Abstract
BACKGROUND AND PURPOSE: To develop a technique for site-specific placement of a thrombus of predetermined volume in an animal model for the purpose of evaluating methods of intravascular thrombolysis and clot retrieval.
METHODS: Six swine were subjected to thrombus injection bilaterally in the ascending pharyngeal artery (APA). Each animal underwent transfemoral angiography while under general anesthesia. A nondetachable balloon catheter and a 3-French microcatheter were then advanced into the common carotid artery through a 7-French guide catheter. With the microcatheter in the proximal APA and the balloon inflated proximally, a bolus of preformed thrombus composed of 0.9 mL of autologous blood and 0.1 mL of bovine thrombin (200 IU/mL) was injected through the microcatheter while local flow arrest was maintained for 15 min. The balloon was deflated and removed. The occluded arteries were observed by serial angiography for 3 hr and then resected for gross examination and hematoxylin and eosin staining.
RESULTS: Each APA was occluded angiographically and did not recanalize during the 3-hr observation period. Persistent, proximal progression of thrombus to the superior thyroid artery origin occurred in three animals. Gross inspection revealed that the resected arteries contained thrombus in the proximal APA but not in the common carotid artery. Histologic examination revealed organized thrombus, without evidence of intimal injury.
CONCLUSION: Our model provides a simple, reliable method for site-specific injection of a thrombus of predetermined volume. Site-specific placement is important for evaluation of the efficacy of thrombolytic agents and techniques. Angiographic evidence of brain revascularization can be used to grade revascularization and clot volume. The ability to specifically localize and estimate clot volume makes our model well suited for the evaluation and comparison of thrombolytic agents and endovascular techniques.
Removal of vascular occlusive thrombus is an effective method for the treatment of cerebral ischemia. Thrombolysis with IV administered tissue plasminogen activator (tPA) is currently the only proved therapy for acute stroke (1). Many authors have proposed that site-specific therapy may result in higher rates of recanalization yet lower rates of systemic complications (2–6). To this end, it becomes necessary to develop a model of site-specific thromboembolism as a means of testing emerging techniques for endovascular therapy. Any such model must be developed in a system large enough to accommodate endovascular devices and must allow for targeted vessel occlusion to permit grading of the response to treatment. We have developed an animal model of thromboembolism in which thrombus is preformed and then introduced in a site-directed manner. This model yields highly reproducible results.
Methods
Animal Care
All procedures were conducted according to the ethical guidelines of the State University of New York at Buffalo Animal Care and Use Committee. Six swine (each weighing between 25 and 30 kg) were used in our study. Animals were housed in the University at Buffalo Laboratory Animal Facility and given free access to food and water until the night before angiography and surgery, when food was withheld. The animals received Telazol (Parke-Davis, Morris Plains, NJ) for sedation. After endotracheal intubation, general anesthesia was induced with Telazol and was maintained with 1.5% to 2% isoflurane throughout all procedures. After arterial perfusion and fixation, the animals were euthanized with an overdose of pentobarbital (100 mg/kg) before tissue removal.
Angiography
Each animal underwent transfemoral angiography while under general anesthesia. A 9-French femoral artery sheath was placed percutaneously after induction of anesthesia. The sheath was continuously flushed with heparinized normal saline (1 U/mL). A 7-French guide catheter was advanced over a guidewire into the common carotid artery. Digital subtraction angiography of the cervical carotid vasculature was performed in anteroposterior, lateral, and contralateral oblique positions to visualize the origin of the ascending pharyngeal artery (APA). The diameter of the APA was measured after calibrating to a washer placed on the neck of the swine. From the oblique position, a roadmap was prepared to guide microcatheter and balloon catheter placement.
Follow-up angiography was performed through the same guide catheter 15 min and 1, 2, and 3 hr after clot placement. Additional angiography of the contralateral common carotid artery permitted assessment of distal extension of the clot via retrograde filling of the distal APA through cerebral anastomoses.
Clot Preparation
Thrombus was prepared for infusion by mixing a predetermined volume of autologous blood with bovine thrombin (200 IU/mL) in a blood-to-thrombin ratio of 9:1. The volume of clot required to occlude a 1-cm segment of the APA was determined by calculating the cross-sectional area of the artery and the volume of 1 cm of that artery. That volume was doubled to allow for serum separation during extracorporeal clot formation. The volume of dead space within the microcatheter to be used for clot infusion was added to that calculation to determine the final volume of the blood and thrombin mixture required for infusion.
