Abstract
BACKGROUND AND PURPOSE: Stable xenon–enhanced CT (sXe/CT) has gained wide acceptance in the assessment of regional cerebral blood flow (rCBF) in patients with intracranial abnormalities. The aim of this study was to test whether the contrast medium (ie, sXe) itself directly induces relevant changes in rCBF, thereby distorting any valid determination of cerebral perfusion by using sXe/CT.
METHODS: To characterize the degree and temporal dynamics of sXe-induced flow activation, a thermal diffusion (TD)-based microprobe was placed subcortically into the frontal lobe on either hemisphere to assess rCBF (TD-rCBF) continuously in 23 patients (mean age, 55 ± 18 years) with severe intracranial insult who were undergoing sXe/CT.
RESULTS: In 35, the sXe/CT studies TD-rCBF rose from 25 ± 17 mL/100 g per minute (range, 5–42 mL/100 g per minute) before sXe administration to 28 ± 21 mL/100 g per minute (range, 6–46 mL/100 g per minute) after arterial sXe saturation was reached. Analysis of the flow activation curve showed a logarithmic shape with an increase in TD-rCBF between 3% and 7% within the first 76 seconds of sXe wash-in (12% after 190 seconds) and showed no further augmentation until the end of the blood flow study.
CONCLUSION: The observed sXe-induced rCBF activation, which showed significant inter- and intraindividual variability, might lead to overestimation of rCBF in patients with severe intracranial insult. The obtained flow activation curve provides essential information that may allow subsequent refinement of the methodology, aiming to further minimize the influence of sXe-induced rCBF activation on rCBF calculations when using sXe/CT technology.
Since its introduction into clinical practice in the early 1980s, stable xenon–enhanced CT (sXe/CT) has been well validated for the determination of regional cerebral blood flow (rCBF) in absolute values for various indications (1). The apparent advantages of the method include quantitative assessment of rCBF with anatomic referencing, short investigation time, and high accuracy, even in low-flow states. However, there has been some concern related to the biological properties of the xenon tracer. Stable xenon (sXe) is a chemically inert, freely diffusible, and highly lipophilic gas with an atomic weight of 131. Its biological and chemical behavior is equal to that of other xenon isotopes, such as xenon-133 and xenon-127. In accordance, sXe should be ideal for rCBF measurements; its narcotic potency, however, suggests that it cannot be physiologically inert (2–4). Therefore, sXe might induce an uncoupling of brain function and metabolism (5), resulting in an increase in rCBF. Various studies using either transcranial Doppler sonography (TCD) (6, 7) or an intravenous xenon-133 clearance technique (8–10) in healthy subjects have shown an increase ranging from 5% to 40% in rCBF due to sXe inhalation. On the basis of these results, the mathematical model underlying sXe-based rCBF determination was remodeled to enable reliable calculation of rCBF values (11). All studies, however, were performed in healthy volunteers or awake patients with occlusive cerebrovascular disease. Whether the results of these studies can be transferred to patients with severe intracranial insult, who often undergo sXe/CT studies in the clinical setting, is unknown. Intubated and artificially ventilated patients with cerebral insult often exhibit severely disturbed metabolic and vascular cerebral regulatory mechanisms, presumably affecting the degree and time course of sXe-induced flow activation. Therefore, knowledge of the degree and real-time course of sXe-induced flow activation is crucial for the reliable assessment of rCBF using sXe/CT if we are to guarantee trustworthy, quantitative rCBF values for clinical decision-making in patients with severe intracranial insult.
Recently, a minimally invasive thermal diffusion (TD)-based flowmetry probe was introduced into clinical practice; it allows online assessment of absolute blood flow in parenchymal organs, such as liver (12), or in brain (13, 14). This microprobe, which has recently been tested under both experimental and clinical conditions (13), permits us to elucidate the effects of sXe on rCBF under clinical conditions in patients with severe cerebral insults who undergo xenon-enhanced CT. The aim of the present study was to investigate the degree of sXe-induced flow activation in patients with severe cerebral insult and to characterize the real-time course in flow activation by means of simultaneously performed TD flowmetry (15).
Methods
Patient Characteristics
The study protocol was approved by the local ethics committee. Twenty-three patients (11 men and 12 women; mean age, 55 ± 18 years) who had sustained a severe traumatic brain injury (n = 4) or subarachnoid hemorrhage (SAH, n = 19) and had a Glasgow Coma Scale score of less than 8 (16) were included in the study. After admission, all patients underwent a ventriculostomy or had an intraparenchymal sensor placed to monitor intracranial pressure (ICP) and an arterial line placed to monitor blood pressure. Patients were intubated and artificially ventilated. Analgesia and sedation were achieved with fentanyl citrate (4–8 g/kg body weight per hour) and midazolam (0.2–0.3 mg/kg body weight per hour). Skeletal muscle relaxation was obtained with pancuronium bromide (0.1 mg/kg body weight per hour).
