Abstract
Summary: Hyperperfusion syndrome is a well-documented complication of carotid endarterectomy, as well as internal carotid artery angioplasty and stent placement. We report a similar complication after distal intracranial (middle cerebral artery [MCA] M2 segment) angioplasty. To our knowledge, this is the first report of hyperperfusion syndrome after intracranial angioplasty of a distal MCA branch.
Hyperperfusion syndrome is a well-described complication of carotid endarterectomy (1–6) and extracranial internal carotid artery (ICA) angioplasty (7–10). To our knowledge, this phenomenon has not been reported after endovascular revascularization of intracranial vessels. The purpose of this report is to describe the occurrence of hyperperfusion syndrome with symptomatic intracranial hemorrhage after balloon angioplasty for middle cerebral artery (MCA) stenosis.
Case Report
The patient was an 81-year-old Caucasian woman who initially presented with transient ischemic attacks (TIAs) despite therapeutic levels of anticoagulation with warfarin. She had been given anticoagulants for atrial fibrillation. The patient's medical history was also significant for congestive heart failure with an ejection fraction of 25% (normal = 75%), resulting in pulmonary hypertension. The TIAs manifested as periods of dysphasia, which became greater in frequency and duration. After switching from warfarin to heparin anticoagulation, angiography was performed 5 days after admission to our institution.
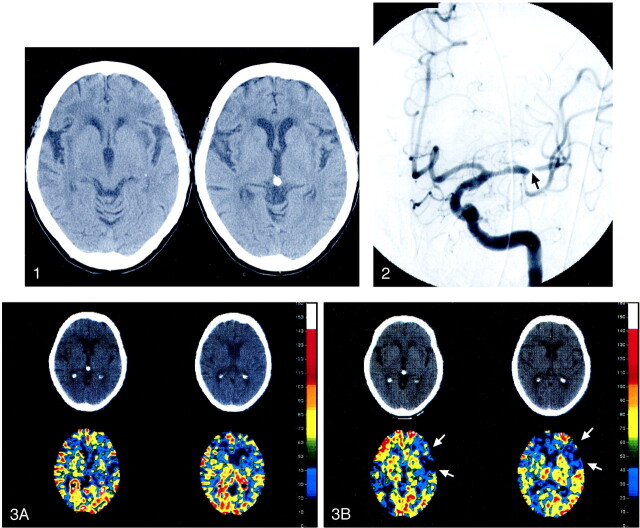
At admission, a CT scan revealed scattered areas of subinsular and deep white matter hypoattenuation, worse in the left cerebral hemisphere than on the right, consistent with either small-vessel ischemic disease or perhaps some degree of striatocapsular ischemic injury in the left MCA territory (Fig 1). There was no evidence of acute intracranial hemorrhage, mass effect with edema, or an acute stroke. MR imaging was not performed because she had a cardiac pacemaker. The diagnostic cerebral angiogram showed a high-grade stenosis (95% diameter narrowing) of the proximal M2 segment of the left MCA (Fig 2) with no collateral filling of the left MCA territory. A xenon CT (Xe-CT) scan obtained before and after an acetazolamide challenge (Fig 3) showed lack of cerebral blood flow augmentation after acetazolamide, most notably in the left frontal lobe, consistent with loss of autoregulation of the vessels in this vascular territory. This autoregulation study was done to better understand the physiologic flow of the left cerebral hemisphere and as an attempt to differentiate embolic from flow-related ischemia in this patient.
fig 1.
Noncontrast head CT. Two contiguous slices at the level of the basal ganglia 6 days prior to left M2 segment balloon angioplasty demonstrate left frontal and subinsular small vessel disease.fig 2. Left ICA angiogram (anterior projection) at the time of initial evaluation reveals a severe, approximately 95%, focal stenosis of the proximal M2 segment (arrow) of the left MCA.fig 3. Xe-CT with axial CT and blood flow map images on two contiguous slices before (A) and after (B) acetazolamide challenge 1 day prior to balloon angioplasty. Loss of cerebrovascular autoregulation is most notable in the left frontal lobe, as demonstrated by paradoxically low cerebral blood flow after acetazolamide administration. Note arrows between areas on the postacetazolamide scans. The color coding scale is defined in units of cc/100 g tissue/min
Owing to her impaired cardiac function, the patient was thought to be a poor candidate for surgical revascularization with an external carotid–internal carotid bypass graft. In light of the patient's worsening TIAs on maximal medical therapy, together with the poor natural history of untreated symptomatic intracranial stenoses, balloon angioplasty was recommended.
