Abstract
BACKGROUND AND PURPOSE: We sought to investigate whether the combination of conventional, diffusion-weighted, and perfusion-weighted MR imaging increases the diagnostic accuracy of balloon test occlusion of the internal carotid artery. We describe perfusion anomalies and patterns of enhancement seen in areas of altered brain perfusion during MR-monitored temporary balloon occlusion of the internal carotid artery.
METHODS: Nine patients underwent balloon occlusion testing under standard angiographic conditions with continuous clinical and EEG monitoring. One patient who failed the test by clinical criteria underwent an external carotid to internal carotid bypass operation, followed by a repeat balloon test occlusion, thereby bringing the total number of procedures to 10. Patients were further imaged at 1.5 T with perfusion- and diffusion-weighted imaging as well as with conventional noncontrast and contrast-enhanced turbo fluid-attenuated inversion recovery (FLAIR) and T1-weighted sequences.
RESULTS: Seven of 10 patients who tolerated unilateral carotid test occlusion without adverse clinical neurologic or EEG changes exhibited delayed first-pass transit of contrast material through the affected cerebral hemisphere, indicative of altered perfusion without significant concurrent cerebral blood flow or blood volume changes. Four of these patients and both symptomatic patients showed pial or subarachnoid contrast staining in areas of altered perfusion without abnormalities on diffusion-weighted images.
CONCLUSION: Our findings indicate that MR perfusion-weighted imaging is safe and easily accomplished in a high-field-strength magnet and that contrast-enhanced turboFLAIR imaging may provide clinically useful MR imaging evidence of abnormal cerebral blood flow and subclinical ischemia.
Temporary balloon test occlusion of the internal carotid artery (ICA) is performed as a preliminary evaluation in patients who are scheduled to undergo endovascular, neurosurgical, or otolaryngologic procedures in which permanent occlusion of the ICA either is indicated preoperatively or presents a significant risk intraoperatively. The purpose of this procedure is to stratify patients according to their risk for stroke during or after permanent occlusion of this vessel. Essentially 100% of patients who fail balloon test occlusion by clinical criteria incur permanent neurologic deficits if the ICA is occluded without a revascularization procedure (1, 2). The majority of patients tolerate the procedure, by clinical criteria, but a small subset of these patients, less than 5%, incur delayed neurologic deficits after permanent vascular occlusion either because of inadequate cerebral blood flow (CBF) or embolic phenomena occurring in the ipsilateral hemisphere (3–7). Approximately 10% of patients pass the temporary balloon test occlusion but have decreased hemispheric blood flow as measured by stable xenon CT. More than 50% of patients have a stroke after permanent ICA occlusion, which has been attributed to low-flow states; this phenomenon is of concern, particularly in patients undergoing long surgical procedures or experiencing perioperative hypotension, hypovolemia, or anemia, in contradistinction to patients undergoing endovascular occlusion with close postprocedural hemodynamic monitoring (2, 8, 9).
In an attempt to identify patients with decreased cerebral perfusion who are at risk for stroke, adjunctive imaging and monitoring techniques have been proposed. These include measurement of cerebral perfusion by stable xenon-enhanced CT (9, 10), single-photon emission CT (11–13), transcranial Doppler sonography (14, 15), and hypotensive challenge (16). In the present study, first-pass perfusion-weighted MR imaging was used in conjunction with diffusion-weighted and contrast-enhanced turbo fluid-attenuated inversion recovery (FLAIR) imaging in an attempt to identify patients who would tolerate temporary balloon test occlusion of a carotid artery without delayed adverse neurologic effects.
Methods
Patient Selection
Two groups of patients were referred to our service for balloon test occlusion procedures: patients undergoing surgical sacrifice of the ICA during resection of a neck, skull base, or intracranial tumor and patients scheduled to undergo endovascular occlusion of the ICA for management of complex carotid artery aneurysms. The human subjects committee approved the protocols used in this study.
