Abstract
BACKGROUND AND PURPOSE: The prognosis of comatose survivors is determined by clinical examination. Early laboratory indicators of poor prognosis (such as evoked potentials) have low sensitivity. The role of MR imaging as a confirmatory study was investigated.
METHODS: We studied fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted (DW) imaging in 10 patients comatose after cardiac arrest.
RESULTS: None of the 10 comatose patients had myoclonus status epilepticus or fixed, dilated pupils on neurologic examination, and none had abnormal somatosensory-evoked potentials. Eight patients showed diffuse signal abnormalities, predominantly in the cerebellum (n = 5), the thalamus (n = 8), the frontal and parietal cortices (n = 8), and the hippocampus (n = 9). One patient showed normal MR imaging results, and one patient had abnormalities in the thalamus and cerebellum and minimal abnormality on DW images; both later awakened. None of the patients with abnormal cortical structures on FLAIR MR images recovered beyond a severely disabled state.
CONCLUSION: MR imaging in comatose survivors may parallel the pathologic findings in severe anoxic-ischemic injury, and extensive abnormalities may indicate little to no prospects for recovery. If confirmed, MR imaging may have a role as a prognosticating test in anoxic-ischemic coma.
Since the landmark study by Levy et al (1) on prognostication in anoxic-ischemic coma, to our knowledge no study has challenged its findings that clinical examination within the first 3 days can accurately determine outcome (2–5). Recent studies have added clinical and electrophysiological modifiers that may further aid in prognostication. We and others have found that myoclonus status epilepticus in comatose patients portends a poor outcome (6–8), and several authors have noted that somatosensory-evoked potentials (SSEP) performed within 24 hours after cardiac resuscitation may accurately predict a persistent vegetative state (9, 10).
In certain circumstances, an additional confirmatory test is needed before poor prognosis in anoxic-ischemic coma can be established. First, many patients are heavily sedated or paralyzed after cardiac resuscitation to combat pulmonary complications, which renders neurologic examination almost useless. Second, early clinical signs of poor prognosis are infrequent, occurring in 20% of patients at most. Further, tests such as CT or SSEP have not been consistently useful because abnormal findings, such as diffuse cerebral edema or absent scalp potentials on SSEP, also are uncommon. Third, families of previously healthy patients struck by severe anoxic-ischemic damage to the brain often demand some form of corroborative testing for assurance before the decision is made to withdraw support.
MR imaging may aid in prognostication, but its availability, its costs, and the complicated transport of patients with the potential for recurrent cardiac arrhythmias have been prohibitive and hindered its study. We performed a preliminary study of MR imaging using fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted (DW) imaging in 10 comatose survivors of cardiac arrest.
Methods
For 12 months, we prospectively followed 27 patients who remained comatose after cardiac resuscitation. All of the patients, who had been admitted to a medical or surgical intensive care unit, underwent a complete neurologic examination. Consent was obtained to perform MR imaging, but in 17 patients with terminal cardiac failure or recurrent cardiac arrhythmias, MR imaging was not judged safe. Ten patients underwent MR imaging with anesthesia support. It took 20 to 30 minutes to complete the study in each patient. The MR images were reviewed without knowledge of any of the clinical features.
Nine of the patients had a noncontrast CT head examination within 24 hours of their anoxic event, and all 10 patients had MR imaging of the head 1 to 15 days after resuscitation (median, 6 days). All MR examinations were performed with a 1.5-T imager (General Electric Signa LX, 8.2.5, Milwaukee, WI). Standard MR imaging was performed in all 10 patients, which included a T1-weighted sagittal sequence (580/14/1 [TR/TE/excitation]), intermediate- and T2-weighted spin-echo axial sequences (2000–2500/30–80/1), and a FLAIR T2-weighted axial sequence (1100/168/2250/1 [TR/TE/TI/excitation]). Echo-planar DW images (9999/93/1, b = 1000 s/mm2 in three orthogonal directions, and b = 0 with FLAIR pre-pulse TI = 2200) were acquired in seven patients. Six patients were given intravenous contrast medium (Magnevist, 0.1 mmol/kg, Berlex Laboratories, Princeton, NJ), and postcontrast T1-weighted axial and coronal images were obtained (400–500/14–20/2). Two patients had follow-up MR imaging of the head, within 4 or 5 days of the first study.
Results
The clinical features are shown in Table 1. Age ranged from 32 to 76 years. Six patients had in-hospital arrest. Eight of 10 patients had a Glasgow coma sum score of 3 after resuscitation, but brain stem reflexes were present in all patients. There were absent motor responses to pain in eight patients, and extensor responses in two patients. One patient showed continuous blinking, and another patient had a persistently downward gaze. None of the patients had a sustained upward gaze or myoclonus status epilepticus. Median SSEP were performed in six patients and were normal or indeterminate (prolonged latencies or unilaterally absent scalp potential). Electroencephalography (EEG), available for five patients, showed a mixed appearance of delta and theta frequencies. None of the patients had an α-coma or burst-suppression pattern. CT scan findings were normal in all nine patients.
