Abstract
The application of nanoparticles for medical purposes has made enormous strides in providing new solutions to health problems. The observation that plant virus-based nanoparticles (VNPs) can be repurposed and engineered as smart bio-vehicles for targeted drug delivery and imaging has launched extensive research for improving the therapeutic and diagnostic management of various diseases. There is evidence that VNPs are promising high value nanocarriers with potential for translational development. This is mainly due to their unique features, encompassing structural uniformity, ease of manufacture and functionalization by means of expression, chemical biology and self-assembly. While the development pipeline is moving rapidly, with many reports focusing on engineering and manufacturing aspects to tailor the properties and efficacy of VNPs, fewer studies have focused on gaining insights into the nanotoxicity of this novel platform nanotechnology. Herein, we discuss the pharmacology of VNPs as a function of formulation and route of administration. VNPs are reviewed in the context of their application as therapeutic adjuvants or nanocarrier excipients to initiate, enhance, attenuate or impede the formulation’s toxicity. The summary of the data however also underlines the need for meticulous VNP structure-nanotoxicity studies to improve our understanding of their in vivo fates and pharmacological profiles to pave the way for translation of VNP-based formulations into the clinical setting.
Keywords: Plant virus; nanoparticle; drug delivery; imaging; biodistribution, toxicity; biocompatibility; nanomedicine
Graphical Abstract
1. Introduction
Nanotechnology uses advanced manufacturing science and engineering to achieve the synthesis or assembly of materials at the nanoscale range. When compared to bulk materials, the uniqueness of nanometric materials arises from their high surface to volume ratio [1]. Furthermore, from a medical viewpoint, NPs operate at the same size scale as cells do; thus, NPs are ideal for navigating systemic and cellular trafficking, i.e. receptor binding and cell internalization followed by cargo delivery. The application of NPs for medical purposes (i.e. nanomedicine) has made enormous strides in proving new solutions to health problems [1,2]. NPs have gained tremendous considerations as high-value tools for diagnosis, therapy, and regenerative medicine targeting various diseases [3,4]. By achieving facile early diagnosis and/or site-specific therapy, nanomedicine holds the promise of promoting the quality of life and affordable disease management. Previous research efforts in nanotechnology for biomedicine have paid-off. Anselmo and Mitragotri [5] recently reported up to 65 nanoparticles under clinical trials and 29 nanoproducts approved either as therapeutics (23) or as imaging agents (6). Despite these success stories of clinical translation and marketed nano-based products, many nanotechnologies are still at an infant stage [6]. Challenges include the need for a cost-effective scale-up manufacturing processes enabling reproducible fabrication of uniform NPs with constant physicochemical, biological and pharmacological properties, while complying with the safety considerations [7].
Among the types of NPs currently investigated, which mostly encompass synthetic systems such as lipid-based NPs, polymeric and inorganic NPs (metallic and metal oxide NPs), one emerging platform includes viruses or viral vectors as naturally occurring NPs. In comparison with synthetic NPs, virus-based NPs are proteinaceous structures with unique properties, including structural uniformity, ease of manufacture through cell culture, fermentation or plant molecular farming, and high degree of quality control and assurance; their production involves genetically programmed viral replication or expression of coat proteins yielding essentially identical NPs with unprecedented monodispersity [8]. In nanomedicine, viruses have evolved as smart vehicles for targeted delivery due to the virus’ general ability to infiltrate, target, manipulate, and deliver cargoes to specific cells [9]. A successful example of intracellular delivery using a mammalian viral vector is the Adeno-associated virus-based technology. Gene therapies marketed as Glybera®, Luxturna® and Zolgensma® are used for the treatment of lipoprotein lipase deficiency, RPE65 mutation-associated retinal dystrophy and spinal muscular atrophy, respectively [10,11]. However, since the immunogenicity, cytotoxicity, inflammatory reaction and other toxic features of mammalian viral vectors affect the viral technology’s cost, complexity and safety, plant viruses or plant viral nanoparticles (VNPs) have emerged as an alternative platform technology [12]. Apart from the common benefits from their viral nature, plant VNPs offer an additional advantage of being non-infectious to mammalian cells, while being a cost-effective technology with a huge potential for large-scale production [9,12]. Like plant VNPs, bacteriophages also make an intriguing class of nanocarriers [13]; and in fact, several bacteriophage therapies have entered clinical testing [14]. Since the present report does not discuss bacteriophages, we refer the reader to recent reviews covering bacteriophages as a promising platform technology for therapeutic applications [15,16], including drug delivery [17,18] and vaccine development [19,20].
