Abstract
BACKGROUND AND PURPOSE: The overall mortality rate of primary pontine hemorrhage (PPH) in recent studies is 40–50%. The aim of the present study was to analyze the predictive value of clinical and neuroradiologic parameters concerning the outcome of patients with PPH.
METHODS: We reviewed the clinical data of 29 consecutive patients (mean age, 59 ± 13.5 years; 12 women, 17 men) with PPH. National Institutes of Health Stroke Scale (NIHSS) and Glasgow Coma Scale (GCS) scores were assessed on admission, and NIHSS, GCS, and Glasgow Outcome Scale (GOS) scores were assessed on discharge. The hemorrhage volume was calculated by using a previously published formula. Clinical manifestations, outcome, and volume and location of the bleeding were correlated.
RESULTS: The mean GCS score on admission was 6.8 ± 3.9 and increased to 9.0 ± 3.9 on discharge. The NIHSS score improved from 29.1 ± 12.5 to 12.1 ± 7.3. Nine patients (31%) died as a result of PPH after 5 ± 3 days. The mean GOS score was 3.0 ± 1.5 (3.9 ± 0.8 for patients who survived). Arterial hypertension was the most common risk factor (90%); other causes were anticoagulation therapy (7%) and amyloid angiopathy (3%). A high correlation was observed between a poor outcome (GOS score < 4) and hematoma volume greater than 4 mL (P = .006), ventral hemorrhage (P < .001), and necessity for mechanical ventilation (P < .001). Patients with dorsally located hematomas less than 4 mL in volume had a significantly better outcome.
CONCLUSION: The prognosis of PPH is better than commonly expected. Most patients with moderate neurologic deficits on admission and dorsally located small hematomas are able to survive PPH with minor neurologic deficits.
Spontaneous primary pontine hemorrhage (PPH) accounts for about 10% of intracranial hemorrhages. PPH frequently causes severe disturbances of consciousness, oculomotor disturbances, tetraparesis, and respiratory failure. In recent studies, the prognosis was found to be highly fatal, with the overall mortality rate being 40–50% (1–3). Previous predictors for a poor outcome were coma on admission, intraventricular extension, and acute hydrocephalus (4–6). Treatment with CT-guided stereotaxic hematoma aspiration was suggested by Takahama et al (7) and Hara et al (8), who found a significantly better functional outcome in patients treated surgically compared with those treated conservatively. Patients with acute hydrocephalus may improve when ventricular drainage is performed, but studies on functional outcome are not present. Murata et al (1) found no improvement in outcome when acute hydrocephalus in PPH was treated with ventricular drainage. However, surgical therapy is not a standard therapy for PPH and might be limited to selected indications in specialized centers.
CT scanning and MR imaging enable identification of even small hematomas, as well as precise description of their extent and location (1, 4, 9). However, the prognosis in recent reports is highly fatal (1, 3). In 1992, Chung and Park (4) reported an overall mortality rate of 60% with a close relationship to the hematoma location. In 1999, Murata et al (1) reported their retrospective evaluation of 80 cases and found an overall mortality rate of 47%. The aim of the present study was to analyze the predictive value of clinical and neuroradiologic parameters on the outcome of patients with PPH.
Methods
We reviewed the clinical data of 29 consecutive patients with PPH who were admitted to our neurologic intensive care unit between 1980 and 2002. Mean age was 59 ± 13.5 years, with a range of 33–84 years (12 women and 17 men). Patients with arteriovenous malformation and cavernoma were excluded from this study by CT, MR imaging, and angiographic findings and based on history because of the different clinical courses.
The National Institutes of Health Stroke Scale (NIHSS), Glasgow Coma Scale (GCS), and Glasgow Outcome Scale (GOS) scores were assessed retrospectively. All patients were investigated with cranial CT. The volume of the hemorrhage was calculated by using the “bedside formula” of Kothari et al (10): hemorrhage volume (in mL) = (A x B x C)/2, where A = greatest hemorrhage diameter (in mm at CT), B = diameter 90° to A (in mm at CT), and C = approximate number of CT sections with hemorrhage × section thickness.
