Abstract
BACKGROUND AND PURPOSE: Increased fat content in vertebrae may indicate bone weakness. Vertebral proton MR spectroscopy (1H MRS) quantitatively measures vertebral fat relative to water. Thus, 1H MRS measurements of percent fat fraction (%FF) and spectral line width (LW) of vertebral bone marrow may differ between subjects with and those without MR imaging evidence of weakened bone.
METHODS: We measured %FF and LW in 22 subjects with (15 men and 7 women, aged 26 to 80 years) and 72 control subjects without (33 male and 39 female subjects, aged 15 to 87 years) MR findings of weakened bone, including prominent Schmorl's nodes, endplate depression, vertebral wedging, and vertebral compression fractures. In those with bone weakness, 1H MRS data were sampled from an intact vertebra, usually L2. Data were analyzed for differences by age and sex. We compared the mean %FF and LW in subjects with and in those without bone weakness by use of Student's t test.
RESULTS: The %FF increased linearly with age in the control subjects, ranging from 20.5% in the second and third decades of life to 49.4% in eighth or ninth decades of life. Across all age groups, male control subjects had a higher aggregate %FF than did female control subjects. Male control subjects tended to have a greater LW than did female control subjects, but differences between the sexes within or across age groups were not significant. Contrary to expectations, LW was greatest in the oldest control subjects and lowest among younger control subjects, but there were insufficient data points to make definitive conclusions. Overall, %FF was a relative 45% higher in subjects with weakened bone compared with control subjects (48.8 vs 33.6 [P < .001]). The subjects with evidence of vertebral bone weakness also had a higher overall mean LW (37 vs 29 Hz [P < .002]), but this finding is of uncertain importance.
CONCLUSION: The %FF was significantly higher within the L2 vertebral body in 22 subjects with weakened bone compared with the control group, suggesting that it could serve as a measure of bone quality. The LW measurements did not yield meaningful conclusions.
The literature suggests that bone strength is multifactorial and may depend not only on bone density but also on bone-marrow quality (1, 2). Osteoporosis, for example, has been shown to be associated with increased fat content in the bone marrow (3–6). Marrow fat may reflect bone weakness (7).
Proton MR spectroscopy (1H MRS) can provide quantitative information about certain bone constituents, most prominently, fat (8–12). Other MR techniques also allow quantitative bone analysis, but these are based on magnetic susceptibility considerations and on T2 relaxation measurements (9, 13–16), which may be too complex for routine clinical applications. Thus, although these alternate techniques hold promise, 1H MRS may be easier to apply and interpret.
We published normative 1H MRS data that included lipid-water ratios (LWR) and spectral line widths (LW) for a group of healthy subjects (17). In this study, we expanded our control group by adding 1H MRS data from individuals with normal lumbar MR imaging findings. In addition, we included 1H MRS data from a group of patients with MR findings of vertebral weakness for comparison with those from the control subjects. We investigated whether 1H MRS can provide diagnostic clues to help appraise vertebral biomechanical competence. If so, it could add important information to diagnostic lumbar MR imaging, in that localized 1H MRS can be easily applied and attached to routine MR imaging without substantial time penalty.
Methods
We studied 72 control subjects (33 male and 39 female subjects, aged 15 to 87 years) without and 22 subjects (15 men and 7 women, aged 26 to 80 years) with MR imaging evidence of vertebral bone weakness. The group of control subjects included 57 people from our previous study (17). The 72 control subjects also included 11 volunteers and 61 subjects who underwent MR imaging to evaluate back pain but who exhibited no imaging signs of bone weakness. The 22 subjects with evidence of bone weakness were clinical patients who underwent lumbar MR imaging for low back pain and who had findings that supported a diagnosis of weakened bone. We excluded patients with known, diffuse marrow disease and a known history of radiation or steroid therapy. All subjects in this study had had 1H MRS added to their routine MR study in accordance with an approved clinical protocol. The 1H MRS sequence added 3 to 5 minutes to the routine MR imaging procedure.
The MR imaging findings of bone weakness included prominent Schmorl's nodes (n = 14), endplate depression (n = 1), wedging of vertebrae (n = 4), and vertebral body compression fractures (n = 3) (Table 1). Schmorl's nodes represent a form of intraosseous disk herniation, which often is associated with bone weakness. Endplate depression is the late result of fractures localized to one of the vertebral endplates. Vertebral wedging reflects an old compression fracture with no obvious fracture lines or fracture fragment(s) but with anterior height loss. Vertebral compression fractures are vertebrae with inhomogeneous tissue changes, sometimes associated with obvious fracture lines, fracture fragments, or bone displacement.
