Abstract
Summary: Treatment of dissecting pseudoaneurysms of the distal cervical internal carotid artery with preservation of the parent artery by using stents or coils has become routine. Tortuosity remains a significant obstacle to successful endovascular treatment in some cases. We report the use of a stent-coil technique to treat a nonhealing dissecting pseudoaneurysm and associated stenosis with anatomic preservation of a redundant loop involving the stented arterial segment. This was accomplished by using a Neuroform dedicated intracranial stent.
Spontaneous dissections of the cervical internal carotid artery may heal incompletely, leading to a persistent dissecting aneurysm in as many as one third of cases or hemodynamically significant stenosis in less that 10% of cases (1). Spontaneous resolution of these late sequelae is rare (2). Endovascular treatment of these lesions with stents or coils or both has emerged as a viable treatment option (3–7). Tortuosity of the cervical internal carotid artery is common, with an increased propensity for dissections to occur on redundant loops of the vessel (8). Endovascular treatment in these cases remains difficult with existing self-expanding and balloon-expandable stent technology secondary to relative inflexibility of the delivery apparatus or of the stent itself after deployment. Development of more flexible stents and delivery systems affords opportunity for successful endovascular treatment of these difficult lesions, as demonstrated by our case.
Case Report
A 34-year-old woman with no notable medical history had lightheadedness, left visual blurring, and left neck pain while jogging. She subsequently experienced left hemispheric transient ischemic attacks consisting of right-hand numbness. An imaging workup included MR angiography, which suggested occlusion of the left internal carotid artery. Cerebral angiography confirmed occlusion of the cervical left internal carotid artery with a flame-shaped configuration to the occlusion, suggesting dissection (Fig 1A). Warfarin was administered, and the patient’s ischemic symptoms resolved. A Xenon-133 cerebral blood flow study demonstrated diminished perfusion in the left hemisphere with poor augmentation after Diamox. Extracranial-to-intracranial bypass was considered, but repeat angiography demonstrated recanalization of the dissected left internal carotid artery (Fig 2A). Follow-up CT angiography after 3 months of anticoagulant therapy demonstrated a pseudoaneurysm of the distal cervical left internal carotid artery. Confirmatory angiography documented the presence of this pseudoaneurysm as well as a significant stenosis, measuring approximately 90%, just distal to the pseudoaneurysm (Fig 2B). Because of the configuration of the pseudoaneurysm and complicating stenosis on a redundant loop of the high cervical internal carotid artery, carotid sacrifice was contemplated; however, a trial balloon occlusion test produced reversible perfusion defects in the left frontal lobe. The patient then received anticoagulants for several more months until a highly flexible intracranial nitinol stent (Neuroform, Target/BSC/Smart therapeutics, Fremont, CA) became commercially available. She remained neurologically asymptomatic, except for generalized fatigue and occasional headaches. Informed consent for angioplasty and stent placement, as well as coil embolization of the pseudoaneurysm, was obtained. In addition, consent was obtained to receive treatment of her aneurysm with the Neuroform stent through our institutional review board–approved protocol. Three days before her procedure, dual antiplatelet therapy was instituted with clopidogrel, 75 mg per day, and aspirin, 650 mg per day. Warfarin was stopped after the institution of subcutaneous enoxoparin therapy in anticipation of endovascular intervention.
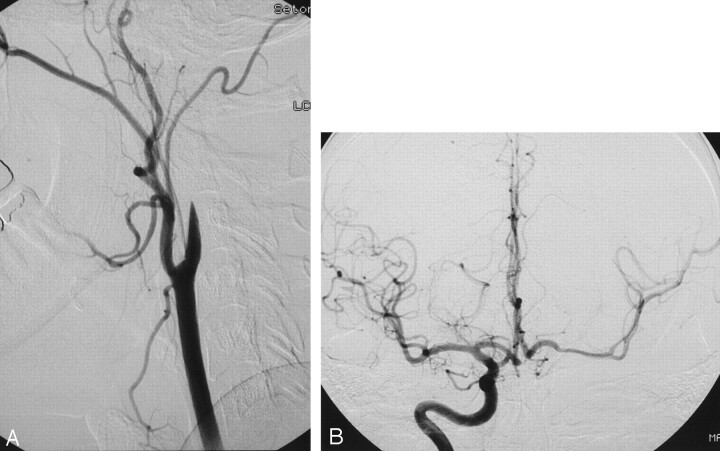
Fig 1.