Patients were divided into two groups: a “good” and a “poor” outcome group, according to the GOS score (1, death; 2, persistent vegetative state; 3, severe disability; 4, moderate disability; 5, good recovery) (11). The good outcome group was defined as patients with a GOS score of 4–5, who required none or only partial assistance in daily life. The poor outome group comprised patients with a GOS score of 1–3. An additional comparison was made between the survivors and the patients who died as a consequence of the PPH. Then, we evaluated the clinical and neuroradiologic differences between the good outcome group (12 patients with GOS score ≥4) and the poor outcome group (17 patients with GOS score <4), as well as between the survivors and the patients who died.
Based on clinical outcome, the patients where further divided into groups of those with a hematoma volume of 4 mL or greater and those with a hematoma volume less than 4 mL, to evaluate the clinical significance of hematoma volume.
According to the CT classification of Chung and Park (4), the hemorrhages where divided into three types: dorsal, ventral, and massive. The hematoma was classified as dorsal when the location of the PPH was uni- or bilateral tegmental, sparing the base pontis (Fig 1). The ventral type consisted of those hematoma occupying the ventral base pontis and the junction between the bilateral tegmentum (Fig 2). The massive type defined hematomas occupying the base pontis and the bilateral tegmentum with extension to the midbrain (Fig 3).
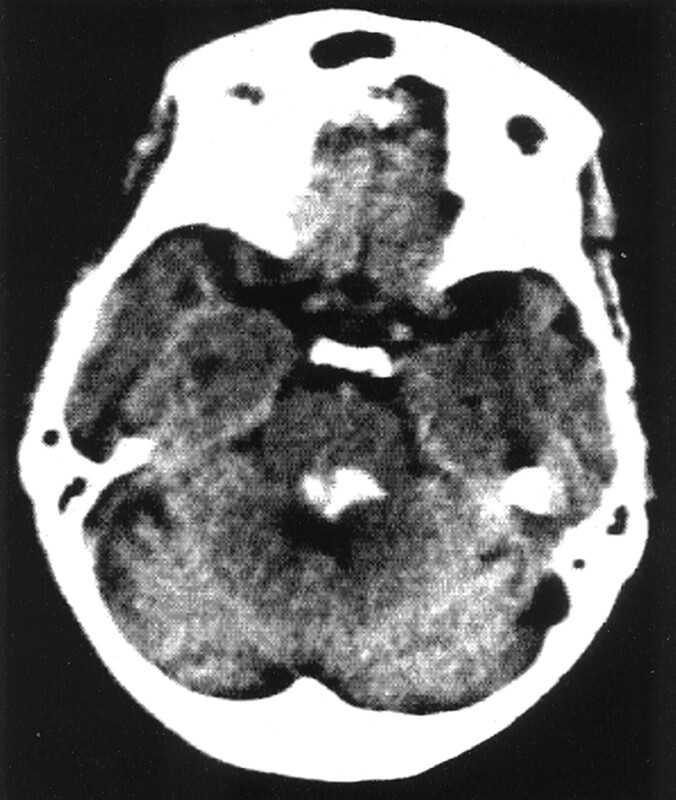
Fig 1.
Dorsal PPH.
CT scan in a 65-year-old man who was alert on admission, with mild gait ataxia. Hematoma volume is 2.5 mL. Patient outcome was excellent (GOS score 5).
Fig 2.