The patient was premedicated with aspirin 325 mg and a loading dose of clopidogrel (300 mg) given orally on the night before the procedure. A heparin drip (400 U/h) was continued overnight. At the time of the procedure, partial thromboplastin time (PTT) measured 79.4 s (normal = 25–40 s), and international normalized ratio (INR) measured 1.2. An activated clotting time (ACT) at the beginning of the procedure measured 172 s. There were no fixed neurologic deficits prior to endovascular revascularization.
The patient was placed under general anesthesia in the neuroangiography suite. Neurophysiological monitoring was used to detect clinically significant cerebral ischemia (11) and consisted of EEG and somatosensory evoked potentials after median nerve stimulation. After a 6F vascular sheath was placed in the right common femoral artery, a bolus of heparin (3000 U) was administered intravenously. A repeat ACT was 260 s. Owing to the tortuosity of the left ICA, a 5F Simmons I catheter (Cook; Bloomington, IN) was initially used to selectively catheterize this vessel and was subsequently exchanged over an exchange-length J-wire for a 6F Envoy MPC guide catheter (Cordis; Miami, FL). The tip of the guide catheter was placed in the distal cervical portion of the left ICA, at approximately the level of the C2 vertebral body.
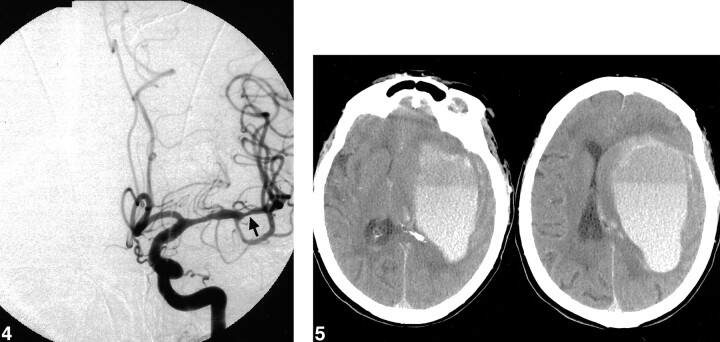
By use of a roadmapping technique and fluoroscopic guidance, a 2.0-mm (maximum diameter) × 10-mm Ranger over-the-wire balloon dilation catheter (Scimed; Maple Grove, MN) was advanced coaxially through the guide catheter over a Choice PT (Scimed; Maple Grove, MN) 0.014-in microwire. After traversing the stenotic left MCA M2 lesion with the microwire, the distal 5–6 mm of the balloon was advanced over the microwire to cross the lesion completely. The balloon was then slowly inflated over a span of 10 s to a maximum pressure of 4 atm, corresponding to a putative balloon diameter of 1.94 mm. At fluoroscopy, the waist of the stenotic lesion was reduced, and the balloon was intentionally underinflated relative to our estimate of the normal diameter of this proximal M2 segment (2.5 mm). Estimation of the diameter of arteries at the base of the brain was based on Wollschlager et al (12, 13). This postmortem angiographic study found the mean diameter of the M1 segment to be between 2.51 and 2.55 mm. The balloon remained inflated for 1 minute. Final postoperative left ICA angiography performed approximately 20 minutes after balloon dilation showed a reduction in the focal stenosis from approximately 95% to 55% diameter narrowing (Fig 4). The angiogram also demonstrated good filling of the left MCA territory with no evidence of vessel cutoff, vessel dissection, vessel perforation, or intraluminal thrombus. No neurophysiological changes were identified during or after the angioplasty to suggest cerebral ischemia. The final neurophysiological examination was performed after the postoperative angiogram. The patient was kept normotensive (mean arterial pressure 80–90 mm Hg) throughout the procedure, except at the time of the balloon inflation, when the mean aterial pressure (MAP) was raised to slightly above 90 mm Hg, with the intention of maintaining adequate left MCA territory perfusion.
fig 4.