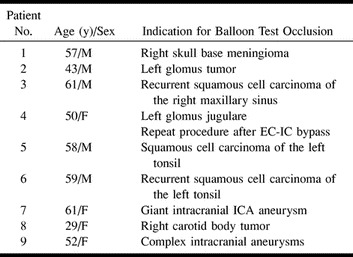
Nine patients (five men and four women) had a total of 10 balloon test occlusion procedures; patient 4 had a repeat procedure after undergoing external carotid to internal carotid (EC-IC) bypass. Patient demographics and clinical indications for balloon test occlusion are summarized in Table 1.
TABLE 1:
Patient demographics and indications for balloon test occlusion
ICA Test Occlusion
Unilateral ICA balloon test occlusion was carried out in the interventional MR therapy suite under MR guidance outside the 5-gauss line. Bilateral common femoral arterial punctures were performed with the Seldinger technique. A common carotid arteriogram was obtained to evaluate the patency of the ICA and collateral flow from the ipsilateral external carotid artery. In accordance with published protocols, 5000 U heparin was given intravenously to prevent thrombus formation, and a 7F balloon catheter (Medtronic, Minneapolis, MN) was placed into the proximal cervical ICA. Arterial pressures (systolic, diastolic, and mean) were taken as baseline controls. The test occlusion balloon was then inflated with contrast material under fluoroscopic monitoring. Arterial occlusion was assessed with a repeat angiogram, and then a second set of arterial pressures was recorded. The patients underwent continuous neurologic monitoring, including assessment of memory, sensory, and motor functions, and continuous 12-lead EEG monitoring. The contralateral ICA and the posterior circulation were also studied to assess collateral flow during occlusion. The procedure was judged a clinical success if the patient's neurologic status and EEG tracings remained stable and if occluded arterial back pressure persisted at a mean value of greater than 40 mm Hg for 30 minutes. After clinical assessment, the EEG leads were removed from the scalp and the patient was moved into the MR imager gantry for the imaging part of the protocol. This required an additional 20 minutes of internal carotid balloon occlusion, during which periodic neurologic testing was performed.
Patients who could not tolerate temporary carotid balloon occlusion did not participate further in this study. Patients who successfully tolerated carotid balloon test occlusion for 30 minutes, as defined above, were considered to be acceptable surgical candidates for permanent carotid occlusion at subsequent surgery.
Imaging Protocol
Before and after internal carotid balloon test occlusion, all patients underwent diffusion-weighted imaging of the brain (4100/117 [TR/TE], matrix = 128 × 256, b = 1000 s/mm2). A bolus injection of 0.1 mmol/kg gadopentetate dimeglumine was then administered, followed by perfusion-weighted MR imaging of the brain (317/30) on eight levels with 60 acquisitions per level. Computer processing of the acquired images enabled calculation of a cerebral perfusion map. Axial turboFLAIR images (6000/125, TI = 2000, 19 slices, each 5-mm thick, with a gap of 2.5 mm) and axial T1-weighted images (445/12, 19 slices, each 5-mm thick, with a gap of 2.5 mm) were obtained in some patients by using the contrast agent administered earlier for the hemodynamic-weighted studies.
In summary, each patient underwent the following neuroimaging procedures: pre–balloon test occlusion MR imaging, consisting of noncontrast diffusion-weighted, contrast-enhanced perfusion-weighted, and contrast-enhanced T1-weighted and turboFLAIR sequences; and carotid balloon test occlusion in the therapy suite under MR guidance with intra–test occlusion MR imaging, consisting of noncontrast diffusion-weighted, contrast-enhanced perfusion-weighted, and contrast-enhanced T1-weighted and turboFLAIR sequences (Figs 1 and 2).
fig 1.
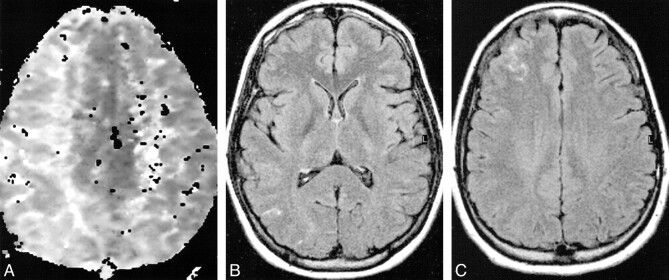
Patient 4: 50-year-old woman undergoing left internal carotid balloon test occlusion in whom neurologic symptoms developed 27 minutes into the procedure.