TD Flowmetry
At the time of placement of the ICP monitoring system, a one-way bolt was inserted through a coronar burr hole over either hemisphere. The TD microprobe (Thermal Technologies Inc., Cambridge, MA) was inserted through the bolt and placed subcortically at a depth of 2 cm in a standardized fashion. The sample volume of the probe approximates 27 mm3 and allows continuous recording of rCBF in absolute flow values (TD-rCBF, mL/100 g per minute) with a sampling rate of one value per second (1 Hz). Since the probe was placed subcortically, the obtained TD-rCBF values represent white as well as gray matter flow, assuming a higher white matter content within the compartment measured. The accuracy of the probe placement was confirmed radiographically as well as by determining the initial conductivity and temperature values of the probe.
Stable Xenon–Enhanced CT
A 4.5-minute wash-in protocol was used to determine rCBF using sXe/CT technology (DDP Inc., Houston, TX). Blood flow studies were conducted with a GE CT Pace (General Electric Medical Systems, Milwaukee, WI) using parameters of 120 kVp, 100 mA, 3-second rotation time, and 10-mm slice thickness, resulting in a cumulative radiation dose of approximately 240 mSv per slice and study. Patients received a mixture of medical-grade 30% sXe, 60% oxygen, and 10% air, and a three-level investigation was performed. The end-tidal carbon dioxide (CO2) level was adjusted to approximately 35 mm Hg throughout the study. The rCBF study consisted of a baseline period with a duration of 42 seconds, followed by a 270-second sXe wash-in period, in which the gas mixture was delivered to the patient. During the wash-in period, the corresponding xenon build-up curve was obtained, showing a steady state of tidal and end-tidal sXe concentration after about 90 seconds, indicating saturation of the arterial blood with sXe. At the end of the study, data analysis was performed with a standardized data review and correction protocol to gain rCBF values in distinct regions of interest.
Study Protocol
After adequate positioning in the CT scanner, the patient was connected to the sXe inhalation unit. As soon as mean arterial blood pressure (MABP), end-tidal CO2, and ICP were constant, an initial determination of conductivity and temperature values of the TD probe were performed to ensure correct positioning and functioning. A conventional CT study was executed. The three sections for measurement of rCBF with sXe/CT were determined, and one section was centered on the position of the tip of the TD microprobe (Fig 1).
fig 1.
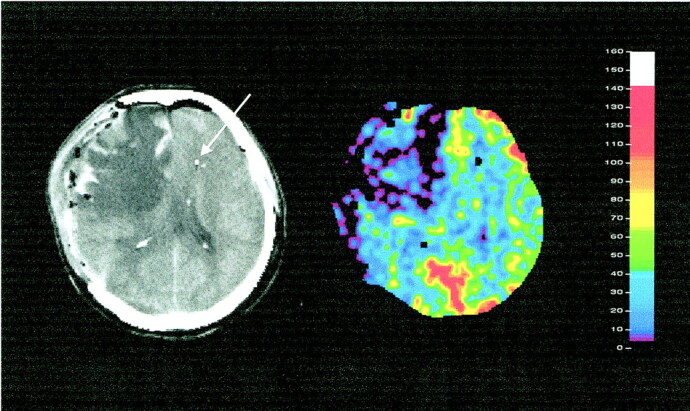
sXe-enhanced CT blood flow study in a patient with severe head injury. Left, anatomic reference section (arrow indicates tip of TD-rCBF probe); right, corresponding rCBF (mL/100 g per minute) image shows severe right hemispheric hypoperfusion
The TD-rCBF measurement was then initiated again and the sXe/CT blood flow study started after the TD-rCBF readings were stable. Values were recorded during the complete blood flow study and stored for off-line analysis. ICP, MABP, and end-tidal CO2 were recorded during the baseline period and at the end of the wash-in period.
Data Analysis and Statistics
The obtained TD-rCBF values were processed in two different ways. In the first approach, the values recorded during baseline and during the steady-state condition of sXe inhalation were averaged. Steady state was assumed when tidal and end-tidal sXe concentrations were equal during the sXe wash-in. The second approach aimed to assess the temporal changes in TD-rCBF due to sXe inhalation. The wash-in period was divided into seven periods of 38 seconds each. For that purpose, the mean relative changes in relation to baseline were computed (and termed wash-ins, 1–7). This time frame was chosen because it provides periods of almost the same duration as baseline (42 seconds). Under these conditions, the number of data points making up the average values for each period are quite comparable. Statistical comparisons for all parameters were executed by using paired t tests after normal distribution was achieved with Fisher's z transformation.