TABLE 1:
Summary of results by decades of age*
To be included in this study, a subject was required to undergo 1H MRS, usually of the L2 vertebral body, and a sagittal T1-weighted MR imaging of the lumbar spine to allow for visual assessment of lumbar vertebrae. Most subjects had single-voxel spectra. Eleven control subjects from our earlier study also had multivoxel 1H MRS (2D chemical-shift imaging). One of our authors (D.S.) reviewed the clinical MR images of patients who had undergone 1H MRS and determined that MR imaging showed bone weakness. The L2 vertebra was targeted for 1H MRS data sampling, because it usually is less affected by spondylosis or degenerative disk disease. In the patients with bone weakness, 1H MRS measurements were made in vertebrae that were not compressed or otherwise abnormal, usually L2. If L2 was compressed or otherwise altered in structure, a neighboring vertebra was used.
The 1H MRS techniques and voxel placement have been described in detail (17). All measurements were performed on a Siemens Vision 1.5-T unit (Erlangen, Germany). The stimulated echo-acquisition mode sequence was used; its advantages over point-resolved spectroscopy have been discussed (17). The software applied is part of the 1H MRS package for the Siemens Vision system (Siemens, Erlangen, Germany) and has United States Food and Drug Administration approval.
The 1H MRS divides the global MR signal from bone into two major segments: water and lipid. The water signal comes mostly from red marrow, and most lipid signals stem from yellow marrow. The spectra of 1H MRS have a water peak and a lipid peak (10, 18–22). Although the lipid signal is composed of at least eight fractions (23), it is the methylene group at 1.6 ppm that contributes the largest signal. The two major signals (water and lipid) are separated by 3.1 ppm. Signal peaks can vary from subject to subject and are influenced not only by the water/lipid proton quantity but also by the surface coil in use, the distance between the voxel and surface coil, and by the tissue environment. Therefore, instead of measuring the absolute signal peak for signal quantification, we derived a lipid-water ratio (LWR) for each voxel. Relative vertebral fat content can be expressed in LWR, fat fraction (FF), or percent fat fraction (%FF). For the purpose of clarity and consistency, we will use %FF as the preferred standard measurement in the remainder of this study. All values are derived from this equation: FF = LWR / (LWR +1). A spectrum with a lipid peak of 50 mm and a water peak of 25 mm has a LWR of 2 (50/25). The FF and %FF are derived directly from LWR: an LWR of 2.0 results in a FF of 0.66 (2/(2+1) and in an %FF of 66% (FF × 100).
In 42 control subjects and 16 patients with weakened bone, the 1H MRS spectra also were evaluated for LW. The smaller number of samples for this evaluation reflects its late addition to the 1H MRS studies. In a few subjects, the LW was erroneously omitted. The LW is determined by measuring the signal's full width at half height (FWHH). The LW stems largely from susceptibility produced by the inhomogeneity of the magnetic field caused by trabeculae. The potential of LW to serve as a measure of bone density was first suggested by Schick et al (9). The LW, commonly measured in Hz, depends on the distribution of the static magnetic field, which influences water and lipid peaks in the same way. We used the water peak for LW measurements, because it always consists of a single peak and thus is more suitable for FWHH (9). The LW measurements for control subjects are presented in our previous article (17). For this analysis, we obtained additional LW measurements for control subjects. All 22 subjects with MR findings of bone weakness had LW measurements, in addition to LWR and %FF.
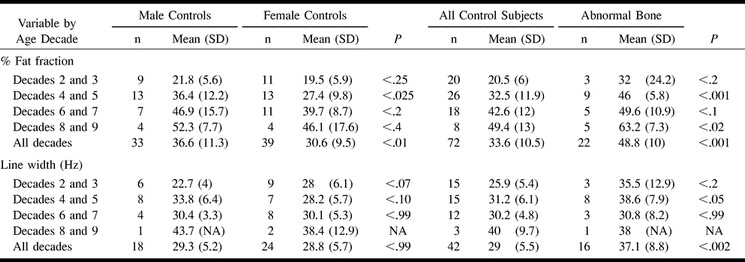
The 1H MRS data (LWR, %FF, LW) for control and abnormal vertebrae are listed by decade of age and summarized in Table 1. The data for %FF and LW were separately arranged in a manner that allowed analysis of age and sex and comparison of control and abnormal bone (Table 2). Statistical evaluation was conducted with Student's t test. Commercially available software (Excel; Microsoft, Redmond, Washington) was used.