Being amenable to both chemical and genetic modifications, VNPs are being extensively investigated as a template for biomaterial design [21]. A recent review by Eiben et al. [22] discusses various biomedical applications of VNPs from in vitro settings, outside patients such as biosensing and tissue engineering, to in vivo applications, such as their use as tools for prophylaxis, diagnosis and therapy. Another review discusses the use of VNPs as therapeutic reagents or molecular platform technology for drug discovery and delivery research [23]. The methods for cargo encapsulation and tailoring VNPs for drug delivery and imaging applications, also have been reviewed [24,25]. In addition, Hefferon [26] discussed the repurposing of VNPs as cost-effective nano-systems for vaccine expression and epitope presentation, and the associated potential applications have been detailed elsewhere against life-threatening diseases such as cancer [27] and infectious diseases [28]. As much as commendable efforts have been made to provide evidence advocating the potential of VNPs for immunotherapy and targeted delivery of therapeutic and diagnostic agents, it is utmost important to consider thorough characterization of the risks and benefits of VNP-based formulations in disease models [29]. Indeed, the nanosized dimension of NPs being similar to that of biomolecules, the intermolecular interactions following product administration and during particle distribution and clearance are evident, and require special attention for better understanding of the NPs’ risk-benefit trade-offs [30]. The area of VNP nanoengineering for biomedical applications has grown out of its infancy; proof-of-principle has been demonstrated both in vitro and in vivo [21,26,31–35]. Therefore, at this stage, time has come to critically focus on the pharmacology to realize the clinical potential of VNPs nanotechnology. Nikitin et al. [36] reviewed the biosafety of plant viruses in conjunction with human and environmental exposures. However, there was no critical consideration of key parameters that determine the biocompatible or toxic responses to VNPs, when used as therapeutic reagents or nanocarriers for drugs and contrast agents.
In general, NPs toxicity depends upon both the formulation characteristics (i.e. the NP’s physicochemical properties, such as size, morphology and surface chemistry), and pharmacological parameters such as dose, administration route and tissue distribution [37–44]. As such, although native or empty plant viruses (i.e. virus-like particles or VLPs) are generally thought to be biocompatible and biodegradable [36], VNPs acting as nanocarriers for drug delivery and imaging, in particular those targeted to specific tissues, may alter the biodistribution and clearance of the cargos and lead to toxic accumulation or metabolism in the tissues. Therefore, it is crucial to encourage in-depth organ-function assessment to better characterize the risk and benefits of a specific composition of VNP formulation, instead of relying on limited tissue-response studies that evaluate degeneration, apoptosis or necrosis [23].
Herein, we present data from toxicity studies and examine the toxicological relevance of the key parameters that affect the biomedical performance of VNPs as therapeutic adjuvants or nano-vehicles for cargo delivery. The formulation strategies, administration routes and biodistribution profiles of VNPs have been reviewed in effort to demonstrate the need for extensive organ-function studies to enhance their toxicological understanding and safely boost clinical translation.
2. Insights into VNPs formulations
In medical applications, nanomaterials are used as a well characterized in a mixture prepared according to a precise formula or formulation [45]. The nature, composition and properties of NPs determine the functional features of the formulation in biological systems [46]. Viral particles are typically composed of hundreds to thousands protein coat units, which are genetically programmed to self-assemble into a hollow structure for nucleic acid encapsulation [47]. Additional cargo can be appended to their exterior as well as interior surface, and the natural cargo can be replaced with the cargo of interest. In addition to various functionalization strategies; VNPs come in various but distinctive sizes and shapes, and their size and shape can be precisely tailored [33,48]. In this section, we present the fundamental structural composition of VNPs and highlight their molecular and morphological modification strategies to repurpose VNPs for drug delivery and imaging applications.