Results
A total of 35 simultaneous sXe-CT and TD-rCBF measurements were performed in 23 patients. The number of investigations performed ranged from one to four per patient.
Systemic Parameters and ICP
The MABP was 112 ± 19 mm Hg before and 113 ± 20 mm Hg at the end of the sXe/CT study. Furthermore, ICP increased slightly, from 15 ± 5 mm Hg to 18 ± 5 mm Hg (not significant) during the sXe wash-in period. Accordingly, the calculated cerebral perfusion pressure ([CPP]; CPP = MABP − ICP) remained almost unchanged, amounting to 95 ± 20 mm Hg before versus 95 ± 19 mm Hg at the end of the wash-in period. End-tidal CO2 remained nearly constant throughout the performed investigations (35 ± 3 mm Hg versus 36 ± 4 mm Hg; not significant).
Changes in rCBF during sXe Inhalation
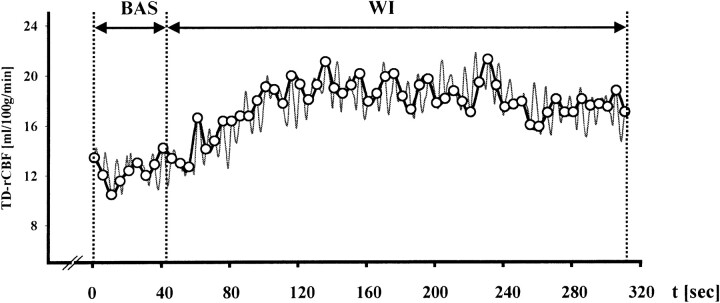
In 15 patients studied, administration of sXe resulted in an increase in TD-rCBF, as exemplified in Figure 2, which depicts an original registration that was obtained in a patient with severe traumatic brain injury.
fig 2.
Graph shows an original TD-rCBF recording obtained during a 30% sXe/CT blood flow study in a patient with head injury. Baseline (BAS): preinhalation TD-rCBF recording (duration, 42 seconds); wash-in (WI): TD-rCBF during sXe inhalation (duration, 270 seconds)
The mean TD-rCBF for baseline condition was 25 ± 17 mL/100 g per minute (n = 35; range, 5–42 mL/100 g per minute). The inhalation of 30% xenon during the 4.5-minute wash-in protocol resulted in an increase of TD-rCBF to 28 ± 21 mL/100 g per minute (n = 35; P = .012 versus baseline; range, 6–46 mL/100 g per minute) under steady-state conditions. This increase corresponds to a 12% rCBF activation due to sXe inhalation.
The TD-rCBF increased in 20 investigations and remained almost unchanged in 11 studies (alterations in TD-rCBF < 1 mL/100 g per minute). In four cases, TD-rCBF decreased as a result of sXe inhalation. In these studies, the decrease in TD-rCBF was accompanied by a concomitant increase in ICP, to 23 ± 4 mm Hg versus 18 ± 5 mm Hg before sXe inhalation. In these patients, the decrease occurred in calculated CPP from 91 ± 10 to 85 ± 11 mm Hg (not significant). Therefore, a uniform pattern of rCBF changes during sXe inhalation could not be detected. When correlating baseline with the mean difference between baseline and steady state (ΔTD-rCBF), applying linear regression, no significant correlation (n = 35, r = .507, y = 0.2x − 1.68) was obtained.
A total of 16 repeated measurements was recorded in six patients. In all except one patient, in whom TD-rCBF increased in all four investigations, TD-rCBF showed variable reactivity to sXe application. Each of these patients had no significant increase in TD-rCBF during sXe inhalation at least once. These observations did not correlate with the clinical condition or with the intracranial insult of the patients.
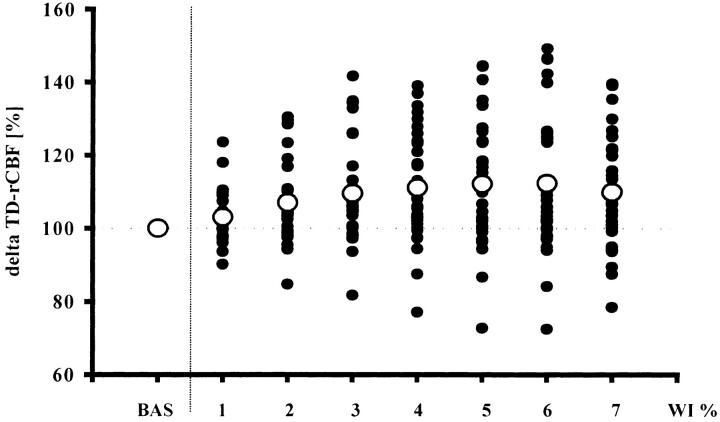
The analysis of the 38-second epochs (wash-ins 1–7) during the sXe wash-in showed a dynamic flow activation curve due to xenon inhalation (Fig 3). Within the first 38 seconds of xenon wash-in, the increase in TD-rCBF approached 3%, reached 7% after 76 seconds, and rose further to 11% after 152 seconds. Peak values were reached during wash-in 5 (190 seconds) and remained almost unchanged thereafter until the end of the wash-in procedure (Fig 3).
fig 3.