TABLE 1:
Clinical features in 10 comatose patients after cardiac arrest
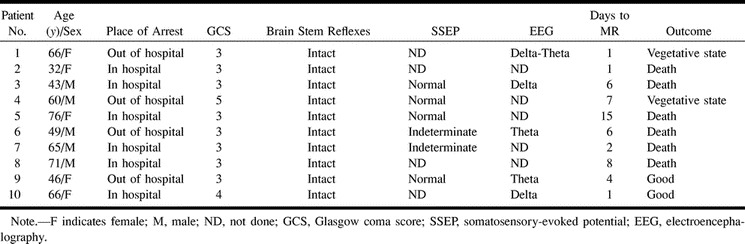
The MR imaging features are shown in Tables 2 and 3. Apparent diffusion coefficient (ADC) mapping and quantification are shown in Table 3. Normal values are 700–800 × 10−6 mm2/s; decreased ADC values represent areas of restricted diffusion, presumably because of infarction. Areas with increased ADC should represent vasogenic edema. In six patients, MR imaging showed diffuse signal abnormalities in the cortex in both hemispheres, frontal and parietal lobes, cerebellum, thalamus, and hippocampus (Fig 1). In two patients, effacement of the sulci and T2-weighted signal abnormalities were found in white matter (Fig 2). None of these eight patients recovered beyond a severely disabled state, and six patients died within several weeks from withdrawal of support or systemic complications. Two patients awakened within 3 days with no major neurologic deficit. One patient had normal MR images, and one patient had abnormalities in the thalamus and cerebellum and minimal abnormalities on DWI (Fig 3).
TABLE 2:
MR characteristics in 10 patients with anoxic-ischemic coma
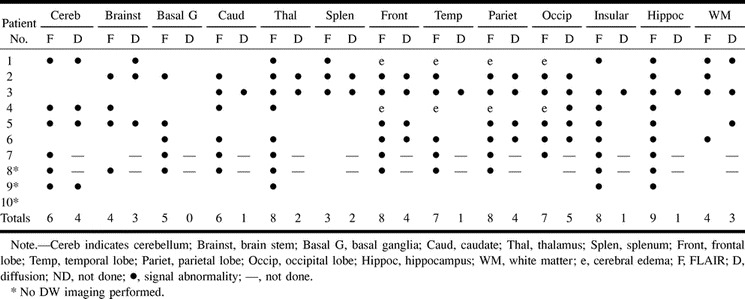
TABLE 3:
Apparent diffusion coefficient mapping and quantification
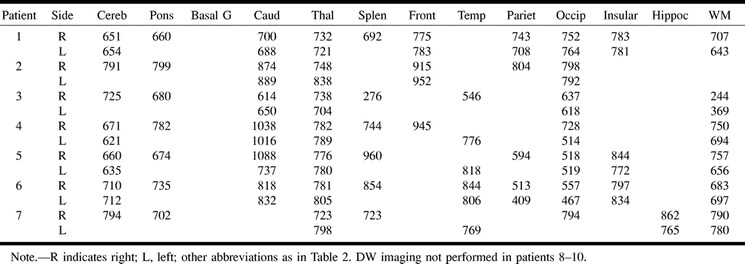
fig 1.

Patient 3. A and B, Axial FLAIR images show widespread T2 signal abnormalities. C and D, throughout the cortices of both cerebral hemispheres. DWI shows intense signal at these locations
fig 2.
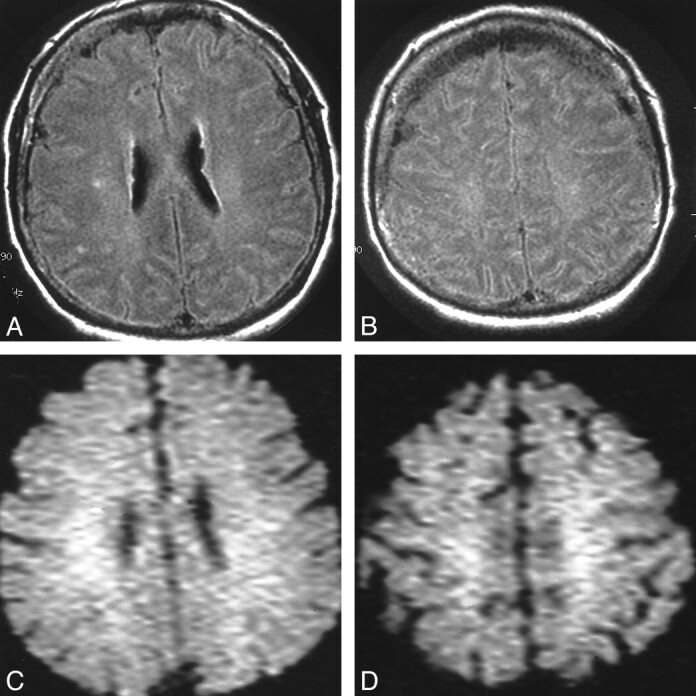
Patient 1. A and B, Axial FLAIR images show cerebral edema with effacement of the sulci and T2 signal abnormalities in white matter. C and D, DW images show only a corresponding white matter abnormality
fig 3.