2.1. Plant VNPs
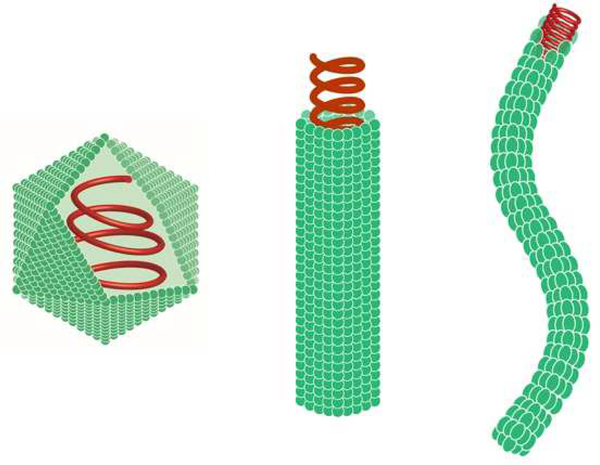
Plant viruses are emerging as naturally occurring therapeutic adjuvants for vaccine applications or protein-based vehicles for drugs and contrast agents. This application involves the use of virions with active or deactivated nucleic acids (denoted herein VNPs or inactivated VNPs) or their capsids deprived of nucleic acids (i.e. virus-like particles or VLP) [49]. In the following we refer to VNPs as this includes the subclass of the VLPs; if important for the design and application or biological affects observed we will detail the specifics for the formulation. Table 1 summarizes the structural characteristics of plant viruses discussed in this report; including Cowpea mosaic virus (CPMV), Cowpea chlorotic mottle virus (CCMV), Hibiscus chlorotic ringspot virus (HCRSV), Johnson grass chlorotic stripe mosaic virus (JgCSMV), Papaya mosaic virus (PapMV), Physalis mottle virus (PhMV), Potato virus X (PVX), Red clover necrotic mosaic virus (RCNMV), Sesbania mosaic virus (SeMV), Tomato bushy stunt virus (TBSV) and Tobacco mosaic virus (TMV). Typical structural morphologies of plant viruses are illustrated in Figure 1.
Table 1:
Summary of typical structural characteristics of plant viruses discussed rn this review
| Plant virus (family) | Protein constitution | Nucleic acid | Capsid shape | Size (nm) | Ref. |
|---|---|---|---|---|---|
| CPMV (Comoviridae) | 60 asymmetrical CPs (24 and 41 kDa subunits) | Two positive sense single stranded RNAs | icosahedral | 30 | [50,51] |
| CCMV (Bromoviridae) | 180 CPs (20.3 kDa) | Three positive sense single stranded RNAs | icosahedral | 28 | [52,53] |
| HCRSV (Tombusviridae) | 180 CPs (37 kDa) | One positive sense single-stranded RNA | icosahedral | 30 | [54] |
| JgCSMV (Tombusviridae) | 180 Cps (41 kDa) | One positive sense single-stranded RNA | icosahedral | 30 | [55] |
| PapMV (Alphaflexiviridae) | 1400 CPs (23.8 kDa) | One positive sense single-stranded RNA | helical, filamentous | 13 × 530 | [56,57] |
| PhMV (Tymoviridae) | 180 CPs (21 kDa) | One positive sense single-stranded RNA | icosahedral | 30 | [58] |
| PVX (Flexiviridae) | 1300 CPs (28 kDa) | One positive sense single linear RNA | helical, filamentous | 13 × 515 | [59] |
| RCNMV (Tombusviridae) | 180 CPs (37 kDa) | Two positive sense single stranded RNAs | icosahedral | 35 | [60,61] |
| SeMV (Solemoviridae) | 180 CPs (29 kDa) | One positive sense single-stranded RNA | icosahedral | 30 | [62,63] |
| TBSV (Tombusviridae) | 180 CPs (41 kDa) | One positive sense single-stranded RNA | icosahedral | 33 | [64,65] |
| TMV (Virgaviridae) | 2130 CPs (18 kDa) | One positive sense single-stranded RNA | helical, rigid | 18 × 300 | [66,67] |
Figure 1:
Overview of plant VNP shapes; from left to right: icosahedron, rigid tubular, and filamentous. Green = coat proteins; red = RNA. Not drawn to scale. This Figure was prepared by Dr. Duc Le (Radbound University, Nijmegen, Netherlands).
2.2. Modification of VNPs
The use of VNPs as nanoscale vehicles for drugs and contrast agents lies on their ability to undergo structural modifications in a predictable way. The engineering of modified VNPs is done genetically, chemically or through self-assembly. The genetic modification (or genetic fusion) is achieved through manipulation of the viral genome to incorporate non-native biological structures (e.g. amino acids, peptides and proteins) in the viral coat protein. This often aims to display targeting ligands [68], antigenic structures [69], or specific amino acids as functional handles enabling further modification [70,71]. The integration of small proteins as tags (e.g. SNAP-tag) for further functionalization is also possible with genetic programming [72,73]. In addition, one shall keep in mind that while often depicted as rigid protein structures, the VNPs are stimuli-responsive NPs that undergo conformational changes in response to pH and salt concentrations in the bathing medium; therefore enabling the opening of pore structures to allow gating and trapping of small molecules [74,75]. For instance, RCNMV achieves cargo encapsulation through infusion following the cycle of stimulus-triggered opening and closure of capsid pores: the removal/chelation of calcium and magnesium induces formation of 11–13 Å pores (allowing cargo diffusion), while re-addition of the aforementioned cations leads to pore closure (leading to cargo entrapment) [61,75]. Taking this a step further, another promising encapsulation approach used for VNPs cargo loading is the self-assembly process (or caging) of purified coat proteins around a target moiety [76,77]. Reconstitution of hydrid VLPs (devoid of their genome but loaded with an artificial cargo) has been achieved using the following targets: therapeutic nucleic acids [78], proteins/enzymes used in therapy or bio-catalysis [79], as well as synthetic nanoparticles for bioimaging [79].