TABLE 2:
Summary of results, control and abnormal subjects
Results
Percent Fat Fraction in Control Subjects: Age- and Sex-Related Differences
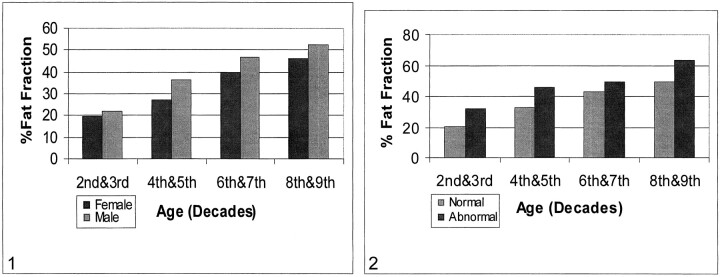
There was a linear rise in %FF with age, ranging from 20.5% for the second and third decades of life to 49.4% for the eighth and ninth decades. In the aggregate, male subjects had higher %FFs than did female subjects in all age categories (Table 2, Fig 1). This sex difference was most pronounced in the fourth through fifth and sixth through seventh decades, but was significant only for the fourth and fifth decades (P < .025) and for the total group of 72 control subjects (P < .01).
fig 1.
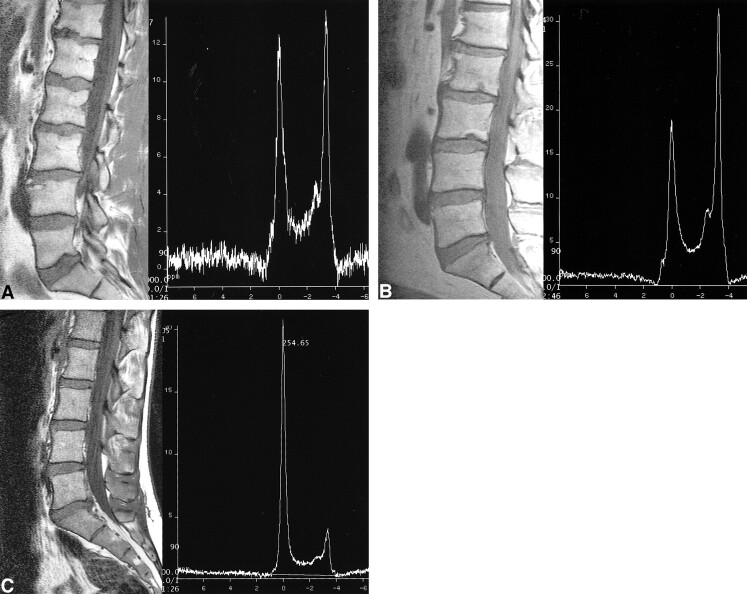
Relationship of age, sex, and percent fat fraction (%FF). Patients are grouped by decades of age; within each decade group, male and female subjects are separately represented. There is a linear increase of %FF with age and a difference in this variable between male and female subjects.fig 2. %FF for both normal and abnormal bone, by decades of age. Abnormal bone has a higher %FF in all age groups
Percent Fat Fraction in Subjects with Weakened Bone
In the first age grouping (second and third decades), the %FF in the abnormal group was 56% higher than that in the control subject group (32 vs 20.5 [Tables 1 and 2, Fig 2]). It was consistently higher in all other age groups. The difference in %FF between subjects with and those without weakened bone was significant for the fourth and fifth (P < .001) and eighth and ninth decades (P < .02). For the group as a whole, the %FF was a relative 45% higher in the subjects with weakened bone, which also was significant (P < .001).
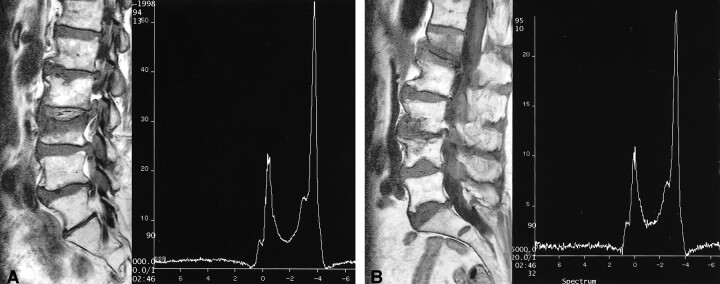
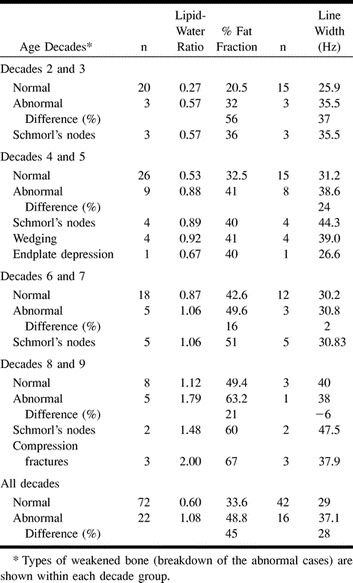
There were 14 subjects with Schmorl's nodes (Fig 3). This constituted our largest disease category. The %FF of subjects with Schmorl's nodes was consistently higher in all age categories compared with age-matched control subjects (Table 1). The %FF in these patients was a relative 76% higher in the second and third, 23% higher in the fourth and fifth, 20% higher in the sixth and seventh, and 22% higher in the eighth and ninth decades of life. Compression fractures (Fig 4) were observed only in the eighth and ninth decades. These three individuals had a %FF that was 36% higher than that of age-matched control subjects (Table 1). Wedging of vertebrae was found in four individuals, all in their fourth or fifth decade of life (Table 1). Their %FF was 26% higher than that of age-matched control subjects.