A 34-year-old woman presenting with neck pain and right arm numbness after jogging. A lateral view from an acute left common carotid angiogram (A) demonstrates flame-shaped occlusion of the proximal left internal carotid artery consistent with acute dissection. An anteroposterior intracranial view from an acute right internal carotid angiogram (B) shows good cross-filling through the anterior communicating artery.
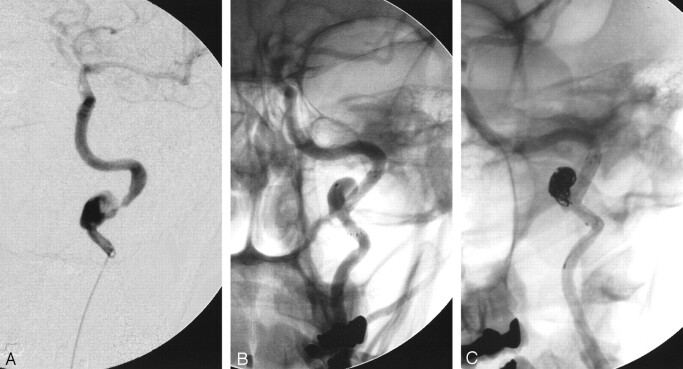
Fig 2.
After transfer to consider extracranial-intracranial bypass, a repeat left common carotid angiogram 1 week after initial presentation (A) shows recanalization of the left internal carotid artery with slow flow into the intracranial circulation and dissection of the distal cervical internal carotid artery. A follow-up left common carotid angiogram (B) obtained to confirm a pseudoaneurysm suspected because of CT angiography findings shows a dissecting pseudoaneurysm of the distal cervical internal carotid artery situated on a redundant loop of vessel with an associated significant stenosis.
Her procedure was performed under conscious sedation administered by our anesthesia team. Standard groin access allowed positioning of a 7F-long sheath (Brite-tip, Cordis Endovascular, Miami Lakes, FL) in the distal cervical left common carotid artery. The patient was systemically anticoagulated with intravenous heparin to maintain an activated clotting time of approximately 260 seconds. Baseline left common carotid angiograms were then obtained, including a rotational angiogram, and detailed measurements of the arterial width were obtained. We measured the petrous internal carotid artery at approximately 3.1–3.2 mm and the more proximal cervical internal carotid artery at approximately 3.6 mm. The pseudoaneurysm measured approximately 10 mm × 6 mm in long and short dimension. A preshaped Prowler 14 microcatheter (Cordis Neurovascular) and a Synchro wire (Target/BSC) were used to cross the neck of the pseudoaneurysm and negotiate the significant stenosis of the distal cervical internal carotid artery. Some difficulty was encountered in accessing the stenotic segment secondary to an acute angle and the severe stenosis. Ultimately, we achieved access beyond the stenosis and positioned the Prowler 14 microcatheter in the petrous segment of the internal carotid artery. Contrast medium injection then documented positioning of this catheter within the true lumen. We then positioned the 300-cm Transcend exchange wire with its tip in the cavernous segment of the internal carotid artery and withdrew the Prowler microcatheter. The stent delivery apparatus for a 3.5 × 13-mm Bx Velocity stent (Cordis cardiovascular) was then advanced across the level of stenosis of the distal cervical internal carotid artery by using road mapping technique. We then deployed this stent with balloon inflation to approximately 10 atmospheres (nominal pressure) for approximately 5–6 seconds. This was deflated after witnessing full expansion of the stent. The balloon was withdrawn, and contrast medium injection showed patency with improved caliber of the lumen at the level of the previous stenosis. The Neuroform stent delivery apparatus was advanced over the Transcend 14 wire and, with some difficulty, was advanced in a coaxial fashion through the proximal end of the velocity stent. We then deployed the 4 × 15-mm Neuroform stent in a telescoping fashion within the proximal two thirds of the Velocity stent and extending proximally across the pseudoaneurysm with its proximal aspect along a straight segment of the internal carotid artery. This provided good coverage of the neck of the pseudoaneurysm, with anatomic preservation of the redundant loop of the internal carotid artery (Fig 3B).