While genetic engineering mostly addresses biologics incorporation, chemical functionalization achieves attachment of both biological and nonbiological structures to VNPs. The attachment of small molecules, such as the chemotherapy doxorubicin, is achieved either covalently or non-covalently, i.e. using electrostatic or π–π stacking interactions [80,81]. Using bioconjugate techniques manifold functional moieties have been conjugated to VNPs; these include macromolecules such as proteins and synthetic polymers as well as small molecules such as oligopeptides, contrast and small molecule drugs [82–84]. Covalent bonding is also commonly used for display of targeting molecules (e.g. folic acid) [85–87], peptide-based targeting ligands (e.g. Asp-Gly-Glu-Ala (DGEA) peptide) [88–90], or proteins, such as antibodies conferring targeting or therapeutic functions [91] or coatings with serum album to act as stealth or shield protecting from immune surveillance [92]. Similarly, synthetic polymers, such as polyethylene glycol (PEG) or polydopamine have been coated onto VNPs either covalently [93] or by coating [94] to extend circulation time and improve biocompatibility (as detailed further in section 3.2).
Because of their proteinaceous nature, VNPs are modified by means of chemical reactions routinely employed in protein derivatization protocols, a.k.a. bioconjugate chemistry [95]. These include classical bioconjugation reactions that address solvent-exposed residues of lysine, cysteine and aspartic/glutamic acid using different chemistries such as N-hydroxysuccinimidyl (NHS) chemistry, Michael addition reaction and carbodiimide activation [96]. Bioorthogonal reactions such as copper-catalyzed azide-alkyne cycloaddition (a.k.a. “click chemistry”) [97,98], azo coupling reaction (diazonium coupling, which involves activated aniline and tyrosine residue) [99], as well as pH-sensitive hydrazone conjugation (or condensation between aldehyde and hydrazide moieties) [100] have also been adapted for VNPs. It is worth mentioning also nitrilotriacetic acid (NTA) complexation, a noncovalent attachment approach, that is used to display a NTA-labelled cargo on viral NPs harbouring a polyhistidine-tag (His-tag) for pH-dependent controlled release [101]. This conjugation strategy takes advantage of the noncovalent bonding/complexation between nickel-NTA and His-tag moieties [102,103].
Lastly, morphological manipulation of VNPs has emerged as a strategy for controlling particle properties [104–106], since protein cage shape is crucial to the effectiveness of VNPs in a given application [48]. The protocols for production of spherical TMV particles from the rod-shaped TMV particles have been established [104,106,107]. The study by Bruckman et al. [93] demonstrated different circulation profiles between spherical and rod-shaped TMV particles, with rods having slightly improved pharmacokinetics vs. their spherical counterparts. Shukla et al. [108] witnessed the impact of particle shape on the in vivo behaviour comparing PVX and CPMV, which are VNPs of filamentous and spherical shapes, respectively. PVX exhibited greater tumor homing and penetration properties compared to CPMV. In another study, TMV rods and icosahedral (sphere-like) CPMV exhibited distinctive diffusion rates in a spheroid model of extracellular matrix [109], and the outcome was consistent with the observation that elongated VNPs show advantageous flow and margination properties [110]. Noteworthy, the field of bionanotechnology has sought to produce a range of elongated high aspect ratio (AR) VNPs such as TMV (size 300 × 18nm; AR 17), PVX (size 515 × 13 nm; AR 40) and grapevine virus A (size 800 × 12 nm; AR 67) [109]; while synthetic engineering affords only carbon nanotubes and filomicelles, also elongated materials but with limited applications due to poor biocompatibility [111] and micron-size regime [112], respectively. Many studies have explored the TMV system as an advanced platform technology for bioengineering of protein-based nanomaterials of different aspect ratios (e.g. AR 2.7–16.5 [112,113] and uncommon shapes (e.g. disks) [115,116] to further interrogate the impact of particle shape on the in vivo performance of NPs (as discussed in depth in section 3.2.1). A recent review from Wege’s group discussed the spatial and structural synthetic biology used to produce proteinaceous nanoobjects of unusual shapes (e.g. branched, disk-like or tubular nano-assemblies), from TMV coat protein and variable RNAs equipped with TMV’s origin of assembly site [117].