fig 3.
Comparison of patients with and a patient without Schmorl's nodes, the most common pathologic finding.
A, Schmorl's nodes at the lower thoracic and lumbar levels in a 38-year-old man. The 1H MRS shows a water peak (left) and a lipid peak (right). The LWR is 1.1, which yields a %FF of 52. This is relatively high for this age group (see fig 2).
B, Schmorl's nodes at the inferior endplates of L2 and L3 in a 60-year-old man. The 1H MRS shows the lipid peak (right) to be much higher than the water peak (left). The LWR measured 1.76, which yields a %FF of 64. This is relatively high for this age group (see fig 2).
C, Normal bone in a 17-year-old girl. The LWR measured 0.19, which yields a %FF of 16.
fig 4.
Examples of compression fractures.
A, An 87-year-old man with recent compression fracture of L3 and Schmorl's node at the superior endplate of L2. The 1H MRS at L2 revealed a LWR of 2.3, for a %FF of 70, which is relatively high for this age group.
B, An 80-year-old woman with recent compression fracture of L1, probable fracture at the inferior L3 vertebral body, and inferior endplate depression at L4 and L5. The 1H MRS at L2 shows a very prominent lipid peak. The LWR measured 2.4, for a %FF of 71, which is relatively high for this age group.
Line Width in Control Subjects: Age- and Sex-Related Differences
The LW was almost identical in fourth and fifth versus sixth and seventh decades (31.2 vs 30.2 Hz [Tables 1 and 2]). The LW was highest in the eighth- and ninth-decade group (40 Hz) and was lowest in the second- and third-decade group (25.9 Hz). There were some differences by sex, but these were not statistically significant (Table 2).
Line Width in Subjects with Weakened Bone
The LW of subjects with weakened bone was larger than that of control subjects in two age categories (second- and third-decade and fourth- and fifth-decade groups): 37% and 24%, respectively (Tables 1 and 2). The LW was almost even in the sixth- and seventh-decade group (+2%) and slightly lower in the eighth- and ninth-decade group (-6%). In the entire group, the subjects with weakened bone had an average LW 28% greater than that of control subjects.
Discussion
Change of Vertebral Fat Content with Age
Vertebral marrow fat has been reported to increase with age (24–26). Quantitative histologic studies on age-related changes in bone have shown that the change from hematopoietic to fatty marrow is gradual, steady, and progressive (24).
In this study, additional normative measurements were generated for certain 1H MRS data, foremost of which was %FF. We had observed that the fat content of vertebrae increases linearly with age (17). This observation has been confirmed (27) and is further supported by the current data from 72 subjects. The %FF of subjects in the age category of 11 to 30 years (second- and third-decade group) was 20.5% versus 49.4% in the age category of 71 to 90 years (eighth- and ninth-decade group [Table 1, Fig 2]). Kugel et al (27), in a recent analysis of age differences in the proton spectrum of vertebral bone marrow, also observed an increase in %FF with increasing age, from 24% in the age group of 11 to 20 years to 54% in the group aged 61 years or older.
Differences in Vertebral Fat Content by Sex
The current, larger data pool supports the trend shown in our previous report. In the entire population, male control subjects had a higher %FF than did female control subjects in all age categories. This difference was most pronounced in the age groups of 31 to 50 years and 51 to 70 years but was statistically significant only among control subjects aged 31 to 50 years (P < .025), in which the %FF was a relative 32.8% higher in men, and among the total group of 72 control subjects (P < .01).
