Abstract
Summary: We describe an MR protocol for the noninvasive imaging of the subarachnoid space, which we use in patients with suspected neurocysticercosis in this space. It consists of a fluid-attenuated inversion recovery sequence performed 5 minutes after the continuous inhalation of 100% O2 with a resultant increase in the signal intensity of the CSF that leads to a greater conspicuity of cyst walls in relation to the cortex and the extraventricular CSF.
Initial trials have shown the safety of intrathecal administration of gadopentetate dimeglumine in humans (1–3). The administration of paramagnetic contrast agents by the intrathecal route, however, has not yet been approved by regulatory agencies in most countries, including the United States Food and Drug Administration.
On the other hand, artifactual CSF hyperintensity on fluid-attenuated inversion recovery (FLAIR) images has been shown in patients ventilated with 100% O2 (4–6). We currently use this approach as a noninvasive cisternography technique that allows the detection of cystic lesions in the subarachnoid space, because detection of such lesions is frequently difficult with conventional MR imaging sequences. In neurocysticercosis patients, the resultant hyperintensity of the CSF increases the conspicuity of lesions in the cisterns, sulci, and along the cortical surface.
Among the main complications of neurocysticercosis in the subarachnoid space are hydrocephalus, meningitis, and arteritis (7). The prompt detection of lesions in the extraventricular CSF spaces may help in the diagnosis and management of neurocysticercosis, cases in which medical treatment over surgical approaches is favored (8, 9), and the prevention of complications.
Technique
Initially, a FLAIR sequence (1.0 T; TR, 11,000 ms; TE, 140 ms; TI, 2600 ms) is obtained with the patient breathing room air. Next, 100% O2 is delivered for 5 minutes by using a nonbreathing face mask connected to a ventilator, after which another identical FLAIR sequence is obtained. Images are evaluated with a window width of 950 and window level of 650.
It is essential that the mask fit the face well to prevent room air from entering the ventilator system (4, 5), because high concentrations of O2 are needed to increase the signal intensity of the CSF. Supplemental oxygen at 50% (4) or 60% (6) will not cause the intended hyperintensity in the CSF on FLAIR sequences.
Discussion
FLAIR is very sensitive for the detection of parenchymal lesions. The acquisition of FLAIR sequences before and after the inhalation of 100% O2 may improve the detection of subtle cisternal and extraventricular subarachnoid space lesions because of the resultant hyperintensity of the CSF. In neurocysticercosis endemic areas, this noninvasive MR cisternography may resolve the not uncommon diagnostic dilemma of a patient (symptomatic or not) with subtle cisternal widening or subtle superficial cortical lesions.
This method enables both detection of neurocysticercosis lesions in the extraventricular CSF spaces not evident on conventional FLAIR images (Fig 1) and the capacity to rule out the presence of such lesions within the subarachnoid space. Superficial parenchymal lesions may also become more evident (Figs 2 and 3). It should be noted that arachnoid cysts could also become more prominent with this technique. Therefore, one should take into account epidemiologic data and other clinical, laboratory, and imaging findings such as presence of a cyst with a scolex, other cysts, or multiple parenchymal lesions (calcified or not) to diagnose neurocysticercosis (10).
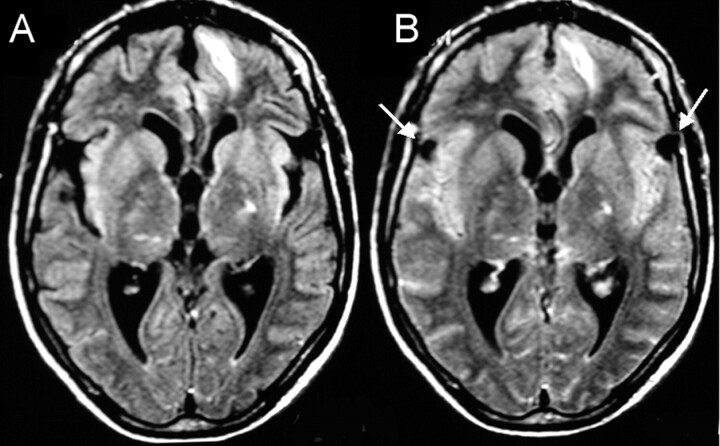
Fig 1.
A 40-year-old man with parenchymal neurocysticercosis and suspected subarachnoid involvement.
A, MR FLAIR image (1.0 T; TR, 11,000 ms; TE, 140 ms; TI, 2600 ms) shows no extraaxial lesions.
B, FLAIR image after 100% O2 for 5 minutes shows two small cysts in the Sylvian fissures confirming racemous neurocysticercosis.
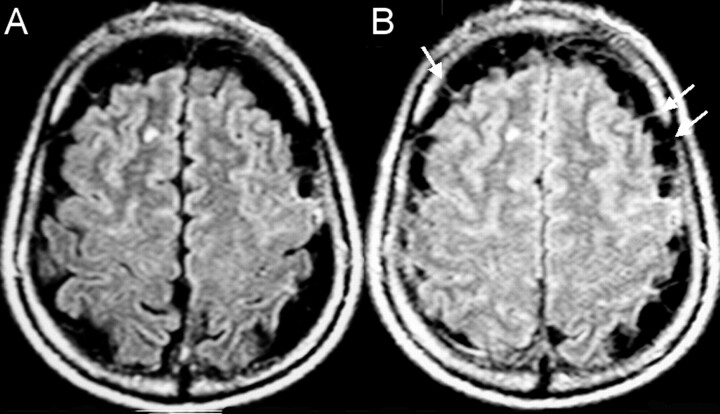
Fig 2.
A 57-year-old man with multiple vesicular lesions in the brain parenchyma.
A, FLAIR image shows prominent CSF space along the cerebral convexities.