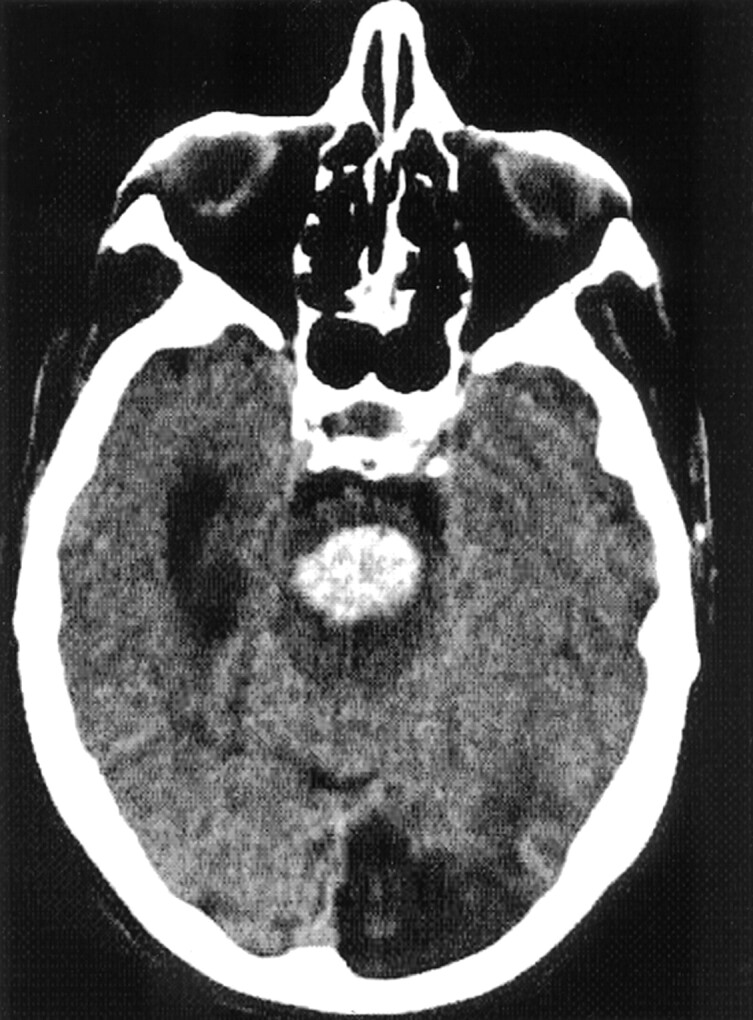
Ventral PPH.
CT scan in an 80-year-old woman who was in a coma on admission. The hematoma volume is 6 mL. The patient received mechanical ventilation and died after 1 week.
Fig 3.
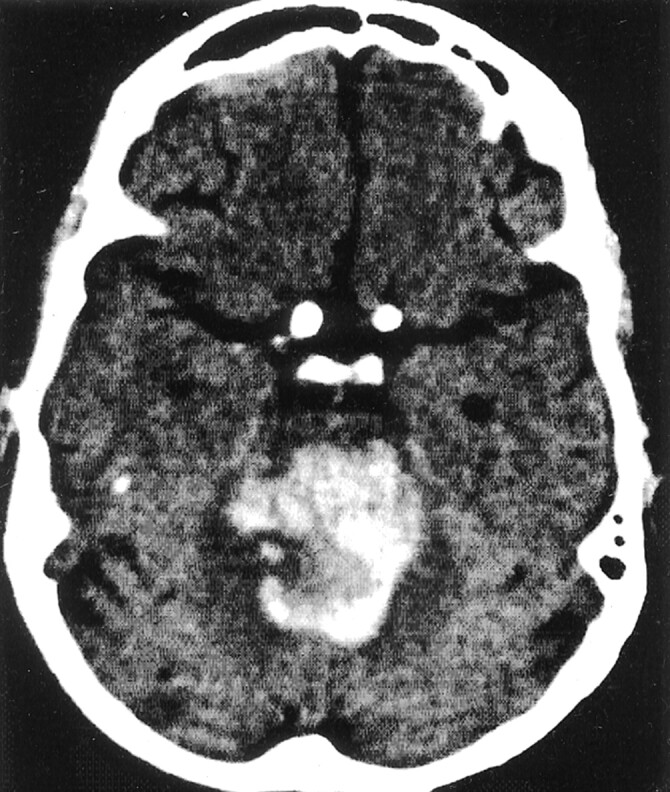
Massive PPH.
CT scan in a 74-year-old woman who was in a coma on admission. The hematoma volume is 15 mL. The patient died 2 days after admission.
Furthermore, we investigated the difference in age. Patients were divided into two groups according to age: those aged 65 years or older (elderly group, 11 patients with mean age 72 ± 7.0 years) and those younger than 65 years (middle-aged group, 17 patients with mean age 50 ± 7.0 years).
Statistical Evaluation
The following factors were assessed to determine possible predictive values: age, hematoma volume, hematoma location (dorsal, ventral, massive), initial level of consciousness (alert, drowsy, stuporous, coma), pupillary abnormalities (anisocoria, pinpoint pupils, mydriasis), need for mechanical ventilation, and GCS and NIHSS scores on admission and discharge.