This sex-related difference is supported by reports that suggest that bone marrow in men has a higher lipid content or that red marrow in women contains less fat and is more cellular than that in men (28–31). A number of reports disagree with these hypotheses, however (32–34). Kugel et al (27) also studied sex-specific differences in the proton spectra of vertebrae. They observed that the proportion of fat in the vertebral bone marrow in female subjects was less than that in male subjects. This difference was largest in the age group of 31 to 50 years (male vs female subjects, 12%).
Increased fat content among male subjects could be interpreted to denote weakened bone. This is unrealistic and contrary to clinical experience. We believe that increased marrow fat found in men is a constitutional phenomenon and reflects their normal tissue texture. Only when fat content increases beyond the norm can it be interpreted as abnormal. As is true for dual-energy x-ray absorptiometry (DXA), the availability of nomograms is crucial. Similar to bone-mineral density, measurements are both age- and sex-dependent.
Relationship of Line Width to Age and Sex
The density and spatial orientation of the trabeculae influence the microscopic homogeneity of the magnetic field inside the marrow (9, 20, 32). This led Schick et al (9) to use LW as a variable in MR bone densitometry. Increased bone density is expected to cause greater magnetic field inhomogeneity and wider spectral peaks. Conversely, decreased bone density narrows the signal peaks.
We previously explored LW measurements among control subjects of various ages and also searched for sex-related differences. The earlier findings did not support the expected LW narrowing with age. In this report, for which additional LW measurements were obtained, the cumulative data show that LW was slightly higher among the older age groups, a finding that runs contrary to expectation (Table 2, Fig 2). The collected data lead us to conclude that LW cannot be used to appraise bone density. Men are known to have a higher bone density than women (35, 36), as are younger compared with older subjects. Although we expected a widened LW in men and in the younger age groups, it was not significantly larger in men or among the young. The LW probably is influenced by factors other than bone trabecula. These data lead us to conclude that LW is not suitable for osteodensitometry. At this time, we are uncertain about the diagnostic value of LW. More data from a larger subject pool probably are needed.
Relationship of Vertebral Fat Content to Bone Weakening
Our data show that patients with MR imaging findings of bone weakness have a consistently higher %FF compared with control subjects within the same age group (Tables 1 and 2, Fig 2). The difference ranged from a relative 16% (sixth- and seventh-decade group) to 56% (second- and third-decade group).
It is known that increased marrow fat content can lead to bone weakness (3, 4, 7, 37). Conversion of red to yellow marrow with aging is a well-known phenomenon (38). In osteoporosis, an increase in bone marrow fat cannot be ignored (5). Histologic and histomorphologic measurements of osteoporotic vertebrae have shown reductions in cancellous bone accompanied by a decrease in hematopoetic marrow and a corresponding increase in fat cells. Dunnill et al (24) observed that with aging, the increase in marrow fat is even more marked than the decrease in cellular marrow, presumably because fatty marrow is replacing bone as well as hematopoetic tissue. This process also may occur under certain pathologic conditions. Additional fat cells are necessary to replace age-related trabecular bone loss and to fill bone resorption cavities (5, 24–26, 32, 39, 40).
Hypothetically, increased marrow fat content could affect bone strength in various ways. First, there could be a passive increase in marrow fat. Trabecular rarefication with thinning is commonly reported as the main cause of bone weakness in osteoporosis. Trabecular thinning results in open spaces among the trabecular mesh. Some have suggested that there is compensatory filling of these spaces with fatty marrow (5, 24–26, 32, 39–41). Therefore, increased vertebral fat content could mirror bone loss.
Second, vertebral bone marrow could act as a biomechanical support medium. Some believe that vertebral marrow quality is another important determinant of vertebral strength (2). The intertrabecular spaces that contain bone marrow have been reported to act as energy dampers. Marrow composition may affect elasticity of the bone superstructure; red marrow is said to contribute to hydrostatic strengthening, whereas fatty marrow is associated with greater compressibility of vertebrae. The combination of hematopoetic marrow with the collagen and hydroxyapatite forms a comparatively tough material. Fatty marrow would cause hydrostatic weakening of a vertebra.
Third, bone marrow relates to the regulation of bone turnover. Marrow fat is observed to influence the quality of trabecula (7). Nuttal et al (42) have reported that bone-marrow stromal cells can undergo adipogenesis or osteoblastogenesis. There is a reciprocal relationship between adipocytes and osteoblast phenotypes; excessive expression of one versus the other may have significance in the context of osteoporosis. Thus, a decrease in osteoplastogenesis in bone marrow may result in increased adipogenesis (43). Predominance of fatty marrow therefore could be a result of increased adipogenesis and have a negative effect on osteogenesis.