Left ICA angiogram (anterior projection) following left M2 segment balloon angioplasty (arrow). The focal stenosis was reduced to approximately 55%, with no evidence of vessel dissection, perforation, or thrombus formation.fig 5. Head CT 2 hours following angioplasty, at the time of acute-onset right hemiparesis and aphasia. A massive left frontal intraparenchymal hemorrhage is seen with subfalcine herniation
The patient was extubated uneventfully and was transferred to the intensive care unit. After the procedure, the patient was neurologically normal, again suggesting there was no cerebral ischemia at the conclusion of the angioplasty. She continued to receive a heparin drip with a PTT of 82.9 s, appropriate for our target range of 2.5–3.0 times normal. Blood pressure was maintained at normotensive levels, with an MAP ranging from 80–90 mm Hg.
Two hours after the procedure, the patient suddenly developed right hemiparesis and aphasia. A head CT scan was obtained immediately and revealed a very large left hemispheric parenchymal hematoma with left-to-right midline shift centered at the level of the corona radiata (Fig 5). The heparin drip was discontinued, and anticoagulation was reversed with protamine sulfate.
Owing to the patient's clinical state and lack of a realistic chance for a reasonable recovery, surgical evacuation of the hematoma was not feasible. After complete neurologic evaluation, the patient was declared brain-dead 1 day later.
Discussion
Percutaneous transluminal intracranial angioplasty has been reported to have good angiographic and short-term clinical outcomes (14), as well as mid-term clinical outcomes that appear superior to the natural history of stroke in patients with symptomatic intracranial atherosclerotic disease (15). The major acute complications these authors describe include vessel elastic recoil, dissection, perforation, and occlusion (due to dissection or platelet aggregation) (14, 15). Delayed (subacute or periprocedural) vessel occlusion is another major complication (13) requiring vigilance in detection and appropriate use of anticoagulant and antiplatelet agents. Our experience in this case, however, suggests that potential hemorrhagic complications also should not be overlooked.
Hyperperfusion was first theorized by Spetzler et al (16) as a possible cause of intracerebral hemorrhage following surgical resection of arteriovenous malformations due to loss of autoregulation in the surrounding normal brain parenchyma. This phenomenon was further elucidated in 1981 by Sundt et al (1), who described a triad of complications, including atypical migrainous phenomena, transient focal seizure activity, and intracerebral hemorrhage after carotid endarterectomy. Numerous reports have subsequently documented the risk of hyperperfusion syndrome when using other cerebral revascularization techniques, including extracranial carotid angioplasty and stent placement (7–10). Estimates of the incidence of hyperperfusion syndrome after carotid endarterectomies range from 0.4% to 2.7% (17, 18).
In our case, the Xe-CT scan before and after acetazolamide administration provided evidence of the loss of cerebrovascular autoregulation, presumably due to the high-grade M2 stenosis causing maximal dilatation of the vessels distal to the narrowing. Reduction of the focal stenosis from 95% to 55% dramatically increased perfusion to this area of impaired autoregulation with disastrous consequences.
Several factors support the conclusion that this parenchymal hemorrhage was not the result of a thromboembolic infarction. Despite the history of worsening TIAs, no fixed persistent neurologic deficits were noted prior to the procedure. No neurophysiological changes were identified at the time of the procedure to suggest cerebral ischemia, and the patient was neurologically intact for 2 hours after the procedure. CT scanning performed immediately after the onset of symptoms showed a large hematoma without evidence of a bland infarct. The final postoperative angiogram revealed no evidence of intraluminal thrombus formation or vessel dissection. Anticoagulation was maintained with heparin before, during, and after the procedure. Therefore, although possible, it is unlikely that there was reperfusion hemorrhage into an area of cerebral infarction.
Other causes of intracranial parenchymal hemorrhage after carotid endarterectomy, such as hypertension (19) or anticoagulant use (20), also seem unlikely in our case. The balloon angioplasty procedure was technically uncomplicated, and the postoperative angiogram revealed no evidence of vessel perforation. The patient was neurologically intact upon extubation and return to the intensive care unit. The patient was maintained at normotensive blood pressures, and although the role of hypertension cannot be excluded, the Xe-CT findings implicate hyperperfusion as the cause of the hemorrhage. The intra- and postoperative anticoagulant regimens were per our routine. The PTT was kept at 2.5–3.0 times the normal value, as our primary concern was to prevent periprocedural vessel reocclusion. It is noteworthy that the patient received only heparin for anticoagulation. She had not yet received her expected dose of clopidogrel (75 mg orally) after the procedure, and we did not use other antiplatelet agents (glycoprotein IIb-IIIa inhibitors) at the time of angioplasty. It should also be noted, however, that because aspirin and clopidogrel were given the night before the procedure, platelet function was also, to some degree, not normal.