Correlations among the clinical manifestations, outcome, hemorrhage volume, and location of the bleeding were assessed by means of a two-tailed Fisher exact test. The level of probability of P < .05 was regarded as significant.
Results
Arterial hypertension was the most common cause (90%) of PPH; others causes were anticoagulation therapy (7%) and amyloid angiopathy (3%). The hematoma volume range was 1–15 mL, and the mean value was 5.0 ± 3.0 mL. A ventral hemorrhage was found in 15 (52%) of the 29 patients (Fig 2), and a dorsal location in nine patients (31%) (Fig 1). A massive PPH with extension to the midbrain was found in five (17%) of the 29 patients (Fig 3). Intraventricular extension, particulary in the fourth ventricle, was found in seven patients (24%). Mean GCS score on admission was 6.8 ± 3.9 and increased to 9.0 ± 3.9 on discharge. The NIHSS score improved from 29.1 ± 12.5 to 12.1 ± 7.3. Nine patients (31%) died as a consequence of PPH after 5 ± 3 days. Mechanical ventilation was necessary in 18 patients and in 16 of the 17 patients who died. The mean GOS score was 3.0 ± 1.5.
The CT findings and clinical features of all patients are stratified in Table 1 and Table 2, respectively. A high correlation was observed between a poor outcome (GOS score < 4) and hematoma volume greater than 4 mL (P = .006), ventral hemorrhage (P < .001), massive hemorrhage (P = .002), coma on admisson (P = .03), and necessity for mechanical ventilation (P < .001). In patients with dorsally located hematomas less than 4 mL in volume, a significantly better outcome was found. Pupillary abnormalities and hypertension did not correlate to outcome. Age older than 65 years and intraventricular hemorrhage were not significant predictors of a poor outcome. Acute hydrocephalus was significantly more common in patients who died, but had no significant influence for functional outcome if the patient survived.
TABLE 1:
CT parameters in 29 patients with PPH stratified by outcome
| CT Parameter | Severe Disability, GOS Score < 4 (n = 17) | Mild Disability Good Recovery, GOS Score ≥ 4 (n = 12) | P Value* | Death (n = 9) | Survivors (n = 20) | P Value* |
|---|---|---|---|---|---|---|
| Mean hematoma volume (mL) | 6.6 ± 2.7 | 2.8 ± 1.6 | 7.9 ± 2.6 | 3.7 ± 2.1 | ||
| Hematoma ≥ 4 mL | 14 (82) | 3 (25) | .006 | 9 (100) | 8 (40) | .003 |
| Hematoma < 4 mL | 3 (17) | 9 (75) | .006 | 0 | 12 (60) | .003 |
| Hematoma location | ||||||
| Dorsal | 0 | 9 (75) | <.001 | 0 | 9 (45) | .011 |
| Ventral | 12 (70) | 3 (25) | <.001 | 4 (44) | 9 (45) | .23 |
| Massive | 5 (29) | 0 | .002 | 5 (55) | 0 | <.001 |
| Intraventricular hematoma extension | 6 (35) | 1 (8) | .18 | 5 (55) | 2 (10) | .01 |
| Hydrocephalus | 7 (41) | 2 (16) | .23 | 6 (66) | 3 (15) | .01 |
Note.—Except for mean hematoma volume, data are number (%) of patients.
Two-tailed Fisher exact test.
