Abstract
Summary: Capillary telangiectasias are being recognized with increasing frequency on MR imaging studies. Most are located in the brain stem and show slightly increased signal intensity on T2-weighted images, low signal intensity on T2*-weighted images (reflecting the presence of deoxyhemoglobin), and contrast enhancement. These findings are considered fairly typical for capillary telangiectasia, and pathologic correlation is not generally pursued. We present a case of a proven capillary telangiectasia in the basal ganglia. The imaging features of the lesion were identical to those described for capillary telangiectasias in the brain stem.
Capillary telangiectasias are vascular malformations that are occult on catheter angiograms but are being increasingly recognized by MR imaging (1, 2). They are generally asymptomatic and found in the pons. Typical MR imaging features include a variable T1 appearance, high signal intensity on T2-weighted images, contrast enhancement, and lack of mass effect (1, 2). Although they are known to occur outside of the pons, reports describing capillary telangiectasia in the cerebral hemispheres are rare (1). Because most of the lesions are asymptomatic and have fairly typical imaging findings, they are infrequently excised for biopsy. We present the imaging findings and histologic confirmation of an unusually large capillary telangiectasia involving the basal ganglia.
Case Report
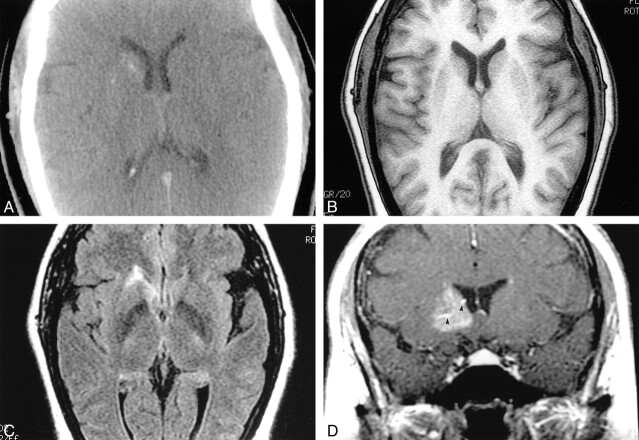
A 41-year-old white woman presented at a local hospital with a 20-year history of headaches that had recently become worse during her menstrual period. During the last 6 months, the characteristics of her headaches changed, becoming more intense. Other than the headache, there were no other complaints. The patient had a history of hypertension, which was appropriately controlled, and a family history of diabetes mellitus. There was no history of tobacco, alcohol, or drug use; recent travel, or exposure to toxins. The physical and neurologic examinations were normal. Noncontrast brain CT showed slight hyperattenuation in the right basal ganglia with punctate calcifications (Fig 1A). MR imaging showed that the lesion was not visible on T1-weighted images and was bright on fluid-attenuated inversion recovery (FLAIR) (Fig 1B and C) and T2-weighted images, but had no mass effect. Following administration of gadopentate dimeglumine, there was significant enhancement confined to the right basal ganglia (Fig 1D). Because a tumor could not be excluded, a stereotactic biopsy was performed and the specimen showed gray and white matter containing numerous dilated and blood-filled capillaries (Fig 2). Some of the vessels were sclerotic, though the majority were thin-walled. No gliosis, inflammation, neoplasia, or hemosiderin (suggesting prior hemorrhage) was found. Scattered small calcifications were present.
fig 1.
Capillary telangiectasia.
A, Noncontrast CT shows slight hyperattenuation in the right basal ganglia with puntacte calcifications.
B, Noncontrast MR T1-weighted image is normal.
C, Axial FLAIR image (slightly below B) shows high signal intensity in the head of the right caudate nucleus.
D, After gadopentate dimeglumine administration, a coronal T1- weighted image shows diffuse enhancement in the right basal ganglia anteriorly. There is a suggestion of large veins (arrowheads) in the malformation.
fig 2.