Finally, although the postoperative arteriogram did not demonstrate a vessel rupture or dissection, the possibility of an evolving perforation cannot be completely ruled out, as the family declined our request for a postmortem examination. However, given the lack of subarachnoid hemorrhage, the preprocedural Xe-CT data, and the other postoperative conditions described above, we conclude that hyperperfusion syndrome is the most likely cause of this massive intraparenchymal hemorrhage.
This case presents a great dilemma in the treatment of intracranial atherosclerosis. The loss of cerebrovascular reserve and the patient's clinical symptoms of medically refractory TIAs strongly suggested the need for cerebral revascularization. Furthermore, the patient's preprocedural head CT demonstrated only small vessel disease without large territorial infarctions, so it did not appear that an area of subacute infarction would be reperfused. In this poor surgical candidate with the poor natural history of an untreated M2 stenosis, the morphology of the M2 stenosis suggested that balloon angioplasty was technically feasible. The dilemma is how to revascularize a territory that already suffers from loss of autoregulation, and how to counterbalance the opposing complications of vessel occlusion versus intracerebral hemorrhage in the periprocedural period.
The optimal treatment for patients with symptomatic intracranial atherosclerosis is unknown, and no randomized trial has, at present, demonstrated the superiority of any specific antithrombotic agent in preventing recurrent cerebral ischemic events. In our institution, we currently reserve intracranial angioplasty for patients who have TIA or a minor stroke despite antithrombotic therapy.
Thijs and Albers (21) recently reported their study to determine the prognosis of patients with symptomatic intracranial atherosclerosis who fail antithrombotic therapy, defined as those patients who had ischemic events while taking either warfarin therapy (INR ≥ 2.0) or an antiplatelet agent that has been shown to be effective for secondary prevention of stroke. They concluded that such patients have extremely high rates of recurrent TIA, stroke, or death (55.8%), and that recurrent ischemic events typically occur within a few months after failure of standard medical therapy. The high recurrence risk observed in their study warrants testing of alternative treatment strategies, such as intracranial angioplasty.
If feasible, preprocedural evaluation with diffusion-weighted MR imaging might be useful to identify areas of subtle infarction, potentially at risk for reperfusion injury. Perfusion studies such as Xe-CT, single-photon emission CT, or perfusion MR imaging might be useful to determine the degree of revascularization necessary to improve but not overwhelm a vascular territory. If sophisticated imaging techniques are unavailable, evaluating the extent of periventricular white matter disease may be useful to identify patients with impaired autoregulation (22, 23).
During the procedure, perhaps less aggressive, staged angioplasty with only small, incremental improvements in a focal stenosis may reduce risk of hyperperfusion by allowing a restoration of autoregulation. Deliberately underdilating in a setting such as this may be of benefit to reduce the risk of hyperperfusion injury. This provides another argument to the one already posed by previous authors that underdilatation is a benefit because it reduces the risk of rupture (14, 15).
In the periprocedural period, very tight control of blood pressure is required after the procedure, although our case was complicated by the patient's severe congestive heart failure. The optimal anticoagulant-antiplatelet regimen is unclear. While adequate anticoagulation is necessary to prevent subacute vessel occlusion after angioplasty and/or stent placement, this must be balanced against the risk of intracerebral hemorrhage. At our institution, we currently use a combination of heparin and clopidogrel in the first 24 hours after the procedure. The addition of an antiplatelet agent, such as currently available glycoprotein IIb-IIIa inhibitors, would very likely reduce the risk of vessel reocclusion but could also prevent rapid reversal of anticoagulation in the event of an intracerebral hemorrhage.
In summary, this case demonstrates that the hyperperfusion syndrome can occur after intracranial angioplasty. Further investigation is warranted to identify patients at risk for this complication and to determine the optimal periprocedural management to reduce this risk.
Footnotes
Address reprint requests to Huy M. Do, MD, Assistant Professor of Radiology, Stanford University Medical Center, 300 Pasteur Drive, Stanford, CA 94305-5105.