After calculating the required volume of blood and thrombin, autologous blood was drawn from the femoral artery sheath into a plastic syringe previously filled with the required volume of thrombin. The syringe was left standing for 15 min to permit clot formation before injection.
Clot Injection
During extracorporeal clot formation, a 0.85-inch nondetachable silicone balloon (NDSB) catheter (Target Therapeutics, Fremont, CA) and a 3-French microcatheter (Rapid Transit; Cordis, Miami Lakes, FL) were advanced side-by-side through the guide catheter into the common carotid artery. Under roadmap guidance, the microcatheter was advanced over a 0.014-inch guidewire (Transcend, Cordis) into the APA, just distal to the origin of the superior thyroid artery. The NDSB was then positioned within the common carotid artery, over the origin of the APA (Fig 1).
Fig 1.
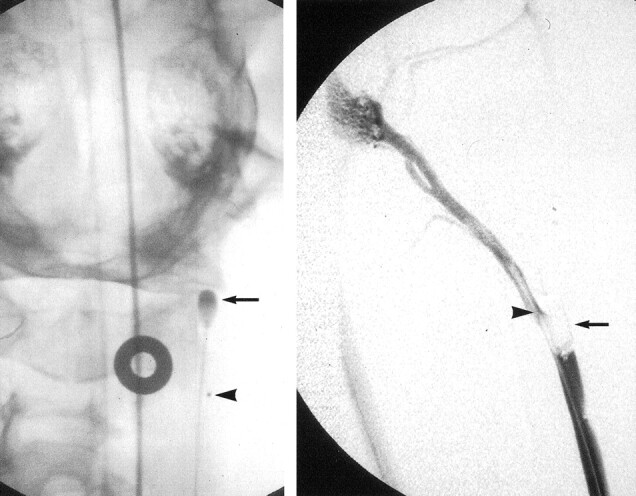
Nondetachable silicone balloon catheter is positioned within the common carotid artery, over the origin of the APA.
Left, Balloon catheter (arrow) and microcatheter (arrowhead) positioning for clot injection seen on anteroposterior projection. The catheters are advanced side-by-side to the level of the origin of the APA. (The APA is the anatomic correlate of the internal carotid artery in humans.)
Right, Microcatheter (arrowhead) is then advanced into proximal APA, just distal to origin of superior thyroid artery. The balloon (arrow) is then inflated within the carotid artery to occlude the origin of the APA.
After inflation of the NDSB, angiography performed through the guide catheter confirmed occlusion of the carotid artery at the level of the APA origin. The preformed clot was then injected through the microcatheter, which was subsequently removed without deflating the NDSB. After 15 min of occlusion, the NDSB was deflated and removed and persistent occlusion of the APA was documented angiographically. Serial follow-up angiograms were obtained, as described above. During each session of angiography, the presence or absence of distal flow and the presence or absence of filling of the superior thyroid artery proximal to clot placement were recorded.
Tissue Removal and Preparation
The guide catheter used for follow-up angiography was positioned proximal to the thrombosed lesion. The common carotid artery was perfused with a fixative solution (10% formalin with 25% glutaraldehyde) for 15 min. An incision was made at the midcervical region, and a segment of the common carotid artery that included the proximal APA was resected. The resected specimen was stored in formalin and then cut for hematoxylin and eosin staining and for gross examination.
Results
Angiographic Results
The radius of the APA in each animal was approximately 2 mm, with a typical cross-sectional area of 0.125 cm2. Thus, an estimated prepared volume of 0.25 mL would be necessary to allow for serum separation and to form a 1-cm-long occlusion. Angiography performed immediately after clot injection confirmed occlusion of the APA in all 12 injected arteries. Angiography performed during the observation period confirmed persistence of APA occlusion in each case (Fig 2). In five arteries, the clot initially placed distal to the origin of the superior thyroid artery progressed proximally to occlude the superior thyroid artery at 1 hr. Recanalization to the origin of the superior thyroid artery was observed in three of the arteries, whereas in the other two, the superior thyroid artery remained occluded at 3 hr. The APA remained occluded at 3 hr in every case.
Fig 2.
Follow-up digital subtraction anterolateral projection angiograms obtained after clot injection.
Left, APA is occluded just distal to origin of superior thyroid artery (arrow).
Center, One hour later, clot has progressed proximally to occlude superior thyroid artery.