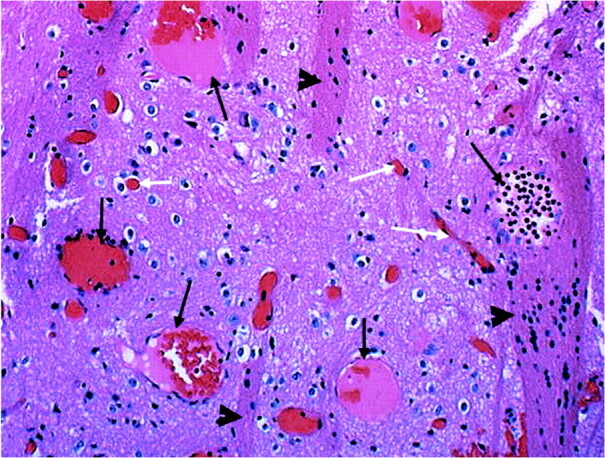
Microscopy. Medium power view (10×). Specimen shows gray matter containing several large, thin-walled vascular spaces (long black arrows). Note normal size capillaries (white arrows). The presence of “pencil fibers” (arrowheads) confirms localization to the basal ganglia. Pencil fibers are the white matter tracts of the basal ganglia. They are an integral part of the histologic composition of the striatum, versus the thalamus, where the neurons are scattered throughout a meshwork of white matter. There is no hemorrhage, hemosiderin-laden macrophages, calcifications, or gliosis
Discussion
Capillary telangiectasias account for 16%–20% of all intracerebral vascular malformations at autopsy and are being increasingly recognized by imaging (3). Most are incidental findings, as these malformations are nearly always asymptomatic. CT findings are normal in most cases but MR imaging shows capillary telangiectasia to be hypo- or isointense to brain on T1-weighted images (1–3). On T2-weighted images (particularly on the late echo), they are nearly always slightly hyperintense (1–3). Gradient-echo imaging shows them to be of low signal intensity (1, 2). Macroscopic hemorrhage and calcifications are rare in capillary telangiectasia, suggesting that the findings on T2*-weighted images are probably related to the presence of deoxyhemoglobin in the slow-flowing blood (4). Capillary telangiectasias are devoid of mass effect, and after gadopentate dimeglumine administration they enhance faintly in a “brushlike” or “stipple” pattern (1, 2). Curiously, about two thirds of capillary telangiectasias show an enlarged vessel believed to represent a draining vein (1, 2). This observation has led some authors to consider the concept of “transitional malformations” or “capillary malformations” (5). Transitional malformations are those containing more than one type of vascular malformation. For example, capillary telangiectasias have been associated with venous angiomas, cavernous angiomas, and even arteriovenous malformations (6). It has also been suggested that isolated capillary telangiectasias are the result of obstructed venous drainage and are acquired, rather than congenital (2). Indeed, only a few capillary telangiectasias have been reported in children (2). When symptoms occur, they are most likely due to the associated vascular malformations, although occasional capillary telangiectasias alone may be symptomatic. Hemorrhage seen in association with capillary telangiectasia almost always arises from an associated vascular malformation and only rarely from the capillary telangiectasia (7, 8). Capillary telangiectasias grow very slowly or not at all. In one series, no changes were noted in the size and configuration of capillary telangiectasias at 40-month follow-up (1).
Most capillary telangiectasias are in the pons, but they may also be found in the cerebral and cerebellar hemispheres and in the spinal cord. In one series of 18 presumed capillary telangiectasias, only two were found outside of the brain stem (temporal lobe and head of caudate nucleus) (2). In that study, three of 18 patients died of causes unrelated to capillary telangiectasia and histologic confirmation was available. The typical microscopic features of capillary telangiectasias are numerous thin-walled “capillary-type” ectatic blood vessels interspersed in a background of normal brain tissue. Capillary telangiectasias are typically devoid of calcification, gliosis, extraluminal hemorrhage, and hemosiderin-laden macrophages. The lesions vary in size from a few millimeters to about 2 cm.