B, FLAIR image after 100% O2 for 5 minutes does not show the expected increased signal intensity of the CSF due to the presence of multiple clustered extraaxial cysts. The walls of the cysts (arrows) are better visualized after 100% O2.
Fig 3.
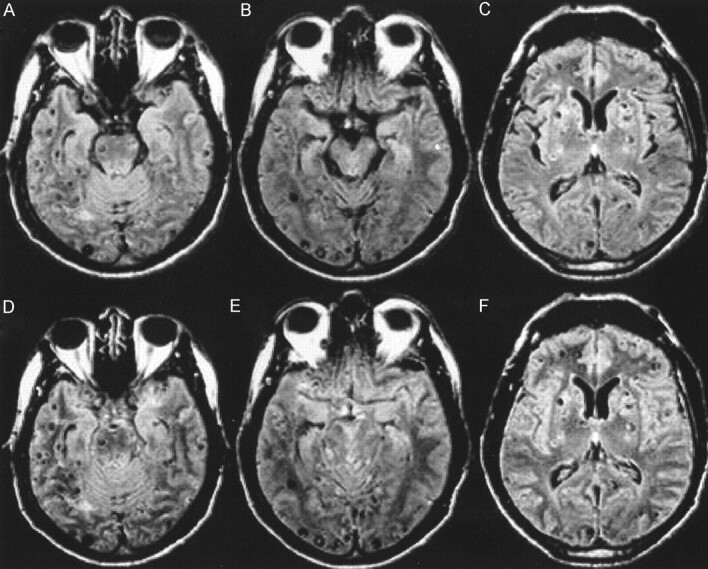
A 34-year-old male patient with known neurocysticercosis.
A, B, and C, FLAIR images show multiple vesicular lesions in the parenchyma, mainly along the cortical surfaces. The perimesencephalic cisterns are prominent and contain hyperintense foci that could represent the expected flow artifact or cystic lesions with scoleces (B).
D, E, and F, FLAIR after 100% O2 shows increased signal intensity in the sulci and basal cisterns, allowing the visualization of a greater number of cortical lesions in the frontal, temporal, and occipital lobes. Also, the increased signal intensity of the CSF confirms that there is no cystic lesion in the perimesencephalic cisterns (E).
The development of hyperintensity in the CSF after the administration of O2 is likely due to an increase in the concentration of oxyhemoglobin in the blood, with a paramagnetic effect in the CSF (5, 11). We did not observe an improvement in the detection of intraventricular lesions with this method. The increase of signal intensity of the CSF is less pronounced or even absent within the ventricles when compared with the cortical sulci, fissures, and cisterns probably because of the combination of a less prominent vasculature along the ventricular walls and the dilution of oxyhemoglobin in the ventricles (5).
The evaluation of other types of lesions may also benefit from this technique. For instance, diffusion-weighted images allow the diagnosis of epidermoid cysts but are not able to delineate the contours of an arachnoid cyst, which would be possible with MR cisternography. Another theoretical application would be the better demonstration of pathologic superficial vessels, such as an arteriovenous malformation, or suspected superficial cortical lesions.
Conclusion
Noninvasive MR cisternography is a new, easy, low-cost technique that can be used in the evaluation of cortical and extraventricular subarachnoid lesions, such as those of neurocysticercosis.
References
- 1.Zeng Q, Xiong L, Jinkins JR, et al. Intrathecal gadolinium-enhanced MR myelography and cisternography: a pilot study in human patients. AJR Am J Roentgenol 1999;173:1109–1115 [DOI] [PubMed] [Google Scholar]
- 2.Jinkins JR, Rudwan M, Krumina G, Tali ET. Intrathecal gadolinium-enhanced MR cisternography in the evaluation of clinically suspected cerebrospinal fluid rhinorrhea in humans: early experience. Radiology 2002;222:555–559 [DOI] [PubMed] [Google Scholar]
- 3.Tali ET, Ercan N, Krumina G, et al. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol 2002;37:152–159 [DOI] [PubMed] [Google Scholar]
- 4.Braga FT, Hernandez Filho G, Rocha AJ, et al. Relationship between the concentration of supplemental oxygen and signal intensity of the cerebral spinal fluid on FLAIR MR imaging. AJNR Am J Neuroradiol 2003;24:1863–1868 [PMC free article] [PubMed] [Google Scholar]
- 5.Deliganis AV, Fisher DJ, Lam AM, Maravilla KR. Cerebrospinal fluid signal intensity increase on FLAIR MR images in patients under general anesthesia: the role of supplemental O2. Radiology 2001;218:152–156 [DOI] [PubMed] [Google Scholar]
- 6.Frigon C, Jardine DS, Weinberger E, et al. Fraction of inspired oxygen in relation to cerebrospinal fluid hyperintensity on FLAIR MR imaging of the brain in children and young adults undergoing anesthesia. AJR Am J Roentgenol 2002;179:791–796 [DOI] [PubMed] [Google Scholar]
- 7.Rocha MS, Brucki SM, Ferraz AC, et al. [Cerebrovascular disease and neurocysticercosis]. Arq Neuropsiquiatr 2001;59:778–783 [In Portuguese] [PubMed] [Google Scholar]
- 8.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis 2002;2:751–762 [DOI] [PubMed] [Google Scholar]
- 9.Proaño JV, Madrazo I, Avelar F, et al. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med 2001;345:879–885 [DOI] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001;57:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippi CG, Ulug AM, Lin D, et al. Hyperintense signal abnormality in subarachnoid spaces and basal cisterns on MR images of children anesthetized with propofol: new fluid-attenuated inversion recovery finding. AJNR Am J Neuroradiol 2001;22:394–399 [PMC free article] [PubMed] [Google Scholar]