Relative changes (in %) in baseline values in TD-rCBF due to sXe inhalation as seen in 35 blood flow studies. Solid circles represent individual changes in TD-rCBF at various time periods; open circles depict mean changes in TD-rCBF as compared with baseline. BAS indicates baseline; WI % represents seven periods, each of 38 seconds' duration, of sXe inhalation
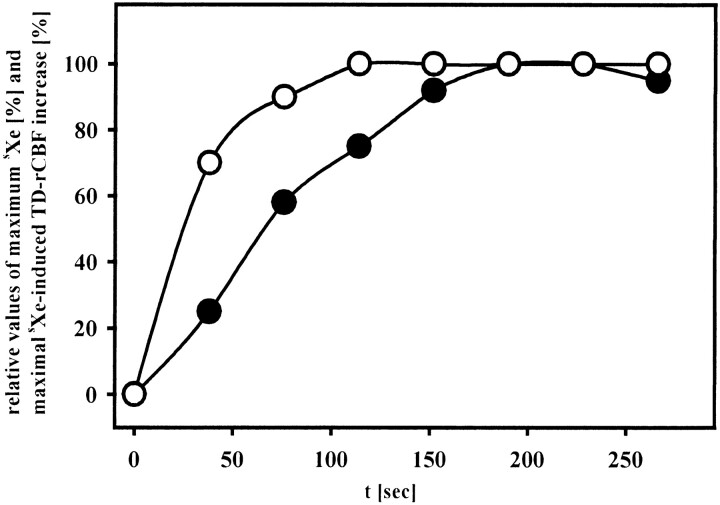
The time course of the sXe-induced rCBF activation was associated with the arterial saturation of sXe during the wash-in procedure in a delayed fashion. While 50% of the maximum end-tidal sXe was reached after approximately 23 seconds' inhalation time, the corresponding 50% of the maximum of the sXe-induced flow activation was approached after nearly 76 seconds. This difference was even more pronounced for the maximum values. The sXe build-up was accomplished about 90 seconds after the beginning of sXe wash-in. However, the maximum sXe-induced flow activation was observed after 190 seconds, leading to a time difference of nearly 100 seconds between maximum sXe-induced flow activation and maximal arterial sXe concentration (Fig 4).
fig 4.
Time course of arterial sXe concentration (open circles) and sXe-induced flow activation (solid circles). The x-axis displays relative values of end-tidal sXe concentration and TD-rCBF, respectively, with each parameter expressed in percentage of maximum response observed
Discussion
The main findings of the present study are that inhalation of sXe results in rCBF activation in patients with severe intracranial insult who have been sedated with analgesics and artificially ventilated; that the time course of flow activation is nonlinear and delayed in relation to end-tidal sXe concentration; and that patients undergoing sXe/CT blood flow studies show a considerable intra- and interindividual variation in sXe-induced flow activation.
Xenon-Induced Activation of rCBF
In previous animal studies of rCBF alteration due to sXe inhalation, most of the investigations described activation in rCBF with varying magnitude and time course. Gur and coworkers (17) demonstrated a 17% alteration in rCBF in baboons breathing 34% sXe by using microspheres for quantitative rCBF measurement. Similarly, flow activation of 22% and 81%, respectively, were found during 35% and 40% sXe inhalation, applying intravenous xenon-133 clearance and autoradiography (18, 19). In general, flow activation appeared to be most pronounced within the second minute after starting sXe inhalation (17, 19). However, discrepant results were obtained in monkeys by Love et al (20), who did not find a significant alteration of brain perfusion during the inhalation of 34% sXe using TCD.
In healthy volunteers, inhalation of various sXe concentrations, ranging from 26% to 33%, resulted in a 13% to 29% increase of CBF as measured by a xenon-133 injection technique (7–10). Use of TCD technology to monitor changes in brain perfusion revealed a 20% to 40% increase in blood flow velocity (BFV) in healthy subjects (6, 21, 22). A comparable degree of flow activation was found in patients with cerebrovascular insult in whom Witt et al (23) and Holl et al (24) found an 18% increase in BFV during sXe inhalation. The corresponding increase in BFV in healthy volunteers ranged from 20% to 40% (6, 21, 22).