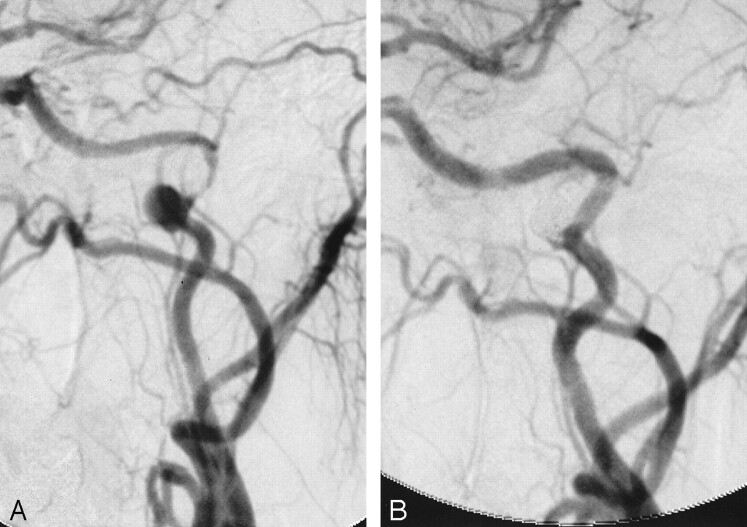
Fig 3.
Endovascular treatment was performed approximately 8 months after the patient’s initial diagnosis. She was treated with anticoagulant therapy for the entire period. A selective microcatheter injection of the left internal carotid artery (A) demonstrates the pseudoaneurysm and associated stenosis. An unsubtracted left internal carotid angiogram after stent placement but before coil placement (B) shows anatomic correction of the stenosis with the Velocity stent and neck bridging of the pseudoaneurysm with preservation of the redundant loop by using the Neuroform stent. A microcatheter is seen within the aneurysm. An unsubtracted left internal carotid angiogram after coiling (C) shows near-complete obliteration of the pseudoaneurysm sac with endovascular coils. The last coil placed into the aneurysm remains attached in the image.
We then used the preshaped Prowler 14 microcatheter and Synchro wire to achieve catheterization of the pseudoaneurysm. We attempted to advance an 8 × 30 T18 Guglielmi detachable coil (Target/BSC) into the pseudoaneurysm, but the entire length of the coil could not be advanced without backing the microcatheter out into the lumen of the internal carotid artery. This coil was withdrawn, and ultimately the first coil placed was a 7 × 30 T10 standard coil. Multiple additional coils of diminishing size were then placed into the pseudoaneurysm until we began to note that the catheter was backing out into the lumen of the internal carotid artery . The last 5-mm coil was placed without difficulty and detached. Because there was only trivial filling of the base of the pseudoaneurysm, we elected to stop at this point. Baseline postembolization angiograms of the head and neck were then obtained, which documented no evidence of intracerebral emboli. Figure 4 shows her pre- and post-treatment angiograms. Her neurologic examination remained unchanged. We performed right-groin angiography to assess the sheath entry site and then used a duet thrombin collagen plug to achieve hemostasis. The patient did well after the procedure, with no new neurologic deficit. Her heparin anticoagulation was continued overnight and was stopped the following morning. She returned home the day after her procedure, receiving dual antiplatelet therapy with clopidogrel and aspirin.
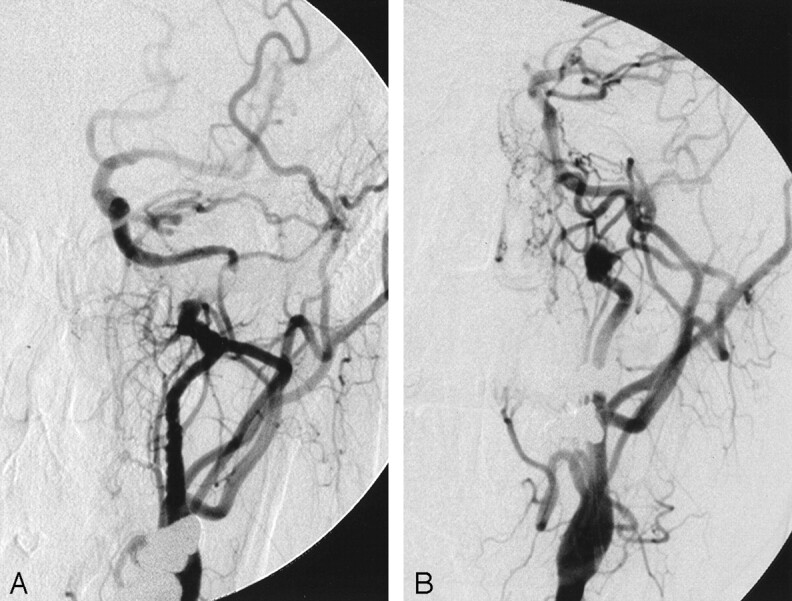
Fig 4.