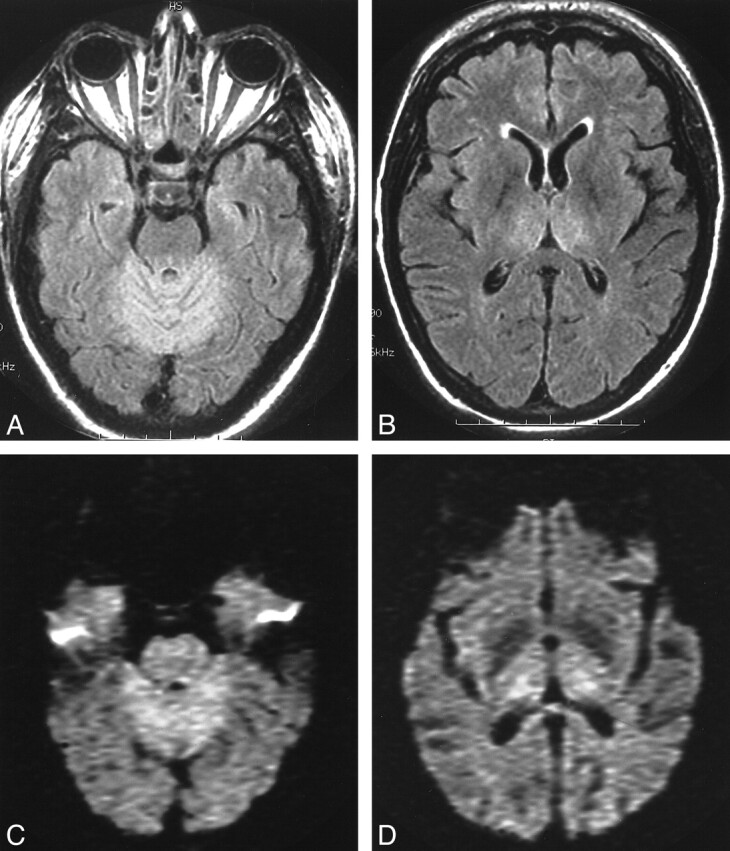
Patient 9. A and B, Axial FLAIR images at the level of the superior cerebellum and thalamus show symmetrical T2 signal abnormalities. C and D, DW images at the same level show abnormalities at same locations
Discussion
Outcome in comatose survivors of resuscitation is determined by the presence of heart failure or recurrent cardiac arrhythmias. Patients who survive this critical period may remain comatose and pose a difficult problem in planning future treatment. Our study of MR imaging shows its potential for use in prognostication in comatose survivors of cardiac resuscitation. The MR abnormalities in comatose patients who never awakened were diffuse and extensive, involving the cortex, thalamus, and cerebellum. The two patients who awakened had normal or localized findings on MR images. We believe that this study may have value because no other clinical or laboratory features were present that would have predicted poor outcome. Clinical neurologic examination revealed intact brain stem reflexes and absence of myoclonus status epilepticus. None of our patients who underwent electrophysiological tests showed abnormal median SSEP (9, 10) or burst-suppression patterns on EEG.
We do acknowledge the difficulty of performing MR imaging in these critically ill patients. In fact, our preliminary cohort suggests only one in three patients may be eligible for MR imaging. In our major teaching hospital, the proximity of the MR imager to the intensive care units allowed us to perform MR imaging with anesthesia support during the acquisition of data. None of the patients had any life-threatening cardiac arrhythmias or hypotension during transport or MR imaging, but we excluded patients in need of inotropic or antiarrhythmic agents. Thus, the applicability of our data to the entire population of comatose survivors of cardiac arrest remains very uncertain, and we should continue to expect major logistical difficulties with MR imaging.
The MR abnormalities can be widespread and represent the well-described pathologic patterns seen at autopsy. Selective vulnerability has been reported for anoxic-ischemic injury; the middle lamina of the cortex, caudate nucleus, putamen, globus pallidus, thalami, and Purkinje cells may be at particular risk. In this MR study, these predisposed locations were affected, although border zone infarcts were absent. The presence of cortical involvement in our preliminary series appeared to distinguish between poor outcome and possible recovery.