The present study describes the effect of sXe inhalation on rCBF measured in absolute values in patients with severe cerebral insult. Using a TD intraparenchymal microprobe with high temporal resolution, we were able to characterize the real-time course of sXe-induced flow activation. These measurements revealed a mean increase of TD-rCBF amounting to 12% collated to baseline during 30% sXe wash-in. Although this increase of blood flow was measured regionally, the changes may nevertheless be fully comparable. Compared with studies using the xenon-133 injection technique (7–10) in healthy volunteers, our results suggest a lower degree of sXe-induced flow activation. The TD-based microprobe was placed subcortically, and thus one may assume mainly blood flow in the white matter being measured. This localization may be explained, at least in part, by the low level of rCBF found in most patients, and may therefore be considered largely comparable to the low-flow compartment measured by xenon-133 clearance techniques. However, changes in blood flow measured by the TD-based microprobe should be well comparable to other changes found in the high-flow compartment using xenon-133 clearance techniques, since there is good evidence in favor of similar reactivity of rCBF measured in gray and white matter both experimentally (25, 26) and clinically (27, 28). In addition, we have recently shown good agreement between TD-rCBF values and sXe/CT measurements obtained from the same brain region (13).
In all patients in the present study, end-tidal CO2, MABP, and CPP did not change significantly during the blood flow studies, excluding hypercapnia or changes in CPP causing the observed changes in TD-rCBF. However, alterations in cerebral vasoreactivity, including autoregulation, CO2 reactivity, and chemical regulation of rCBF, are well-known phenomena in patients with severe traumatic brain injury and SAH (29–33). Furthermore, our patients were sedated with analgesics, which may also affect cerebral vasoreactivity. These factors may therefore explain the wide range of individual rCBF changes observed during sXe inhalation (−29% to +44% relative to baseline), whereas, in healthy volunteers, xenon-induced rCBF activation ranged from −4% to +44% of the preinhalation value (10). Alterations in cerebrovascular reactivity appeared most obvious in the four patients who exhibited a decrease in TD-rCBF during 30% sXe wash-in. This decrease in brain perfusion along with a marked increase in ICP may be explained by initial vasodilatation and accompanying increase of cerebral blood volume. With the cerebral compliance exhausted, this increase of cerebral blood volume may result in an impairment of cerebral microcirculation. However, continuous multimodal neuromonitoring during sXe inhalation would be necessary for clarification.
Although a decrease of cerebral compliance may be manageable in the intensive care setting (ie, under stable conditions), it may exacerbate when the patient is transferred to the CT scanner. Considering that a decrease in TD-rCBF up to 29% occurred in four of 35 sXe/CT investigations, the possibility of a significant decrease in rCBF for at least 3 minutes during sXe application should be kept in mind when studying patients with severe cerebral insult.
However, the potential overestimation of rCBF due to sXe-induced rCBF activation in critically ill patients undergoing sXe/CT blood flow studies might become clinically important, too. This is most evident in diagnostic blood flow studies aiming to detect a critical decrease in rCBF, in which advanced patient care relies on the recognition of threshold values for rCBF (eg, the identification of potentially salvageable brain tissue in acute stroke and the detection of cerebrovascular vasospasm after SAH). Moreover, sXe-induced flow activation possibly limits the capability of the methodology to study physiological activation processes, such as cognitive and motor activation tasks. For these, the degree of task-related rCBF activation is known to be lower than the observed augmentation in rCBF caused by the contrast medium sXe itself (34, 35).
The exact mechanism by which sXe leads to an increase in rCBF remains unclear. Volatile anesthetics, such as halothane and isoflurane, have been shown to cause direct vasodilatation in vitro (36–38) and in vivo (39, 40). The proposed mechanisms include nitric oxide–related effects, alteration in calcium homeostasis, and release of excitatory amino acids and free radicals (41, 42). Whether any of these explanations also applies to sXe-induced flow activation remains to be established.
Temporal Course of Xenon-Induced Flow Activation
So far, techniques available for the measurement of rCBF in patients allow discontinuous recording only. However, application of the TD-based microprobe, as used in the present study, enables quantitative measurement of rCBF in absolute values and its changes during sXe inhalation with high temporal resolution. Continuous recording of the cerebrovascular effects induced by sXe inhalation have thus far been limited to studies using TCD technology. At best, however, this approach yields changes in BFV only as indirect conditions produced by changes in rCBF.
In healthy volunteers breathing 33% sXe for 4.5 minutes, Obrist et al (8) obtained a linear increase in BFV within the first 180 seconds, which then approached a plateau of 35% over baseline at the end of the inhalation period. A flat and almost linear increase in BFV up to 18% relative to baseline values was obtained in patients with cerebrovascular disease (24). In this study, a steady increase in BFV was perceived throughout the complete sXe inhalation time, whereas other investigations demonstrated only an insignificant increase in BFV within the first 90 seconds of 30% sXe inhalation, followed by a gradual rise in BFV that peaked at about 300 seconds of inhalation and reached approximately 135% of the preinhalation BFV values (6, 23).