Single-frame images from left common carotid rotational angiography before (A) and after (B) endovascular treatment demonstrate correction of the arterial stenosis, near-complete obliteration of the dissecting pseudoaneurysm by coils, and anatomic preservation of the distal cervical redundant loop of the internal carotid artery.
Follow-up
The patient had no symptoms to suggest left hemispheric or ocular ischemia during 6 weeks of clinical follow-up. She stated that her energy level had improved since treatment. As has been our practice, clopidogrel was stopped after 6 weeks, presuming a significant degree of endothelialization of the stents. Aspirin therapy at 325 mg per day will be continued indefinitely. Close follow-up angiography is planned to evaluate the neck remnant.
Discussion
Spontaneous dissection of the cervical internal carotid artery occurs with an incidence of 2.5–3 per 100,000 population. Although accounting for only approximately 2% of ischemic strokes overall, dissections are important causes of stroke in young or middle-aged individuals, accounting for 10–25% of cases. Risk factors include heritable connective-tissue disorders, significant or relatively minor trauma with extension or rotation of the neck, recent respiratory infection, and perhaps migraine (1). Spontaneous dissection is associated with angiographic changes suggesting fibromuscular dysplasia in 15% of patients, and there seems to be an association with arterial redundancy as well (8). Because most spontaneous dissections heal completely, most practitioners advocate medical therapy with anticoagulants or antiplatelet agents to prevent thromboembolic stroke until healing is documented. More aggressive surgical or endovascular treatment options are reserved for patients who fail to respond to medical treatment or for nonhealing sequelae such as hemodynamically significant stenoses or dissecting pseudoaneurysms.
The long-term natural history of persistent stenoses and pseudoaneurysms following spontaneous carotid dissection has been studied in small case series. One recent prospective observational study of 46 patients with persistent stenoses and occlusions after dissection reported a stroke risk of 0.7% per year for ipsilateral events and 1.4% per year for any stroke while receiving antithrombotic or anticoagulant therapy (9). Although this stroke rate is low, it is approximately double the 0.3% per year (ipsilateral) and 0.6% per year (any stroke) stroke rates reported in the same study for transient stenoses or occlusions after dissection. A retrospective review of 71 patients with cervical arterial dissection (10) reported long-term follow-up on 35 patients with 42 aneurysms, 33 of which were internal carotid aneurysms. During a mean clinical follow-up period of 41.6 months, no patients had transient ischemic attacks, symptoms of stroke, or aneurysm rupture. Most were treated with antiplatelet or anticoagulant therapy. Follow-up imaging showed pseudoaneurysm resolution in 25% of cases, decrease in size in 15% of cases, and no change in 60% of cases. These studies suggest a relatively benign natural history for these sequelae of carotid dissection, which should be taken into account when contemplating more risky surgical or endovascular treatment alternatives to standard antithrombotic or anticoagulant medical treatment. In our case, we perceived the long-term stroke risk from the combination of stenosis and pseudoaneurysm to be higher than either lesion alone, particularly in this young woman, who would be relatively intolerant of carotid occlusion based on her blood-flow studies. This perceived higher risk provided our rationale for treatment.
Many treatment options have been pursued involving deconstructive and reconstructive surgical and endovascular techniques (3–7, 11). Carotid sacrifice with trapping of the aneurysm, either surgically or by endovascular means by using detachable balloons or coils, is an effective treatment but is possible in only a limited population of patients with adequate collateral circulation without concomitant bypass. Surgical reconstructive techniques are also effective, but they are difficult for lesions near the skull base, a common location for dissecting pseudoaneurysms. Also, cranial nerve deficits are not uncommon, occurring in 11 of 25 cases in one surgical series (11). There are many reports of successful endovascular treatment of cervical dissecting pseudoaneurysms by using bare, covered, or overlapping stents with or without secondary coil embolization of the pseudoaneurysm sac (3–7, 12, 13). These reports suggest that endovascular repair can be accomplished with preservation of anatomic flow in the parent vessel and durable occlusion of the pseudoaneurysm sac.