TABLE 2:
Clinical parameters in 29 patients with PPH stratified by outcome
| Clinical Parameter | Severe Disability, GOS Score < 4 (n = 17) | Mild Disability, Good Recovery, GOS Score ≥ 4 (n = 12) | P Value* | Death (n = 9) | Survivors (n = 20) | P Value* |
|---|---|---|---|---|---|---|
| Coma on admission | 11 (64) | 1 (8) | 0.03 | 7 (77) | 2 (10) | .014 |
| Abnormal pupils | 10 (58) | 4 (33) | .26 | 6 (66) | 8 (40) | .24 |
| Scores (mean ± SD) | ||||||
| GCS on admission | 4.3 ± 2.3 | 10.3 ± 3.0 | .001 | 3.1 ± 0.3 | 8.5 ± 3.6 | .03 |
| GCS on discharge | 6.7 ± 4.3 | 12.2 ± 1.5 | <.001 | — | 11.7 ± 1.8 | <.001 |
| NIHSS on admission | 36.4 ± 7.3 | 18.7 ± 11.0 | .008 | 39 ± 2.4 | 24.6 ± 12.7 | .40 |
| NIHSS on discharge | 30.4 ± 13.6 | 8.6 ± 4.6 | <.001 | — | 12.1 ± 7.3 | <.001 |
| Age (mean, 59 ± 13 y) | ||||||
| ≥65 years (n = 11) | 8 (47) | 3 (25) | .20 | 7 (77) | 4 (20) | .01 |
| <65 years (n = 18) | 9 (52) | 9 (75) | .20 | 2 (22) | 16 (80) | .01 |
| History of hypertension | 15 (88) | 9 (75) | .62 | 9 (100) | 15 (75) | .15 |
| Mechanical ventilation | 16 (94) | 2 (16) | <.001 | 9 (100) | 9 (45) | .005 |
Note.—Except for scores, data are number (%) of patients.
Two-tailed Fisher exact test.
Discussion
The spectrum of PPH is diverse, ranging from small hematomas less than 1 mL to massive bleeding with a greater than 8-mL hematoma destroying the entire base pontis and tegmentum, leading to intraventricular hemorrhage and early hydrocephalus.
In most patients with centrally located hematomas, consciousness disturbances, oculomotor disturbances (nystagmus, palsy), and irregular breathing patterns that require intubation and mechanical ventilation occur. Recent studies predict a fatal outcome in patients with coma on admission, large hematomas, intraventricular extension, hydrocephalus, and pupillary abnormalities (1–3). Initial level of consciousness and size and location of the hematoma were significantly correlated to poor outcome in our patients. Death did not occur in patients who were alert on admission. This indicates that the initial level of consciousness is a major sign for predicting outcome, as shown in previous studies (1–3, 9). Results of the present study also show that hematoma volume is a reliable predictor of outcome, as was found in previous studies (3, 5, 6, 12). As expected, good recovery was seen most frequently in patients with hemorrhages less than 4 mL and dorsally located hematomas. In 1992, Chung and Park (4) suggested a new CT classification for PPH: type 1, small unilateral tegmental; type 2, basal tegmental; type 3, bilateral tegmental; and type 4, massive, in which case survival rate is highest in the unilateral tegmental type and lowest in the massive type. Murata et al (1) found that the horizontal diameter of the hematoma is the most reliable predicting factor of outcome. Other authors emphasize cross-sectional diameter as well as the transverse and vertical extension of the hematoma as the most exact predictor of outcome (6, 12–14). All these parameters offer a good tool for determining the prognosis of patients with PPH because the size of the hematoma is strongly correlated to the parenchymal damage. In our opinion, particularly in patients with smaller hematomas, differentiation between ventral and dorsal location of the hemorrhage and calculation of hematoma volume by using the “bedside formula” offer easy and reliable tools to anticipate the prognosis. PPHs of the ventral and massive types result from rupture of parenchymal midpontine branches of the basilar artery. The bleeding vessel is probably a paramedian perforator in its distal portion and forms the initial hematoma at the junction of the tegmentum and the basis pontis. The vessels causing the dorsal, or in other studies called tegmental, type of PPH have the same origin, but the degree and direction of the hematoma differ. It is caused by rupture of the penetrating, long circumferential vessels that enter the tegmentum dorsally and course medially (15, 16). In our series, no patient with a dorsally located hematoma died. Among the patients who died, 44% had a ventral and 55% had a massive hemorrhage. The hematoma location was highly significantly correlated to functional outcome (GOS score): patients with a dorsally located hematoma had a good outcome, and patients with a ventral or massive hematoma had a a poor outcome. No intraventricular extension was seen in patients with dorsal hematomas. A possible mechanism causing the unfavorable outcome in patients with ventral hematomas could be the effect on the cranial nuclei leading to dysarthria, dysphagia, aspiration pneumonia, and abnormal breathing patterns requiring mechanical ventilation. The poor prognosis of the massive type can be explained by the rapid brain stem destruction with early coma, need for mechanical ventilation due to the effect on the reticular activating system in the upper third of the pontine tegmentum, and acute hydrocephalus.