A, Perfusion map shows delayed perfusion in the left hemisphere and no response in the right frontal borderzone.
B, Axial turboFLAIR image (9000/110, TI = 2500) is normal.
C, Contrast-enhanced axial turboFLAIR image shows curvilinear regions of hyperintensity in the subarachnoid spaces or pial surface on the left frontal region.
D, Contrast-enhanced T1-weighted fat-saturation image (710/17) shows areas of short T1 relaxation in the corresponding regions, but more subtle than on the turboFLAIR image.
Perfusion MR Imaging
Cerebral blood volume (CBV) was measured from the MR signal intensity changes based on the differences between tissue T2 rates with and without contrast agent, the brain signal before and after contrast injection, the TE, and a proportionality constant. Coronal brain images were acquired at the level of the optic chiasm, which enabled imaging of the carotid arteries as they enter the brain, as well as most of the vascular territory of the middle cerebral artery. Rapid imaging of the transit of contrast agent through the carotid arteries yielded an arterial input function, which in turn permitted the calculation of mean transit time (MTT) of the contrast agent. The regional CBF was then calculated as CBF = CBV/MTT. The CBV and MTT were used in this study for qualitative analysis of brain perfusion.
Data Analysis
From the perspective of experimental design and data analysis, the subject population had two salient features: first, each patient served as his or her own control in the comparison of cerebral perfusion before, during, and after the carotid balloon occlusion test; and second, since the occlusion was a planned event, the experimental parameters and results were referable to each individual subject, and the intra- and intersubject variability were, thus, inherently quantifiable. Finally, the contrast-enhanced perfusion MR assessments were used to define the quantitative threshold, or minimal cerebral perfusion values, for individual patients. This information, obtained preoperatively, was then evaluated in terms of its utility in relation to subsequent surgical and postoperative management using standard statistical methodologies.
Results
Nine patients underwent a total of 10 unilateral carotid artery balloon test occlusion procedures. Seven patients tolerated the procedure without clinical or EEG changes. Patient 3 developed immediate neurologic deficits, which resolved after 12 hours without clinical or imaging evidence of stroke. Patient 4 had a temporary neurologic change at 27 minutes, which resolved immediately after balloon deflation. Both symptomatic patients underwent EC-IC bypass before final ICA occlusion. Repeat balloon test occlusion in patient 4 after EC-IC bypass was performed but the enhanced turboFLAIR images were of poor diagnostic quality. All remaining eight patients underwent contrast-enhanced turboFLAIR imaging, but only six had diagnostic postocclusion contrast-enhanced T1-weighted imaging. The clinical and imaging findings are summarized in Table 2. None of the symptomatic or asymptomatic patients showed abnormalities on diffusion-weighted images.
TABLE 2:
Clinical and neuroimaging findings
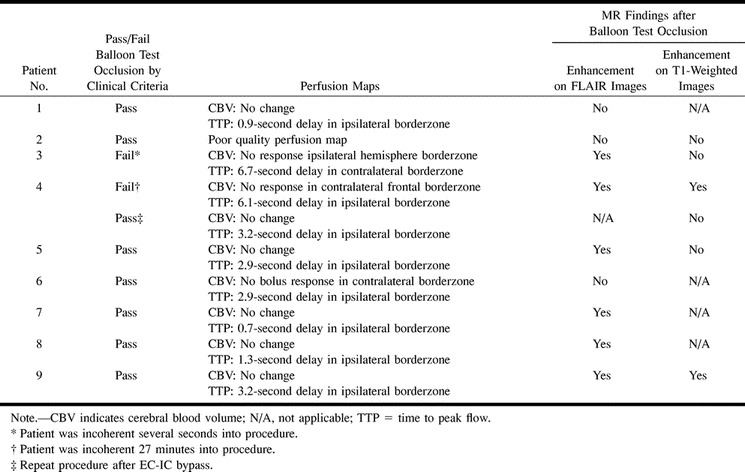
Perfusion maps were abnormal in all cases (Figs 1A and 2A). All patients studied had delayed perfusion to the ipsilateral or contralateral hemisphere, although CBF and CBV were not uniformly affected. One patient had a nondiagnostic perfusion map. Of the remaining nine patients, six showed no change in CBV. The other three patients showed no response to the contrast bolus: in two patients the abnormality was localized to the contralateral hemisphere borderzone white matter, and in the third patient, to the ipsilateral hemisphere borderzone. Both patients who failed the test clinically had severe perfusion abnormalities whereas only one patient who passed clinically showed a lack of perfusion. All patients with diagnostic perfusion maps had perfusion delays. The seven patients who passed clinically had delays ranging from 0.7 seconds to 3.2 seconds, as compared with the nonoccluded hemisphere. Patient 3, who failed clinically, had a 6.7-second perfusion delay in the contralateral hemisphere, and patient 4, who also failed, had a 6.1-second delay in the ipsilateral hemisphere. In both these patients the CBV and MTT abnormalities occurred in opposite hemispheres.