On the basis of these considerations, it appears that bone mineral and bone density are not the only factors to consider when estimating biomechanical competence. Instead, a combination of factors come into play, including above-listed ones (1, 2, 6).
To our knowledge, no reports in the radiology literature have examined the association of increased vertebral marrow fat content and bone weakness. Many reports have focused on MR imaging and compression fractures, with the main interest directed toward distinguishing benign from malignant vertebral fractures (44–48), but they have concentrated only on the compressed vertebra, not on the bone environment. In this study, we performed 1H MRS of uncompressed vertebrae, usually L2, in subjects who had some MR imaging evidence of weakened bone.
Increased vertebral fat content can be suspected on routine MR images when vertebral bone marrow has bright T1 signals. However, 1H MRS allows quantitative assessment of vertebral fat. Our initial findings must be verified in other, similar analyses. If confirmed, 1H MRS should be strongly considered as an add-on to lumbar MR imaging, particularly in patients with clinical suspicion of bone weakness.
Relationship of Line Width to Bone Weakening
Bone tissue may be a silent partner in the shaping of vertebral spectra, by widening the LW in an environment of dense bone (9, 20, 32) and by causing the LW to narrow in an environment of increased marrow fat. Our data suggest no convincing relationship between LW and bone density. In the “all decades” group, the LW in subjects with weakened bone was an average 28% higher compared with age-averaged control subjects (Tables 1 and 2). This statistically significant difference in LW (29 Hz vs 37.1 Hz) runs counter to our expectation and is of uncertain importance. Therefore we believe that LW cannot be used as a measure of bone weakening.
We propose that future 1H MRS studies evaluate all five lumbar vertebrae and use larger voxels for data collection. This will provide a larger tissue sample, similar to that used for lumbar-spine DXA. Improved scanning techniques allow whole lumbar-spine measurements within 5 minutes. Future 1H MRS research also must concentrate on comparisons with a noninvasive, reference-standard assay for bone density, such as DXA. We are presently engaged in a DXA-1H MRS correlation study (49).
Future investigations should also compare sex-related differences in %FF in cohorts with bone weakness. Since our LW measurements did not allow definitive conclusions, further LW data should be compiled to help formulate a more definitive opinion about its diagnostic merits.
Conclusion
The %FF reflects bone fat content relative to water. This analysis suggests that %FF can serve as a measure of bone quality, in that increased %FF values were associated with bone weakness in 22 subjects.
Footnotes
Address reprint requests to Dieter Schellinger, MD, Department of Radiology, Georgetown University Medical Center, 3800 Reservoir Road NW, Washington, DC 20007.
References
- 1.Eriksson SA, Isberg BO, Lindgren JU. Prediction of vertebral strength by dual photon absorptiometry and qualitative computed tomography. Calcif Tissue Int 1989;44:243-250 [DOI] [PubMed] [Google Scholar]
- 2.Kazarian L, Graves G. Compressive strength characteristics of the human vertebral column. Spine 1977;2:1-13 [Google Scholar]
- 3.Demmler K, Otte P, Bartl R, Burkhardt R, Frisch B, Jahn A. Osteopenia, marrow atrophy and capillary circulation. Comparative studies of the human iliac crest and 1st lumbar vertebra. Z Orthop Ihre Grenzgeb 1983;121:223-227 [DOI] [PubMed] [Google Scholar]
- 4.Demmler K, Burkhardt R. Relations between fatty tissue, cancellous bone and vascular pattern of the iliac bone in aplastic anaemia. Bibl Haematol 1978;45:109-117 [DOI] [PubMed] [Google Scholar]
- 5.Ikeda T, Sakurai K. Influence of bone marrow fat on the determination of bone mineral content by QCT. Nippon Igaku Hoshasen Gakkai Zasshi 1994;54:886-896 [PubMed] [Google Scholar]
- 6.Krokowski E, Fricke M. Osteoporosis: more than a bone disease. Med Klin 1975;70:822-899 [PubMed] [Google Scholar]