The presence of intraventricular extension and acute hydrocephalus were not significantly correlated to functional outcome in the survivors, but were significantly more common in patients who died. By contrast to the analyses of Murata et al (1) and Wijdicks and St. Louis (3), we found no correlation between functional outcome and age, but between age and death. A possible explanation for this finding could be that, if the patient with hemorrhage survived because of a favorable clinical course, the functional outcome does not differ significantly, because of a good potential for recovery.
As reported by the authors of two recent studies (1, 3), who predicted hyperthermia in PPH as the important role in determining prognosis, we observed no patient with central hyperthermia.
Our data suggest that the prognosis of PPH is better than commonly expected. Most patients with moderate neurologic deficits on admission and dorsally located small hematomas are able to survive PPH with minor neurologic deficits.
Acknowledgments
The authors thank Professor Klaus Willmes von Hinckeldey, Department of Neuropsychology, Aachen University Medical School, for assistance in the statistical evaluation of the presented data, and Stuart Fellows, PhD, Department of Neurology, Aachen University Medical School, for his support in preparing the manuscript.
References
- 1.Murata Y, Yamaguchi S, Kajikawa H, Yamamura K, Sumioka S, Nakamura S. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J Neurol Sci 1999;167:107–111 [DOI] [PubMed] [Google Scholar]
- 2.Kushner MJ, Bressman SB. The clinical manifestations of pontine hemorrhage. Neurology 1985;35:637–643 [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks EF, St Louis E. Clinical profiles predictive of outcome in pontine hemorrhage. Neurology 1997;49:1342–1346 [DOI] [PubMed] [Google Scholar]
- 4.Chung CS, Park CH. Primary pontine hemorrhage: a new CT classification. Neurology 1992;42:830–834 [DOI] [PubMed] [Google Scholar]
- 5.Weisberg LA. Primary pontine haemorrhage: clinical and computed tomographic correlations. J Neurol Neurosurg Psychiatry 1986;49:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto N, Kaneko M, Hosaka Y, Koga H. Primary pontine hemorrhage: clinicopathological correlations. Stroke 1980;11:84–90 [DOI] [PubMed] [Google Scholar]
- 7.Takahama H, Morii K, Sato M, Sekiguchi K, Sato S. Stereotactic aspiration in hypertensive pontine hemorrhage: comparative study with conservative therapy [in Japanese]. No Shinkei Geka 1989;17:733–739 [PubMed] [Google Scholar]
- 8.Hara T, Nagata K, Kawamoto S, et al. Functional outcome of primary pontine hemorrhage: conservative treatment or stereotaxic surgery [in Japanese]. No Shinkei Geka 2001;29:823–829 [PubMed] [Google Scholar]
- 9.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993 [DOI] [PubMed] [Google Scholar]
- 10.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305 [DOI] [PubMed] [Google Scholar]
- 11.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–484 [DOI] [PubMed] [Google Scholar]
- 12.Masiyama S, Niizuma H, Suzuki J. Pontine haemorrhage: a clinical analysis of 26 cases. J Neurol Neurosurg Psychiatry 1985;48:658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwabara S, Ohta K, Ueda T, Yamane K, Uemura Y, Takahashi M. Clinical study of 20 cases of primary pontine hemorrhage, with special reference to correlations between clinical, computed tomographic, and electroencephalographic findings and outcome. Neurol Med Chir (Tokyo) 1982;22:933–942 [DOI] [PubMed] [Google Scholar]
- 14.Ochiai C, Sano K, Kobayashi S, Sasaki T, Mayanagi Y. Clinical study of pontine hemorrhage with special reference to CT classification and surgical indication [in Japanese]. No To Shinkei 1979;31:803–811 [PubMed] [Google Scholar]
- 15.Kase CS, Caplan LR. Hemorrhage affecting the brain stem and cerebellum. Barrnett H JM, Mohr JP, Stein BM., eds. Stroke: Pathophysiology, Diagnosis and Management. Vol 1. New York: Churchill Livingstone;1986. :621–641.
- 16.Caplan LR, Goodwin JA. Lateral tegmental brainstem hemorrhages. Neurology 1982;32:252–260 [DOI] [PubMed] [Google Scholar]