Contrast-enhanced images obtained during balloon test occlusion showed areas of hyperintensity in the subarachnoid spaces or pial surfaces corresponding in location to areas of perfusion abnormality. These were seen in six of nine diagnostic studies with turboFLAIR sequences and in two of six T1-weighted studies. Both patients with T1-positive findings also had positive findings on turboFLAIR images, while two patients had hyperintensities on only the turboFLAIR images. In the patients with positive findings on both the turboFLAIR and T1-weighted sequences, the areas of hyperintensity were more extensive and more prominent on the turboFLAIR images.
Discussion
Noninvasive MR evaluation of cerebral perfusion requires a combination of high-speed MR imaging and timed bolus administration of contrast agent in order to model the kinetics of first-pass transit of contrast material through the cerebral microcirculation (17–19). Diffusion-weighted imaging similarly requires high-performance echo-planar systems and currently is the most sensitive imaging technique available for evaluation of cerebral ischemia and differentiation of acute versus chronic ischemic foci (20). The diagnostic utility of functional MR imaging, consisting of combined perfusion- and diffusion-weighted neuroimaging, of acute stroke has been demonstrated in numerous studies (21–24). In particular, the results have shown that use of diffusion-weighted contrast-enhanced first-pass perfusion imaging significantly improves 1) early detection of acute stroke, based on underlying cerebral perfusion deficits (22, 23, 25); 2) documentation of successful reperfusion of ischemic brain tissues (26, 27); and 3) differentiation of reversibly and irreversibly injured brain tissues (22).
The primary aim of the present study was to use bolus intravenous injection of contrast material combined with MR perfusion neuroimaging methods to evaluate cerebral perfusion before and during 30-minute balloon test occlusion of one ICA in patients in whom subsequent neuroendovascular and/or neurosurgical procedures were planned.
The purpose of preoperative balloon test occlusion of the ICA is to stratify patients according to risk for incurring permanent neurologic deficits after carotid sacrifice. Adjunctive imaging and monitoring techniques have been added to improve the diagnostic accuracy of this test, although the method of permanent occlusion (ie, intraoperative or endovascular) and, inherently, periprocedural management may still influence the final outcome (9). According to reported studies, balloon occlusion of the ICA generally produces one of three outcomes. Approximately 80% of patients have adequate collateral flow and cross circulation through the circle of Willis and, consequently, tolerate balloon occlusion of the ICA; patients in this group are generally considered to be at low risk for developing clinical neurologic symptoms when their ICA is occluded during subsequent surgery, with less than 5% incurring permanent neurologic deficits (3, 6, 7). A second, much smaller, population of patients clinically fail the challenge; preoperative identification of this patient subgroup is important because these patients generally cannot undergo surgery unless an EC-IC bypass procedure is first performed. The third group of patients, representing 5% to 10% of the total patient population, appear to tolerate the standard 30-minute carotid test occlusion, as demonstrated by neurologic monitoring performed during the test, but subsequently develop delayed adverse neurologic deficits, including stroke, usually within 1 to 3 days after the procedure.