- 7.Lotz JC, Gerhart TN, Hayes WC. Mechanical properties of trabecular bone from the proximal femur: a quantitative CT study. J Comput Assist Tomogr 1990;14:107-114 [DOI] [PubMed] [Google Scholar]
- 8.Schick F, Einsele H, Bongers H, et al. Leukemic red marrow changes assessed by magnetic resonance imaging and localized 1H spectroscopy. Ann Hematol 1993;66:3-13 [DOI] [PubMed] [Google Scholar]
- 9.Schick F, Seitz D, Machann J, Lutz O, Claussen CD. Magnetic resonance bone densitometry: comparison of different methods based on susceptibility. Invest Radiol 1995;30:254-265 [PubMed] [Google Scholar]
- 10.Schick F, Einsele H, Lutz O, Claussen CD. Lipid selective MR imaging and localized 1H spectroscopy of bone marrow during therapy of leukemia. Anticancer Res 1996;16:1545-1552 [PubMed] [Google Scholar]
- 11.Machann J, Schick F, Seitz D, Lutz O, Claussen CD. Examination of trabecular bone structures of the foot skeleton with MRI imaging. Biomed Tech (Berl) 1998;43:202-209 [DOI] [PubMed] [Google Scholar]
- 12.Layer G, Traber F, Block W, et al. 1H MR spectroscopy of the lumbar spine in diffuse osteopenia due to plasmacytoma or osteoporosis. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1998;169:596-600 [DOI] [PubMed] [Google Scholar]
- 13.Majumdar S, Thomasson D, Shimakawa A, Genant HK. Quantitation of the susceptibility difference between trabecular bone and bone marrow: experimental studies. Magn Reson Med 1991;22:111-127 [DOI] [PubMed] [Google Scholar]
- 14.Rosen BR, Fleming DM, Kushner DC, et al. Hematologic bone marrow disorders: quantitative chemical shift MR imaging. Radiology 1988;169:799-804 [DOI] [PubMed] [Google Scholar]
- 15.Wehrli FW, Ford JC, Attie M, Kressel HY, Kaplan FS. Trabecular structure: preliminary application of MR interferometry. Radiology 1991;179:615-621 [DOI] [PubMed] [Google Scholar]
- 16.Davis CA, Genant HK, Dunham JS. The effects of bone on proton NMR relaxation times of surrounding liquids. Invest Radiol 1986;21:472-477 [DOI] [PubMed] [Google Scholar]
- 17.Schellinger D, Lin CS, Fertikh D, et al. Normal lumbar vertebrae: anatomic, age, and sex variance in subjects at proton MR spectroscopy—initial experience. Radiology 2000;215:910-916 [DOI] [PubMed] [Google Scholar]
- 18.Frahm J, Merboldt KD, Hanicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson 1987;72:502-508 [DOI] [PubMed] [Google Scholar]
- 19.Frahm J, Michaelis J, Merboldt KD, Bruhn H, Gyngell ML, Hanicke W. Improvements in localized proton NMR spectroscopy of human brain: water suppression, short echo times, and 1 ml resolution. J Magn Reson 1990;90:464-473 [Google Scholar]
- 20.Schick F, Bongers H, Jung WI, Skaley MA, Lutz O, Claussen CD. Volume-selective proton MRS in vertebral bodies. Magn Reson Med 1992;26:207-217 [DOI] [PubMed] [Google Scholar]
- 21.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med 1993;29:158-167 [DOI] [PubMed] [Google Scholar]
- 22.Vogler JB, Murphy WA. Bone marrow imaging. Radiology 1988;168:679-693 [DOI] [PubMed] [Google Scholar]
- 23.Brix G, Heiland S, Bellemann ME, Koch T, Lorenz WJ. MR imaging of fat-containing tissues: valuation of two quantitative imaging techniques in comparison with localized proton spectroscopy. Magn Reson Imaging 1993;11:977-991 [DOI] [PubMed] [Google Scholar]
- 24.Dunnill MS, Anderson JA, Whitehead R. Quantitative histolological studies on age changes in bone. J Pathol Bacteriol 1967;94:275-291 [DOI] [PubMed] [Google Scholar]
- 25.Lang P, Steiger P, Faulkner K, Gluer C, Genant HK. Osteoporosis: current techniques and recent developments in quantitative bone densitometry. Radiol Clin N Am 1991;29:49-76 [PubMed] [Google Scholar]
- 26.Funke M, Kopka L, Vosshenrich R, et al. Broadband ultrasound attenuation on the diagnosis of osteoporosis: correlation with osteodensitometry and fracture. Radiology 1995;194:77-81 [DOI] [PubMed] [Google Scholar]
- 27.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging 2001;13:263-268 [DOI] [PubMed] [Google Scholar]
- 28.Ishijima H, Ishizaka H, Horikoshi H, Sakurai M. Water fraction of lumbar vertebral bone marrow estimated from chemical shift misregistration on MR images: normal variations with age and sex. Am J Radiol 1996;176:355-358 [DOI] [PubMed] [Google Scholar]