References
- 1.Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 1981;56:533-543 [PubMed] [Google Scholar]
- 2.Reigel MM, Hollier LH, Sundt TM Jr, Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg 1987;5:628-634 [PubMed] [Google Scholar]
- 3.Schroeder T, Sillesen H, Boesen J, Laursen H, Sorensen P. Intracerebral haemorrhage after carotid endarterectomy. Eur J Vasc Surg 1987;1:51-60 [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen LG, Schroeder TV. Defective cerebrovascular autoregulation after carotid endarterectomy. Eur J Vasc Surg 1993;7:370-379 [DOI] [PubMed] [Google Scholar]
- 5.Jansen C, Sprengers AM, Moll FL, et al. Prediction of intracerebral haemorrhage after carotid endarterectomy by clinical criteria and intraoperative transcranial Doppler monitoring. Eur J Vasc Surg 1994;8:303-308 [DOI] [PubMed] [Google Scholar]
- 6.Mansoor GA, White WB, Grunnet M, Ruby ST. Intracerebral hemorrhage after carotid endarterectomy associated with ipsilateral fibrinoid necrosis: a consequence of the hyperperfusion syndrome? J Vasc Surg 1996;23:147-151 [DOI] [PubMed] [Google Scholar]
- 7.Andrews BT, Levy ML, Dillon W, Weinstein PR. Unilateral normal perfusion pressure breakthrough after carotid endarterectomy: case report. Neurosurgery 1987;21:568-571 [DOI] [PubMed] [Google Scholar]
- 8.Schoser BG, Heesen C, Eckert B, Thie A. Cerebral hyperperfusion injury after percutaneous transluminal angioplasty of extracranial arteries. J Neurol 1997;244:101-104 [DOI] [PubMed] [Google Scholar]
- 9.McCabe DJ, Brown MM, Clifton A. Fatal cerebral reperfusion hemorrhage after carotid stenting. Stroke 1999;30:2483-2486 [DOI] [PubMed] [Google Scholar]
- 10.Chamorro A, Vila N, Obach V, Macho J, Blasco J. A case of cerebral hemorrhage early after carotid stenting [letter]. Stroke 2000;31:792-793 [DOI] [PubMed] [Google Scholar]
- 11.Lopez JR. Intraoperative neurophysiological monitoring. Int Anesthesiol Clin 1996;34:33-54 [DOI] [PubMed] [Google Scholar]
- 12.Wollschlager PB, et al. The arteries of the basal ganglia. In: VIII Symposium Neuroradiologicum. Paris; 1967
- 13.Wollschlaeger G, Wollschlaeger PB. The circle of willis. In: Newton TH, Potts DG, eds. Radiology of the Skull and Brain. St. Louis: C.V. Mosby; 1974:1171–1201
- 14.Connors JJ 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg 1999;91:415-423 [DOI] [PubMed] [Google Scholar]
- 15.Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke 1999;30:1065-1069 [DOI] [PubMed] [Google Scholar]
- 16.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg 1978;25:651-672 [DOI] [PubMed] [Google Scholar]
- 17.Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg 1986;64:29-34 [DOI] [PubMed] [Google Scholar]
- 18.Breen JC, Caplan LR, DeWitt LD, Belkin M, Mackey WC, O'Donnell TP. Brain edema after carotid surgery [see comments]. Neurology 1996;46:175-181 [DOI] [PubMed] [Google Scholar]
- 19.Caplan LR, Skillman J, Ojemann R, Fields WS. Intracerebral hemorrhage following carotid endarterectomy: a hypertensive complication? Stroke 1978;9:457-460 [DOI] [PubMed] [Google Scholar]
- 20.Piepgras DG, Morgan MK, Sundt TM Jr, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 1988;68:532-536 [DOI] [PubMed] [Google Scholar]
- 21.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology 2000;55:490-497 [DOI] [PubMed] [Google Scholar]
- 22.Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension 1994;23:565-568 [DOI] [PubMed] [Google Scholar]
- 23.Isaka Y, Okamoto M, Ashida K, Imaizumi M. Decreased cerebrovascular dilatory capacity in subjects with asymptomatic periventricular hyperintensities. Stroke 1994;25:375-381 [DOI] [PubMed] [Google Scholar]