The present results indicate that perfusion MR imaging carried out during balloon test occlusion is clinically feasible and provides further evidence of delayed and decreased hemispheric perfusion. In our study, however, perfusion MR imaging alone and in combination with diffusion-weighted imaging was insufficient for stratifying patients into the three categories described above. In particular, we were unable to identify, either prospectively or retrospectively, those patients who could safely tolerate occlusion of an ICA versus those who are likely to have delayed neurologic deficits after internal carotid occlusion during surgery.
The second objective was to assess the specific diagnostic imaging efficacy of administering an intravenous bolus injection of 0.1 mmol/kg contrast material, as determined by the cerebrovascular transit characteristics of the contrast agent, and to attempt to identify patients at intermediate risk, or those in whom delayed neurologic deficits will develop.
This study, however, did reveal an unexpected, clinically significant finding. We found that contrast enhancement of the pial surfaces or subarachnoid spaces on turboFLAIR imaging in areas of altered perfusion during balloon test occlusion could indicate a potential problem. This finding was discovered early in the study and presented us with a clinical dilemma: should we consider this evidence of clinically silent hypoperfusion, which might suggest an increased risk of delayed neurologic sequelae, or should we ignore the finding?
Intravascular enhancement is a well-accepted finding in cases of early cerebral infarction, depicting regions of slow arterial flow (28–30). Our findings, however, are different from those reported in the literature, because the foci of T1 shortening were located on the pial surfaces or in the adjacent subarachnoid spaces. The ability of FLAIR imaging to depict such changes has been well documented for infectious, neoplastic, and hemorrhagic entities (31–33). Furthermore, several investigators recently reported areas of subarachnoid hyperintensity on FLAIR images after intravenous administration of gadolinium products as well as iodine-based agents (34–37). The mechanism hypothesized to account for contrast extravasation into the CSF is leakage across damaged endothelial cells of pial vessels, presumably secondary to an ischemic event. Even more direct correlation between leakage of contrast agent into the subarachnoid space and an ischemic state has been proposed in cases of moyamoya disease and Sturge-Weber syndrome (38, 39).
Our findings showed subarachnoid or pial hyperintensities on four (57%) of seven diagnostic images in the asymptomatic patients and in both (100%) of the symptomatic patients. As expected, these findings were seen better on FLAIR images than on T1-weighted images (32). In this series, only two (50%) of the four patients with positive findings on FLAIR images showed enhancement on T1-weighted images, and in these cases the findings were less extensive in distribution and more subtle in signal intensity than on corresponding FLAIR images. The agreement between the location of these hyperintensities and the areas of abnormal perfusion adds confidence to the opinion that our findings represent alterations in vascular permeability or hemodynamic changes based on changes in CBF. The possibility that the clinical and imaging findings are due to embolic phenomena was also entertained but was considered less likely. The dose of heparin administered was chosen empirically on the basis of previous reports of balloon test occlusion techniques and was considered adequate, although activated clotting times were not checked (8, 10, 15, 40–42). Furthermore, the clinical and imaging findings in our symptomatic patients do not support a pattern of stump-related embolic disease. Such complications have been described during and after balloon test occlusion, but patients usually exhibit focal motor findings rather than cortical deficits, and the imaging pattern of such complications is usually different (8, 14).
An additional unexpected finding was observed in three patients who showed perfusion abnormalities in the hemisphere contralateral to the artery being occluded. In one symptomatic and one asymptomatic patient there was no detectable perfusion to the contralateral hemisphere, while in one symptomatic patient there was marked delay in perfusion to the contralateral hemisphere. Once again, the contrast-enhanced FLAIR and T1-weighted images were in agreement as to location of perfusion abnormalities and T1 shortening. These findings were interpreted as representing a vascular steal phenomenon during the balloon test occlusion via collateral pathways, across a patent and functional anterior communicating artery.
The findings on diffusion-perfusion weighted imaging and contrast-enhanced images did not allow us to stratify patients according to risk for stroke developing after permanent ICA occlusion. Our approach to patients undergoing balloon test occlusion was deliberately conservative and based on clinical criteria. In the future, more rigorous evaluation of the pial enhancement feature of contrast-enhanced turboFLAIR imaging may be indicated.