The results obtained in the present study revealed a gradual increase in TD-rCBF during sXe wash-in, starting within the first 38 seconds and reaching a plateau about 190 seconds after the start of sXe inhalation, which was stable until the end of the wash-in period. Thus, flow activation, reflected by the maximal increase in TD-rCBF, was attained about 100 seconds after the sXe arterial equilibrium was established.
Although changes in both BFV, measured by TCD, and rCBF, measured by TD-based microprobe, allow dynamic changes to be assessed with high temporal resolution, a direct comparison of the results may not be valid. To investigate the relationship between BFV and rCBF both in healthy subjects and patients, parallel real-time assessment of both parameters are necessary.
In the present study, we were not able to establish continuous TCD monitoring during the sXe/CT blood flow studies, because the head frame and TCD probe we used caused too much noise for a reliable determination of sXe/CT rCBF in distinct regions of interest, which would have led to loss of essential information for rCBF-based clinical management procedures. Therefore, no definite conclusion can be drawn as to whether the observed time course of sXe-induced flow activation is unique for patients with cerebral insult or represents the normal human response to sXe inhalation. This uncertainty also refers to the great interindividual differences with respect to degree and temporal dynamics of xenon-induced flow activation we observed. However, no relationship between intracranial morphology, actual clinical condition (ie, ICP and CPP at baseline), and degree of TD-rCBF changes was obtained when reviewing patient data.
The quantitative and time-related differences in TD-rCBF obtained in patients and TCD-measured BFV observed in healthy volunteers may be of functional importance, since TCD-based data have widely been used to refine the algorithm underlying analysis of sXe/CT data. However, the results obtained with the TD-based microprobe may provide the basis for further systematic investigations aiming to characterize the time course and degree of sXe-induced flow activation in humans.
Conclusion
The present study directly depicts the activation of rCBF due to sXe inhalation in patients with intracranial insult who are undergoing sXe/CT studies of cerebral perfusion. Xenon-induced flow activation, as observed here, might cause an overestimation of actual cerebral perfusion, limiting the application of the methodology to certain indications, such as head trauma, SAH, and other cerebrovascular abnormalities. Inhalation of 30% sXe, as performed in the present study, leads to a nonlinear increase in TD-rCBF, exhibiting a logarithmic rCBF activation curve in patients. Since the knowledge of the degree and real-time course of sXe-induced flow activation is crucial for the reliable assessment of rCBF using sXe/CT technology, further systematic investigations using TD flowmetry will provide evidence for subsequent refinement of the sXe/CT methodology.
Acknowledgments
We thank the staff and nurses of the departments of neuroradiology and intensive care, who made this study possible.
Footnotes
Supported in part by the German Research Foundation (DFG VA 151/5-1) and the German Foundation for Neurosurgical Research.
Presented in part at the Fifth International Conference on Xenon/CT CBF, Tokyo, Japan, September 1999.
Address reprint requests to Peter Horn, MD, Department of Neurosurgery, University Hospital Mannheim, Theodor-Kutzer-Ufer 1–3, 68167 Mannheim, Germany.
References
- 1.Yonas H. The xenon/computed tomography cerebral blood flow method: clouded past, clear present, bright future. In: Tomonaga M, Tanaka A, Yonas H, eds. Quantitative Cerebral Blood Flow Measurements Using Stable Xenon/CT: Clinical Applications. Armonk, New York: Futura; 1995:1–11
- 2.Boomsma F, Rupreht J, Man in 't Veld AJ, de Jong FH, Dzolijc M, Lachmann B. Hemodynamic and neurohumoral effects of xenon anesthesia. Anaesthesia 1990;45:273-278 [DOI] [PubMed] [Google Scholar]
- 3.Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings with additional observations on krypton. Science 1951;113:580-582 [DOI] [PubMed] [Google Scholar]