Right, Later follow-up image obtained 2 hr after clot insertion shows that STA has recanalized (arrow) but APA remains occluded at clot insertion site.
Pathologic Results
Hematoxylin and eosin staining confirmed the presence of solid, organized thrombus in each specimen (Fig 3). The endothelium was intact in each specimen, without evidence of intimal dissection, intramural thrombus, or vessel rupture.
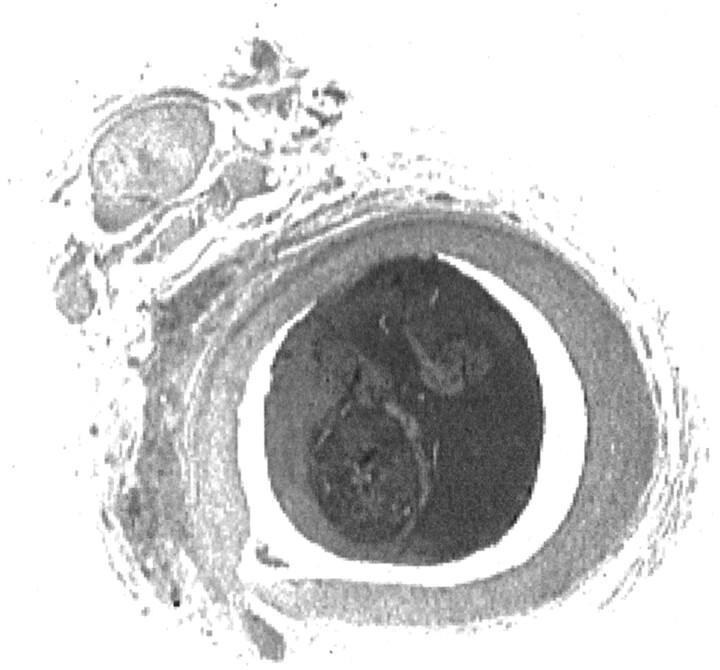
Fig 3.
Cross section of ascending pharyngeal artery obtained 3 hr after clot injection seen at ×15 magnification. Hematoxylin and eosin staining reveals an intact endothelium, with no evidence of vessel wall disruption or subintimal hemorrhage. Solid thrombus fills the vessel lumen.
Gross evaluation of the intraluminal clot revealed solid, thrombin-rich thrombus. The clot filled the vessel lumen entirely. No evidence of recanalization was observed.
Discussion
Acute thrombolytic therapy for stroke is a rapidly developing field. Evidence of clinical benefit associated with IV administered thrombolytic therapy (1) has prompted the investigation of several other techniques for acute revascularization, including local infusion of thrombolytic agents, performance of angioplasty, and use of clot retrieval devices (2, 3, 7, 8). Endovascular revascularization may offer an effective method for local therapy to improve the efficacy of clot lysis while reducing systemic exposure to thrombolytic agents (5, 6). Before any new therapies or devices can be introduced for clinical use, their safety and potential efficacy must be evaluated in the laboratory setting.
Appropriate evaluation of a clot retrieval device or new thrombolytic therapy necessitates a reliable model for thromboembolism in an appropriate vascular distribution. The ideal characteristics of such a model include reliable, site-directed deposition of thrombus of a consistent size and a vascular system that reproduces human hemodynamics, permits angiographic and histologic evaluation, and has sufficiently sized channels for endovascular navigation. Several animal models that use autologous blood for thrombus formation result in consistent, reliable vascular occlusion (9–13). These models typically require exposure of autologous blood to bovine thrombin, either through direct mixing or by drawing blood through tubing primed with thrombin. These techniques reliably produce thrombin-rich blood clots for injection into the target vascular bed. These models, however, use small mammals with vessels that are not conducive to endovascular device navigation. Models described for use in larger animals involve the injection of autologous clot without precise localization of the embolus (14–16). Although this type of model may be useful for evaluation of systemic drug administration, it does not permit reliable evaluation of therapies for local revascularization by using standard grading of revascularization.
Our model uses a vascular system that permits the introduction of catheters and devices intended for human use. The average diameter of the APA in the swine used for our study was comparable with that of the human middle cerebral artery, which is a common location for thrombolytic therapy. Therefore, the vessel diameter in our model is appropriate for the investigation of interventional devices. This model can also be used in similar-sized animals for interventional investigation.