Conclusion
In an attempt to identify patients who are at risk for permanent neurologic deficits as the result of permanent carotid occlusion during balloon test occlusion, imaging was performed in an interventional MR setting. Our findings showed that a protocol consisting of diffusion- and perfusion-weighted imaging as well as conventional spin-echo and inversion-recovery sequences is feasible and safe. Moreover, we found marked alteration in CBF to the affected hemisphere and occasionally to the contralateral hemisphere, presumably due to vascular steal. The areas demonstrating altered perfusion also showed T1 shortening over the pial surfaces or in the subarachnoid spaces, which were more constant and more prominent on FLAIR images than on T1-weighted images. On the basis of our findings and those reported previously, we conclude that this occurrence is due to hemodynamic changes or increased permeability of the leptomeningeal vessels. While these observations did not allow us to prospectively or retrospectively select patients at increased risk for stroke after permanent arterial occlusion, they may indicate subclinical ischemia; therefore, these sequences have become part of our routine MR protocol for evaluation of acute ischemia.
fig 2.
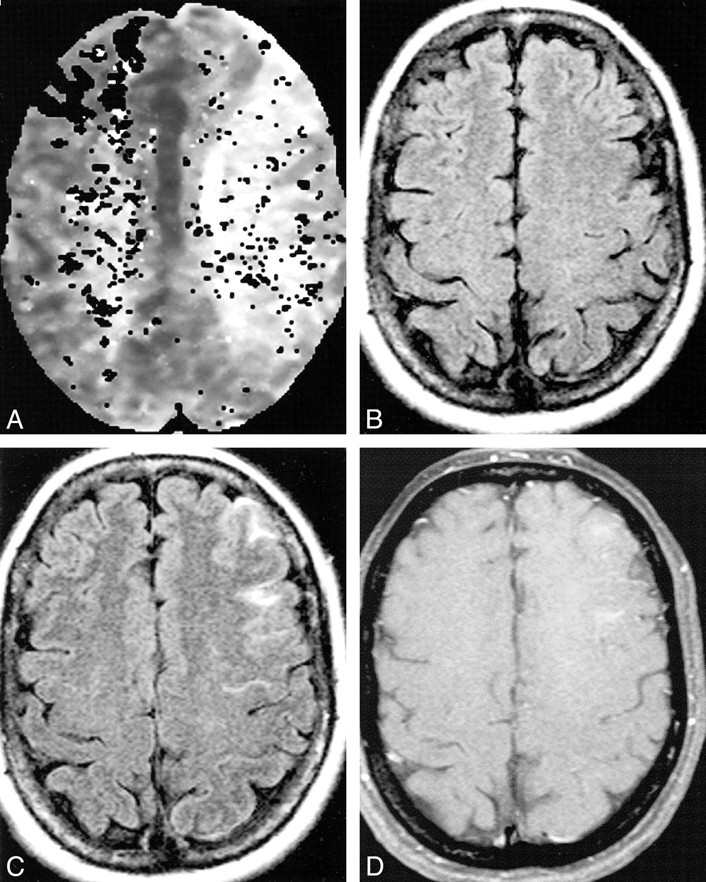
Patient 8: 29-year-old woman undergoing right ICA balloon test occlusion who was asymptomatic throughout the procedure.
A, Perfusion map shows delayed perfusion in the right hemisphere.
B and C, Contrast-enhanced turboFLAIR images (9000/110, TI = 2500) show areas of hyperintensity in the right parietooccipital and right frontal regions, in the right middle cerebral artery—posterior cerebral artery and anterior cerebral artery—middle cerebral artery borderzone distributions of the right hemisphere.
Footnotes
Supported by a grant from Berlex Laboratories.
Presented in part at the annual meeting of the American Society of Neuroradiology, Philadelphia, May 1998.