- 4.Lachmann B, Armbruster S, Schairer W. Anästhesie im geschlossenen System am Beispiel der Xenonnarkose. In: Jantzen JP, Kleemann PP, eds. Narkosebeatmung: Low flow, minimal flow Geschlossenes System. Stuttgart: Schattauer; 1989:125–130
- 5.Christensen MS, Hoedt-Rasmussen K, Lassen A. Cerebral vasodilatation by halothane anesthesia in man and its potentiation by hypotension and hypercapnia. Br J Anaesth 1967;39:927-934 [DOI] [PubMed] [Google Scholar]
- 6.Giller CA, Purdy P, Lindstrom WW. Effects of inhaled xenon on cerebral blood flow velocity. AJNR Am J Neuroradiol 1990;11:177-182 [PMC free article] [PubMed] [Google Scholar]
- 7.Obrist WD, Jardour IT, Marks EC, Wechsler LR, Yonas H. Correlation of transcranial Doppler and cerebral blood flow during xenon inhalation (abstr). Stroke 1992;23:464 [Google Scholar]
- 8.Obrist WD, Jaggi JL, Harel D, Smith DS. Effect of stable xenon inhalation on human CBF. J Cereb Blood Flow Metab 1985;5: (Suppl 1) S557-S558 [Google Scholar]
- 9.Dettmers C, Hartmann A, Tsuda Y. Stable xenon effects on regional cerebral blood flow and electroencephalography in normal baboons and volunteers. In: Wüllenweber R, Klinger M, Brock M, eds. Advances in Neurosurgery 15. Berlin: Springer; 1987:67–71
- 10.Hartmann A, Dettmers C, Schuier FJ, Wassmann HD, Schuhmacher HW. Effect of stable xenon on regional cerebral blood flow and the electroencephalogram in normal volunteers. Stroke 1991;2:182-189 [DOI] [PubMed] [Google Scholar]
- 11.Obrist WD, Zhang Z, Yonas H. Effect of xenon-induced activation on xenon-enhanced computed tomography cerebral blood flow calculations. J Cereb Blood Flow Metab 1998;18:1192-1195 [DOI] [PubMed] [Google Scholar]
- 12.Klar E, Kraus T, Bleyl J, et al. Thermodiffusion for continuous quantification of hepatic microcirculation: validation and potential in liver transplantation. Microvasc Res 1999;58:156-166 [DOI] [PubMed] [Google Scholar]
- 13.Vajkoczy P, Roth H, Horn P, et al. Continuous monitoring of regional cerebral blood flow, experimental and clinical validation: a novel thermal diffusion microprobe and its validation by sXe-CT. J Neurosurg 2000;93:265-275 [DOI] [PubMed] [Google Scholar]
- 14.Thomé C, Vajkoczy P, Horn P, et al. Validation and clinical application of a novel intraparenchymal microprobe for the continuous assessment of regional cerebral blood flow (abstr). J Cereb Blood Flow Metab 1999;19: (Suppl 1) S623 [Google Scholar]
- 15.Horn P, Vajkoczy P, Thomé C, Quintel M, Schilling L, Schmiedek P. Effects of 30% stable xenon on regional cerebral blood flow in patients with intracranial pathology: proceedings of the Fifth International Conference on Xenon/CT CBF in Tokyo, Japan 1999. Keio J Med 2000;49: (Suppl 1) A161-A163 [PubMed] [Google Scholar]
- 16.Teasdale G, Jennet B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;7872:81-84 [DOI] [PubMed] [Google Scholar]
- 17.Gur D, Yonas H, Jackson D, et al. Measurements of cerebral blood flow during xenon inhalation as measured by the microsphere method. Stroke 1985;16:871-874 [DOI] [PubMed] [Google Scholar]
- 18.Hartmann A, Wassmann H, Czernicki Z, Dettmers C, Schuhmacher HW, Tsuda Y. Effects of stable xenon in room air on regional cerebral blood and electroencephalogram in normal baboons. Stroke 1987;18:643-648 [DOI] [PubMed] [Google Scholar]
- 19.Junck L, Dhawan V, Thaler H, Rottenberg DA. Effects of xenon and krypton on regional cerebral blood flow in rats. J Cereb Blood Flow Metab 1985;5:126-132 [DOI] [PubMed] [Google Scholar]
- 20.Love JT, Nemoto EM, Yonas H. Stable xenon does not change internal carotid blood flow in awake monkeys. In: Yonas H, Cerebral Blood Flow Measurement with Stable Xenon-Enhanced Computed Tomography. New York: Raven; 1992:282–285
- 21.Ueltzen J, Harders A, Kohmura E. Die Steigerung der Hirndurchblutung durch Inhalation einer 33% igen Xenon-Sauerstoff-Mischung. In: Becker H, Gaab MR, eds. Hirndurchblutung und die cerebrovaskuläre Reservekapazität. Munich: Urban & Schwarzenberg; 1992:35–39
- 22.Broich K, Bulau P, Hartmann A. The effect of stable xenon inhalation on cerebral blood flow velocities and topographic electroencephalography in normal volunteers. In: Yonas H, ed. Cerebral Blood Flow Measurement with Stable Xenon-Enhanced Computed Tomography. New York: Raven; 1992:292–295