In our patient, although the lesion was large, it was probably not the cause of her symptoms and was located in the basal ganglia. CT showed hyperattenuation and calcification. Noncontrast T1-weighted images were normal but the T2-weighted and FLAIR images showed high signal intensity. The lesion enhanced and showed some enlarged vessels in the malformation. These findings suggest that this lesion is perhaps a transitional malformation and not a pure capillary telangiectasia. The fact that only capillary telangiectasia was demonstrated histologically probably reflects limited sampling typical of needle biopsies. The differential diagnosis of lesions involving the basal ganglia unilaterally includes physiological calcifications, neurofibromatosis type 1, and hemiballismus hemichorea (high signal intensity on T1-weighted images and variable findings on T2-weighted images). All of these lesions are generally bright on T1-weighted images, and thus differ from the lesion in our patient. Other possible lesions, such as infarct, tumors, and Sydenham's chorea (high T2 signal intensity in the head of a caudate nucleus), are bright on T2-weighted images and may enhance (similar to capillary telangiectasia) but would result in symptoms not seen in our patient. Metabolic disorders affecting the basal ganglia generally result in bilateral (although at times asymmetrical) lesions and usually a clear history is elicited from the patients. In our patient, a stereotactic biopsy showed findings of capillary telangiectasia.
In summary, we presented a case of an unusually large, biopsy-confirmed capillary telangiectasia involving the basal ganglia. The lesion was excised at biopsy, as a tumor could not be completely excluded. The imaging features of the lesion in our patient were identical for those described for brain stem capillary telangiectasia. On the basis of our experience with this case, we suggest that lesions outside of the brain stem but conforming to findings described for capillary telangiectasia should be followed, and in absence of change, the diagnosis of capillary telangiectasia may be suggested.
Footnotes
Address reprint requests to M. Castillo, CB # 7510, UNC Radiology, Chapel Hill, NC 27599-7510.
References
- 1.Lee RR, Becher MW, Benson ML, Rigamonti D. Brain capillary telangiectasia: MR imaging appearance and clinicohistopathologic findings. Radiology 1997;205:797-805 [DOI] [PubMed] [Google Scholar]
- 2.Barr RM, Dillon WP, Wilson CB. Slow-flow vascular malformations of the pons: capillary telangiectasias? AJNR Am J Neuradiol 1996;17:71-78 [PMC free article] [PubMed] [Google Scholar]
- 3.Huddle DC, Chaloupka JC, Sebgal V. Clinically aggressive diffuse capillary telangiectasia of the brain stem: a clinical radiologic-pathologic case study. AJNR Am J Neuroradiol 1999;20:1674-1677 [PMC free article] [PubMed] [Google Scholar]
- 4.Auffray-Calvier E, Desal HA, Freund P, Laplaud D, Mathon G, de Kersaint-Gilly A. Capillary telangiectasias: angiographically occult vascular malformations—MRI symptomalogy apropos of 7 cases. J Neuroradiol 1999;26:257-261 [PubMed] [Google Scholar]
- 5.Rigamonti D, Johnson PC, Spetzler RF, Hadley MN, Drayer BP. Cavernous malformation and capillary telangiectasia: a spectrum within a single pathological entity. Neurosurgery 1991;28:60-64 [PubMed] [Google Scholar]
- 6.Chang SD, Steinberg GK, Rosario M, Crowley RS, Hevner RF. Mixed arteriovenous malformation and capillary telangiectasia: a rare subset of mixed vascular malformations—case report. J Neurosurg 1997;86:699-703 [DOI] [PubMed] [Google Scholar]
- 7.McCormick PW, Spetzler RF, Johnson PC, Drayer BP. Cerebellar hemorrhage associated with capillary telangiectasia and venous angioma: a case report. Surg Neurol 1993;39:451-457 [DOI] [PubMed] [Google Scholar]
- 8.Bland LI, Lapham LW, Ketonen L, Okawara SH. Acute cerebellar hemorrhage secondary to capillary telangiectasia in an infant: a case report. Arch Neurol 1994;51:1151-1154 [DOI] [PubMed] [Google Scholar]