References
- 1.de Vries EJ, Sekhar LN, Horton JA, et al. A new method to predict safe resection of the internal carotid artery. Laryngoscope 1990;100:85-88 [DOI] [PubMed] [Google Scholar]
- 2.Sen C, Sekhar LN. Direct vein graft reconstruction of the cavernous, petrous and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery 1992;30:732-743 [PubMed] [Google Scholar]
- 3.Fox AJ, Vinuela F, Pelz DM, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 1987;66:40-46 [DOI] [PubMed] [Google Scholar]
- 4.Andrews JC, Valvanis A, Fisch U. Management of the internal carotid artery in surgery of the skull base. Laryngoscope 1989;99:1224-1229 [DOI] [PubMed] [Google Scholar]
- 5.Weil SM, van Loveren HR, Tomsick TA, Quallen BL, Twe JM. Management of inoperable cerebral aneurysms by the navigational balloon technique. Neurosurg 1987;21:296-301 [DOI] [PubMed] [Google Scholar]
- 6.Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg 1990;72:857-863 [DOI] [PubMed] [Google Scholar]
- 7.Anon VV, Aymard A, Gobin YP, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology 1992;34:245-251 [DOI] [PubMed] [Google Scholar]
- 8.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol 1995;16:749-754 [PMC free article] [PubMed] [Google Scholar]
- 9.Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol 1994;15:829-843 [PMC free article] [PubMed] [Google Scholar]
- 10.Kofke WA, Brauer P, Policare R, Penthany S, Barker D, Horton J. Middle cerebral artery blood flow velocity and stable xenon-enhanced computed tomographic blood flow during balloon test occlusion of the internal carotid artery. Stroke 1995;26:1603-1606 [DOI] [PubMed] [Google Scholar]
- 11.Palestro CJ, Sen C, Muzinic M, Afriyie M, Goldsmith SJ. Assessing collateral cerebral perfusion with technetium-99m-HMPAO SPECT during temporary internal carotid occlusion. J Nucl Med 1993;34:1235-1238 [PubMed] [Google Scholar]
- 12.Ryu YH, Chung TS, Lee JD, et al. HMPAO SPECT to assess neurologic deficits during balloon test occlusion. J Nucl Med 1996;37:551-554 [PubMed] [Google Scholar]
- 13.Tawes RL, Lull R. Value of single photon emission computerized imaging in the treatment of patients undergoing carotid endarterectomy. J Vasc Surg 1996;24:219-225 [DOI] [PubMed] [Google Scholar]
- 14.Schneweis S, Urbach H, Solymosi L, Ries F. Preoperative risk assessment for carotid occlusion by transcranial Doppler ultrasound. J Neurol Neurosurg Psychiatry 1997;62:485-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckert B, Thie A, Carvajal M, Groden C, Zeumer H. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR AM J Neuroradiol 1998;19:577-582 [PMC free article] [PubMed] [Google Scholar]
- 16.Dare AO, Chaloupka JC, Putman CM, Fayad PB, Awad IA. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol 1998;50: [DOI] [PubMed]
- 17.Rosen BR, Belliveau JW, Chien D. Perfusion imaging by nuclear magnetic resonance. Magn Reson Q 1989;5:263-281 [PubMed] [Google Scholar]
- 18.Rempp KA, Brix G, Wenz F, Becker CR, Guckel F, Lorenz WJ. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 1994;193:637-641 [DOI] [PubMed] [Google Scholar]
- 19.Edelman RR, Mattle HP, Atkinson DJ, et al. Cerebral blood flow: assessment with dynamic contrast-enhanced T2*-weighted MR imaging at 1.5T. Radiology 1990;176:211-220 [DOI] [PubMed] [Google Scholar]
- 20.Beauchamp NJ Jr, Ulug AM, Passe TJ, van Zijl PC. MR diffusion imaging in stroke: review and controversies. Radiographics 1998;18:1269-1283 [DOI] [PubMed] [Google Scholar]
- 21.Kucharczyk J, Mintorovitch J, Asgari HS, Moseley ME. Diffusion/perfusion MR imaging of acute cerebral ischemia. Magn Reson Med 1991;19:311-315 [DOI] [PubMed] [Google Scholar]
- 22.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391-401 [DOI] [PubMed] [Google Scholar]