- 29.Richards MA, Webb JA, Jewell SE, Gregory WM, Reznek RH. In vivo measurements of spin lattice relaxation time (T1) of bone marrow in healthy volunteers: the effects of age and sex. Br J Radiol 1988;61:30-33 [DOI] [PubMed] [Google Scholar]
- 30.Vande Berg BC, Lecouvet FE, Malghem J. Sex-related difference in marrow conversion in the proximal femur: does it exist? Radiology 1998;209:587-588 [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Mihara F, Numaguchi Y, Rothman M, Kawaguchi H. Spinal epidural venous plexus: MR enhancement pattern and clinical significance. Presented at the 30th Annual Meeting of the American Society of Neuroradiology, St. Louis, June 1992
- 32.Dooms GC, Fisher MR, Hricak H, Richardson M, Crooks LE, Genant HK. Bone marrow imaging: magnetic resonance studies related to age and sex. Radiology 1985;155:429-432 [DOI] [PubMed] [Google Scholar]
- 33.Ricci C, Cova M, Kang YS, et al. Normal age-related pattern of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 1990;177:83-88 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins JP, Stehling M, Sivewright G, Hickey DS, Hillier VF, Isherwood I. Quantitative magnetic resonance imaging of vertebral bodies: a T1 and T2 study. Magn Reson Imaging 1989;7:17-23 [DOI] [PubMed] [Google Scholar]
- 35.Seeger LL. Bone density determination. Spine 1997;22:49S-57S [DOI] [PubMed] [Google Scholar]
- 36.Myers ER, Wilson SE. Biomechanics of osteoporosis and vertebral fracture. Spine 1997;22:25S-31S [DOI] [PubMed] [Google Scholar]
- 37.Coleman BG, Kressel HY, Dalinka MK, et al. Radiographically negative avascular necrosis: detection with MR imaging. Radiology 1988;168:525-528 [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DG, Rao DM, Dalinka M, et al. Hematopoietic and fatty bone marrow distribution in the normal and ischemic hip: new observations with 1.5-T MR imaging. Radiology 1986;161:199-202 [DOI] [PubMed] [Google Scholar]
- 39.Pykett I, Rosen BR. Nuclear magnetic resonance: in vivo proton chemical shift imaging. Radiology 1983;149:197-201 [DOI] [PubMed] [Google Scholar]
- 40.Riggs BL, Wahner HW, Melton LJ III, Richelson LS, Judd HL, Offord KP. Rates of bone loss in the appendicular and axial skeleton of women: evidence of substantial vertebral bone loss before menopause. J Clin Invest 1986;77:1487-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riggs BL, Melton LJ. Involutional osteoporosis. N Engl J Med 1986;314:1676-1648 [DOI] [PubMed] [Google Scholar]
- 42.Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 2000;27:177-184 [DOI] [PubMed] [Google Scholar]
- 43.Manolagas SC, Bellido T, Jilka RL. Sex steroids, cytokines and the bone marrow: new concepts on the pathogenesis of osteoporosis. Ciba Found Symp 1995;191:187-196 [DOI] [PubMed] [Google Scholar]
- 44.Yuh WT, Zachar CK, Barloon TJ, Sato Y, Sickels WJ, Hawes DR. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology 1989;172:215-218 [DOI] [PubMed] [Google Scholar]
- 45.Yamato M, Nishimura G, Kuramochi E, Saiki N, Fujioka M. MR appearance at different ages of osteoporotic compression fractures of the vertebrae. Radiat Med 1998;16:329-334 [PubMed] [Google Scholar]
- 46.Cuenod CA, Laredo JD, Chevret S, et al. Acute vertebral collapse due to osteoporosis or malignancy: appearance on unenhanced and gadolinium-enhanced MR images. Radiology 1996;199:541-549 [DOI] [PubMed] [Google Scholar]
- 47.Kiel D. Assessing vertebral fractures. National Osteoporosis Foundation Working Group on Vertebral Fractures (erratum published J Bone Miner Res 1995;10:1605). J Bone Miner Res 1995;10:518-523 [DOI] [PubMed] [Google Scholar]
- 48.Rupp RE, Ebraheim NA, Coombs RJ. Magnetic resonance imaging differentiation of compression spine fractures or vertebral lesions caused by osteoporosis or tumor. Spine 1995;20:2499-2503 [DOI] [PubMed] [Google Scholar]
- 49.Schellinger D, Lin C, Lim J, Singer AL, Hatipoglu H, Fertikh D. Use of proton magnetic resonance spectroscopy for analysis of lumbar vertebrae: correlation with bone weakening. Comparison with DXA. Presented at the World Congress on Osteoporosis 2000, Chicago, June 2000:S144