It is our practice to offer endovascular treatment to all symptomatic and occasional asymptomatic individuals who harbor nonhealing dissecting pseudoaneurysms or persistent significant stenoses of the cervical internal carotid artery to reduce their stroke risk and to eliminate the need for chronic anticoagulation. We typically treat these lesions with primary bare stent placement, which often induces flow stagnation within the aneurysm sac and leads to immediate or delayed thrombotic occlusion. We reserve coil embolization for cases in which there is minimal change in the appearance of the pseudoaneurysm after stent placement or persistent pseudoaneurysm filling on follow-up examination. Arterial tortuosity has presented an obstacle to successful endovascular treatment in several of our referral cases. Placement of existing self-expandable stents around tortuous anatomy can be difficult to accomplish because of the relative inflexibility of the delivery apparatus. Also, whereas most current-generation balloon-expandable stent delivery systems are flexible enough to negotiate distal cervical loops, the stents themselves are relatively rigid after deployment, raising concern for complications such as dissection or late intimal hyperplasia when deployed in tortuous, mobile arterial segments. The Neuroform is a nitinol, self-expandable stent that is delivered through a 3F microcatheter. It gained U.S. Food and Drug Administration approval for use with intracranial aneurysms through a humanitarian use exemption. Its 35-μ strut thickness is significantly less than that of other approved stents. This, coupled with its open-cell design, allows for significant flexibility and microcatheter delivery. These characteristics allowed successful endovascular treatment in this patient in whom preservation of the native arterial redundancy in the distal cervical internal carotid artery included in the stented segment was accomplished. Further clinical and angiographic follow-up is needed to assess the long-term efficacy and durability of this approach.
Conclusion
Our case demonstrates that advancing stent technology now potentially allows parent vessel preserving endovascular treatment of difficult distal cervical internal carotid dissecting false aneurysms associated with cervical loops and tortuous anatomy.
References
- 1.Schievink WI, Wouter I. Current concepts: spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001;344:898–906 [DOI] [PubMed] [Google Scholar]
- 2.Guillon B, Brunerau L, Biousse V, et al. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology 1999;53:117–122 [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M, Miller G, Meyer Y, et al. Use of intravascular stents in the treatment of internal carotid and extracranial vertebral artery pseudoaneurysms. AJNR Am J Neuroradiol 1996;17:693–696 [PMC free article] [PubMed] [Google Scholar]
- 4.Malek A, Higashida R, Phatouros C, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol 2000;21:1280–1292 [PMC free article] [PubMed] [Google Scholar]
- 5.Marks MP, Dake MD, Steinberg GK, et al. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology 1994;191:441–446 [DOI] [PubMed] [Google Scholar]
- 6.Hong MK, Satler LF, Gallino R, Leon MB. Intravascular stenting as a definitive treatment of spontaneous carotid artery dissection. Am J Cardiol 1997;79:538. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura JH, Rosenthal D, Jerius H, et al. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent. J Endovasc Surg 1997;4:339–343 [DOI] [PubMed] [Google Scholar]
- 8.Barbour PJ, Castaldo JE, Rae-Grant AD, et al. Internal carotid artery redundancy is significantly associated with dissection. Stroke 1994;25:1201–1206 [DOI] [PubMed] [Google Scholar]
- 9.Kremer C, Mosso M, Georgiadis D, et al. Carotid dissection with permanent and transient occlusion or severe stenosis: long-term outcome. Neurology 2003;60:271–275 [DOI] [PubMed] [Google Scholar]
- 10.Touzé E, Randoux B, Méary E, et al. Aneurysmal forms of cervical artery dissection: associated factors and outcomes. Stroke 2001;32:418–423 [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI, Piepgras DG, McCaffrey TV, Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery 1994;35:809–816 [DOI] [PubMed] [Google Scholar]
- 12.Benndorf G, Campi A, Schneider GH, et al. Overlapping stents for treatment of a dissecting carotid artery aneurysm. J Endovasc Ther 2001;8:566–570 [DOI] [PubMed] [Google Scholar]
- 13.Ellis PK, Kennedy PT, Aires AB, et al. Successful exclusion of a high internal carotid pseudoaneurysm using the Wallgraft endoprosthesis. Cardiovasc Intervent Radiol 2002;25:68–69 [DOI] [PubMed] [Google Scholar]