaemia. J Infect 1996;32:49-51 [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Robinson M, Thong M, et al. Intraventricular chemotherapy in neonatal meningitis. J Pediatr 1977;91:991-995 [DOI] [PubMed] [Google Scholar]
- 18.Durand M, Calderwood S, Weber D, et al. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med 1993;328:21-28 [DOI] [PubMed] [Google Scholar]
- 19.Crane L, Lerner A. Non-traumatic gram-negative bacillary meningitis in the Detroit Medical Center, 1964–1974. Medicine 1978;57:197-209 [DOI] [PubMed] [Google Scholar]
- 20.Domingo P, Barquet N, Alvarez M, et al. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis 1997;25:1180-1187 [DOI] [PubMed] [Google Scholar]
- 21.Breeze R, McComb J, Hyman S, et al. CSF production in acute ventriculitis. J Neurosurg 1989;70:619-622 [DOI] [PubMed] [Google Scholar]
- 22.Berman P, Banker B. Neonatal meningitis: a clinical and pathological study of 29 cases. Pediatrics 1966;38:6-24 [PubMed] [Google Scholar]
- 23.Kim Y, Chang K-H, Song I, et al. Brain abscess and necrotic or cystic brain tumor: discrimination with signal intensity on diffusion-weighted MR imaging. AJR Am J Roentgenol 1998;171:1487-1490 [DOI] [PubMed] [Google Scholar]
- 24.Adams R, Kubik C, Bonner F. The clinical and pathological aspects of influenzal meningitis. Arch Pediatr 1948;65:354-376 [PubMed] [Google Scholar]