Precise localization of the thrombus may be as important as accessibility with interventional devices. Depositing the thrombus in a predetermined location permits accurate measurement for clot burden reduction and grading of revascularization. Before developing our current model, we discovered two key elements that permit precise clot placement. The first is proximal flow reduction. When thrombus infusion was performed under normal flow conditions, even through large catheters, random distal embolization and frequent fragmentation of thrombus occurred. The result was the inability to precisely identify the location of the thrombus for directed treatment. Our first attempt to eliminate distal embolization revealed the second key element, avoidance of clot disturbance immediately after placement. We began by using distal vascular balloon occlusion during clot injection. Although this technique afforded control of the destination of the clot, attempts to withdraw the balloon after deflation resulted in fragmentation and distal embolization of the thrombus. Similarly, proximal occlusion during common carotid artery clot injection failed because of the high backflow from external carotid branches. Ultimately, we found success with proximal occlusion in the lower flow system ofthe APA.
Conclusion
Ascending pharyngeal artery occlusion under proximal flow arrest in the swine is a highly reproducible model for site-specific thromboembolism in a vascular system that is well suited for the introduction of interventional devices. The use of autologous blood maintains coagulation physiology and vascular response. The anatomy of the cerebral circulation in the swine permits some estimation of clot burden. We think that this model will be very useful in testing new devices designed for interventional therapy of stroke.
References
- 1.[No authors listed]. Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP. Recanalization therapies for acute ischemic stroke. Semin Neurol 1998;18:471–484 [DOI] [PubMed] [Google Scholar]
- 3.Barnwell SL, Clark WM, Nguyen TT, O’ Neill O, Wynn ML, Coull BM. Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. AJNR Am J Neuroradiol 1994;15:1817–1822 [PMC free article] [PubMed] [Google Scholar]
- 4.Barr JD, Mathis JM, Wildenhain SL, Wechsler L, Jungreis CA, Horton JA. Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol 1994;5:705–713 [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke: PROACT Investigators: prolyse in acute cerebral thromboembolism. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II Study: a randomized controlled trial: prolyse in acute cerebral thromboembolism. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 7.Nesbit GM, Clark WM, O’ Neill O, Barnwell SL. Intracranial intraarterial thrombolysis facilitated by microcatheter navigation through an occluded cervical internal carotid artery. J Neurosurg 1996;84:387–392 [DOI] [PubMed] [Google Scholar]
- 8.Ringer AJ, Qureshi AI, Fessler RD, Guterman LR, Hopkins LN. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery 2001;48:1282–1290 [DOI] [PubMed] [Google Scholar]
- 9.Busch E, Kruger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res 1997;778:16–24 [DOI] [PubMed] [Google Scholar]
- 10.Kietthubthew S, Kisanuki A, Asada Y, Marutsuka K, Funahara Y, Sumiyoshi A. Pulmonary microthromboembolism by injection of sonicated autologous blood in rabbits with splenic artery ligations. Southeast Asian J Trop Med Public Healthl 1997;28[suppl 3]:138–140 [PubMed] [Google Scholar]
- 11.Overgaard K, Sereghy T, Boysen G, Pedersen H, Hoyer S, Diemer NH. A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. J Cereb Blood Flow Metab 1992;12:484–490 [DOI] [PubMed] [Google Scholar]
- 12.Shuaib A, Yang Y, Siddiqui MM, Kalra J. Intraarterial urokinase produces significant attenuation of infarction volume in an embolic focal ischemia model. Exp Neurol 1998;154:330–335 [DOI] [PubMed] [Google Scholar]
- 13.Krueger K, Busch E. Protocol of a thromboembolic stroke model in the rat: review of the experimental procedure and comparison of models. Invest Radiol 2002;37:600–608 [DOI] [PubMed] [Google Scholar]
- 14.Brown WR, Moody DM, Stump DA, Deal DD, Anderson RL. Dog model for cerebrovascular studies of the proximal-to-distal distribution of sequentially injected emboli. Microvasc Res 1995;50:105–112 [DOI] [PubMed] [Google Scholar]
- 15.Gross DR. Thromboembolic phenomena and the use of the pig as an appropriate animal model for research on cardiovascular devices. Int J Artif Organs 1997;20:195–203 [PubMed] [Google Scholar]
- 16.Kito G, Nishimura A, Susumu T, et al. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods 2001;105:45–53 [DOI] [PubMed] [Google Scholar]