- 23.Witt JP, Holl K, Heissler HE, Dietz H. Stable xenon CT CBF: effects of blood flow alteration on CBF calculations during inhalation of 33% stable xenon. AJNR Am J Neuroradiol 1991;12:973-975 [PMC free article] [PubMed] [Google Scholar]
- 24.Holl K, Nakano S, Yamashita T. The effects of stable xenon gas. In: Tomonaga M, Tanaka A, Yonas H, eds. Quantitative Cerebral Blood Flow Measurements Using Stable Xenon/CT: Clinical Applications. Armonk, New York: Futura; 1995:41–53
- 25.Beck T, Kriegelstein J. Cerebral circulation, metabolism, and blood brain barrier of rats in hypocapnic hypoxia. Am J Physiol 1987;252:504-512 [DOI] [PubMed] [Google Scholar]
- 26.Linder J. Effects of cervical sympathetic stimulation on cerebral and ocular blood flows during hemorrhagic hypotension and moderate hypoxia. Acta Physiol Scand 1982;114:379-386 [DOI] [PubMed] [Google Scholar]
- 27.Ashwal S, Stringer W, Tomasi L, Schneider S, Thompson J, Perkin R. Cerebral blood flow and carbon dioxide reactivity in children with bacterial meningitis. J Pediatr 1990;117:523-530 [DOI] [PubMed] [Google Scholar]
- 28.Hamberg LM, Hunter GJ, Halpern EF, Hoop B, Gazelle GS, Wolf GL. Quantitative high-resolution measurement of cerebrovascular physiology with slip-ring CT. AJNR Am J Neuroradiol 1996;639–650 [PMC free article] [PubMed]
- 29.Meixensberger J. Xenon-133 CBF measurements in severe head injury and subarachnoid haemorrhage. Acta Neurochir Suppl (Wien) 1993;59:28-33 [DOI] [PubMed] [Google Scholar]
- 30.Zauner A, Doppenberg EM, Woodward JJ, Choi SC, Young HF, Bullock R. Continuous monitoring of cerebral substrate delivery and clearance: initial experience in 24 patients with severe acute brain injuries. Neurosurgery 1997;41:1082-1091 [DOI] [PubMed] [Google Scholar]
- 31.Bergsneider M, Hovda DA, Shalmon E, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg 1997;86:241-251 [DOI] [PubMed] [Google Scholar]
- 32.Cruz J. Relationship between early pattern of cerebral extraction of oxygen and outcome from severe acute traumatic brain injury. Crit Care Med 1996;24:953-956 [DOI] [PubMed] [Google Scholar]
- 33.Marion D. Lactate and traumatic brain injury. Crit Care Med 1999;27:2063-2064 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura N, Yamamoto T, Saito T, Fujita H. Analysis of activation in anterior cingulate cortex during cognitive process of selection following somatosensory stimuli: fMRI study with elaborate task paradigms. Magn Reson Imaging 2000;18:397-404 [DOI] [PubMed] [Google Scholar]
- 35.Stippich C, Hofmann R, Kapfer D, et al. Somatotopic mapping of the human primary somatosensory cortex by fully automated tactile stimulation using functional magnetic resonance imaging. Neurosci Lett 1999;277:25-28 [DOI] [PubMed] [Google Scholar]
- 36.Cucciara RF, Theye RA, Michenfelder JD. The effects of isoflurane on canine cerebral metabolism and blood flow. Anesthesiology 1974;40:571-574 [DOI] [PubMed] [Google Scholar]
- 37.Gelman S, Fowler KC, Smith LR. Regional blood flow during isoflurane and halothane anesthesia. Anesth Analg 1984;63:557-565 [PubMed] [Google Scholar]
- 38.Todd MM, Drummond JC. A comparison of the cerebrovascular and metabolic effects of halothane and isoflurane in the cat. Anesthesiology 1984;60:276-282 [DOI] [PubMed] [Google Scholar]
- 39.Flynn NM, Buljubasic N, Bosnjak ZJ, Kampine JP. Isoflurane produces endothelium-independent relaxation in canine middle cerebral arteries. Anesthesiology 1992;76:461-467 [DOI] [PubMed] [Google Scholar]
- 40.Jensen NF, Todd MM, Kramer DJ, Leonard PA, Warner DS. A comparison of the vasodilating effects of halothane and isoflurane the isolated rabbit basilar artery with and without intact endothelium. Anesthesiology 1992;76:624-634 [DOI] [PubMed] [Google Scholar]
- 41.Moore LE, Kirsch JR, Helfaer MA, Tobin JR, McPherson RW, Traystman RJ. Nitric oxide and prostanoids contribute to isoflurane induced hyperemia in pigs. Anesthesiology 1994;80:1328-1337 [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, Okabe E. Selective impairment of endothelium-dependent relaxation by sevoflurane: oxygen free radicals participation. Anesthesiology 1992;76:440-447 [DOI] [PubMed] [Google Scholar]