DW imaging measures the diffusion of free water. Ischemic brain damage results in restricted diffusion of free water, principally because of failure of the energy-requiring active water transport mechanism. The physiological changes of acute ischemia initially manifest as areas of high signal intensity on DW images. Diffusion abnormalities are present within minutes of an acute ischemic episode and, DW imaging will show infarcts earlier than conventional MR imaging, including FLAIR images (11, 12). Inclusion of DW imaging is important if imaging is to be performed within several hours of the ischemic insult. This was highlighted in a study by Arbelaez and coworkers (13), in which two of three patients imaged within 3 hours showed only diffusion abnormalities. DW imaging signal abnormalities may not be present in the subacute period (days 6–12) because of pseudonormalization of the ADC. This is not of any concern, because the FLAIR images remain abnormal, as seen in one patient in this study imaged after 1 week.
Conclusion
Our results confirm those of the early study by Arbelaez and associates (13), who reported similar abnormal changes in the basal ganglia, cerebellum, and cortex. However, comparison with their study is difficult because of inclusion of children and other causes for cardiac arrest (such as sepsis and cocaine overdose) and no correlation of MR imaging with other indicators of a poor prognosis. Further larger studies are needed, but our preliminary observation suggests that FLAIR and DW imaging can help detect extensive ischemic damage in comatose survivors. If our observations are confirmed, withdrawal of intensive care support can be justified, thus reducing the substantial costs of prolonged intensive care of severely crippled comatose survivors of resuscitation. The cost of a single MR study may offset these costs.
NOTE: Author Queries
1 Please indicate any financial support for or previous presentation of the analysis.
2 Please indicate what this symbol means—not done? normal? “ND” is not used in the table; OK to delete?
3 Title correct?
Footnotes
Address reprint requests to E. F. M. Wijdicks, MD, Mayo Clinic, W8B, 200 First Street SW, Rochester, MN 55905.
References
- 1.Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. JAMA 1985;253:1420-1426 [PubMed] [Google Scholar]
- 2.Thomassen A, Wernberg M. Prevalence and prognostic significance of coma after cardiac arrest outside intensive care and coronary units. Acta Anaesthesiol Scand 1979;23:143-148 [DOI] [PubMed] [Google Scholar]
- 3.Mullie A, Verstringe P, Buylaert W, et al. Predictive value of Glasgow coma score for awakening after out-of-hospital cardiac arrest. Cerebral Resuscitation Study Group of the Belgian Society for Intensive Care. Lancet 1988;1:137-140 [PubMed] [Google Scholar]
- 4.Sandroni C, Barelli A, Piazza O, Proietti R, Mastria D, Boninsegna R. What is the best test to predict outcome after prolonged cardiac arrest? Eur J Emerg Med 1995;2:33-37 [DOI] [PubMed] [Google Scholar]
- 5.Attia J, Cook DJ. Prognosis in anoxic and traumatic coma. Crit Care Clin 1998;14:497-511 [DOI] [PubMed] [Google Scholar]
- 6.Wijdicks EF, Parisi JE, Sharbrough FW. Prognostic value of myoclonus status in comatose survivors of cardiac arrest. Ann Neurol 1994;35:239-243 [DOI] [PubMed] [Google Scholar]
- 7.Krumholz A, Stern BJ, Weiss HD. Outcome from coma after cardiopulmonary resuscitation: relation to seizures and myoclonus. Neurology 1988;38:401-405 [DOI] [PubMed] [Google Scholar]
- 8.Young GB, Gilbert JJ, Zochodne DW. The significance of myoclonic status epilepticus in postanoxic coma. Neurology 1990;40:1843-1848 [DOI] [PubMed] [Google Scholar]
- 9.Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet 1998;352:1808-1812 [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Bolton CF, Young B. Prediction of outcome in patients with anoxic coma: a clinical and electrophysiologic study. Crit Care Med 1996;24:672-678 [DOI] [PubMed] [Google Scholar]
- 11.Mintorovich J, Moseley ME, Chileuitt L, Shimizu H, Cohen Y, Weinstein PR. Early detection of cerebral ischemia in cats: comparison of diffusion and T2-weighted MRI and spectroscopy. Magn Reson Med 1990;14:330-346 [DOI] [PubMed] [Google Scholar]
- 12.Mintorovich J, Moseley ME, Chileuitt L, Shimizu H, Cohen Y, Weinstein PR. Comparison of diffusion and T2-weighted MRI for the early detection of cerebral ischemia and reperfusion in rats. Magn Reson Med 1991;18:39-50 [DOI] [PubMed] [Google Scholar]
- 13.Arbelaez A, Castillo M, Muktierji SK. Diffusion-weighed MR imaging of global ischemic cerebral anoxia. AJNR Am J Neuroradiol 1999;20:999-1007 [PMC free article] [PubMed] [Google Scholar]