- 23.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999;212:325-332 [DOI] [PubMed] [Google Scholar]
- 24.Ueda T, Yuh WT, Taoka T. Clinical application of perfusion and diffusion MR imaging in acute ischemic stroke. J Magn Reson Imaging 1999;10:305-309 [DOI] [PubMed] [Google Scholar]
- 25.de Crespigny AJ, Tsuura M, Moseley ME, Kucharczyk J. Perfusion and diffusion MR imaging of thromboembolic stroke. J Magn Reson Imaging 1993;3:746-754 [DOI] [PubMed] [Google Scholar]
- 26.Marks MP, Tong DC, Beaulieu C, Albers GW, de Crespigny A, Moseley ME. Evaluation of early reperfusion and i.v. tPA therapy using diffusion- and perfusion-weighted imaging. Neurology 1999;52 [DOI] [PubMed]
- 27.Schellinger PD, Jansen O, Fiebach JB, et al. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke 2000;31 [DOI] [PubMed]
- 28.Crain MR, Yuh WT, Greene GM, et al. Cerebral ischemia: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol 1991;12:631-639 [PMC free article] [PubMed] [Google Scholar]
- 29.Elster AD, Moody DM. Early cerebral infarction: gadopentate dimeglumine enhancement. Radiology 1996;177:627-632 [DOI] [PubMed] [Google Scholar]
- 30.Essig M, von Kummer R, Egelhof T, Winter R, Sartor K. Vascular MR contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol 1996;17:887-894 [PMC free article] [PubMed] [Google Scholar]
- 31.Noguchi K, Ogawa T, Inugami A, et al. Acute subarachnoid hemorrhage: MR imaging with fluid-attenuated inversion recovery pulse sequences. Radiology 1995;196:773-777 [DOI] [PubMed] [Google Scholar]
- 32.Singer MB, Atlas SW, Drayer BP. Subarachnoid space disease: diagnosis with fluid-attenuated inversion-recovery MR imaging and comparison with gadolinium-enhanced spin-echo imaging: blinded reader study. Neuroradiology 1998;208:417-422 [DOI] [PubMed] [Google Scholar]
- 33.Mathews VP, Caldemeyer KS, Lowe MJ, Greenspan SL, Weber DM, Ulmer JL. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Neuroradiology 1999;211:257-263 [DOI] [PubMed] [Google Scholar]
- 34.Eckel TS, Breiter SN, Monsein LH. Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol 1998;170:503-505 [DOI] [PubMed] [Google Scholar]
- 35.Lev MH, Schaefer PW. Subarachnoid gadolinium enhancement mimicking subarachnoid hemorrhage on FLAIR MR images. AJR Am J Roentgenol 1999;173:1414-1415 [DOI] [PubMed] [Google Scholar]
- 36.Mamourian AC, Hoopes PJ, Lewis LD. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: an in vitro and animal-model investigation. AJNR Am J Neuroradiol 2000;21:105-111 [PMC free article] [PubMed] [Google Scholar]
- 37.Dechambre SD, Duprez T, Grandin CB, Lecouvet FE, Peeters A, Cosnard G. High signal in cerebrospinal fluid mimicking subarachnoid haemorrhage on FLAIR following acute stroke and intravenous contrast medium. Neuroradiology 2000;42:608-611 [DOI] [PubMed] [Google Scholar]
- 38.Benedikt RA, Brown DC, Walker R, Ghaed VN, Mitchell M, Geyer CA. Sturge-Weber syndrome: cranial MR imaging with Gd-DTPA. AJNR Am J Neuroradiol 1993;14:409-415 [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda M, Tsuchida C. “Ivy sign” on fluid-attenuated inversion-recovery images in childhood moyamoya disease. AJNR Am J Neuroradiol 1999;20:1836-1838 [PMC free article] [PubMed] [Google Scholar]
- 40.Lorberboym M, Pandit N, Machac J, et al. Brain perfusion imaging during preoperative temporary balloon occlusion of the internal carotid artery. J Nucl Med 1996;37:415-419 [PubMed] [Google Scholar]
- 41.Graves VB, Perl J III, Strother CM, Wallace RC, Kesava PP, Masaryk TJ. Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol 1997;18:1201-1206 [PMC free article] [PubMed] [Google Scholar]
- 42.Morishima H, Kurata A, Miyashaka Y, Fujii K, Kan S. Efficacy of the stump pressure ratio as a guide to the safety of permanent occlusion of the internal carotid artery. Neurol Res 1998;20:732-736 [DOI] [PubMed] [Google Scholar]