In sum, the structural manipulation of VNPs has laid the foundation for turning particle functionalities to supply various applications. Through multiple cargo loading processes, bioengineered VNPs can be tailored for biomedical applications, including targeted delivery of chemotherapy, immunotherapy and gene therapy, as well as bioimaging for disease diagnosis and treatment follow up. Table 2 illustrates the potential applications of VNPs for biomedicine in conjunction with the nature of cargos.
Table 2:
Examples of cargo-loaded and functionalized VNPs developed for biomedical applications
| Application area/Molecule of interest | VNP | Cargo loading strategy | No. of cargo molecules per VNP | Intended application | Ref. |
|---|---|---|---|---|---|
| Drug delivery (chemotherapy) | |||||
| Aldoxorubicin prodrug (cytotoxic anticancer drug) | PhMV | π–π stacking interactions and covalent attachment to internal cysteine through pH-sensitive hydrazone linkages | 1570 | Breast cancer (pH-dependent drug release) | [118] |
| Cisplatin (cytotoxic anticancer drug) | TMV | Electrostatic entrapment | 1900 | Platinum-resistant (PR) ovarian cancer | [119] |
| Doxorubicin (cytotoxic anticancer drug) | RCNMV | Infusion process | 900–1000 | Melanoma and ovarian cancer | [120] |
| Mitoxantrone (anti-neoplastic agent) | CPMV | Infusion process | 20–50 | Primary brain tumors such as glioblastoma multiforme | [121] |
| Monomethyl auristatin (antimitotic anticancer agent) | PVX | Covalent attachment to external cysteine | 400 | B cell malignancies such as lymphoma | [122] |
| Phenanthriplatin (cytotoxic anticancer drug candidate) | TMV | Electrostatic entrapment | ∼2000 | Triple negative breast cancer | [80] |
| Epitope display (Vaccines and Immunotherapy) | |||||
| Cancer testis antigen NY-ESO-1 | CPMV | Covalent attachment to external lysine | 30–60 | Triple-negative breast cancer, melanoma, myeloma, and ovarian cancer | [123] |
| Hepatitis C Virus E2 epitope | PapMV | Genetic fusion to C-terminus of the viral coat protein | 560 | Hepatitis C | [124] |
| HER2-derived antigen CH401 | CPMV | Covalent attachment to external lysine | 30 | HER2+ tumors such as HER2+ breast cancer | [125] |
| HIV-1 glycoprotein 41-derived 2F5e | PVX | Genetic fusion to N-terminus of the viral coat protein | - | AIDS | [126] |
| Melanoma-associated CTL epitope p15e and tyrosinase-related protein 2 (Trp2) peptides | TMV | Covalent attachment to genetically inserted viral lysine | >1917 | Melanoma | [127] |
| Nucleic acid delivery (Gene therapy) | |||||
| CPG Oligodeoxy-nucleotides ODN1826 | CCMV | Self-assembly/caging | 50 | Colon cancer and melanoma | [78] |
| Flock House virus RNA encoding GFP | TMV | Self-assembly | - | Vaccine/proof of concept | [128] |
| mRNA encoding GFP | TMV | Self-assembly | - | Vaccine/proof of concept | [129] |
| siRNAs targeting GFP and forkhead box transcription factor | CCMV | Self-assembly/caging | Cancer therapy | [130] | |
| Replicons encoding eYFP | CCMV | Self-assembly/caging | - | Vaccine/proof of concept | [131] |
| Protein delivery | |||||
| Bacterial cytochrome P450 | CCMV | Self-assembly/caging | 14 | Bioactivation of chemotherapeutic pro-drugs | [132] |
| Staphylococcus aureus protein A domain B | PVX | Genetic fusion to N-terminus of the viral coat protein | - | Immunoabsorbent for antibody biosensing | [133] |
| Streptokinase | TMV | Covalent attachment to genetically inserted external lysine | ∼767 | Thrombolytic therapy | [134] |
| Tissue plasminogen activator (tPA) | TMV | Covalent attachment to genetically inserted external lysine | 50–100 | Thrombolytic therapy | [135] |
| Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) | PVX | Covalent attachment through Ni-NTA linker conjugated to external lysine | 490 | Breast cancer | [136] |
| Contrast agent delivery (Bioimaging) | |||||
| Alexa Fluor 488/555/647/750 dyes | CPMV | Covalent attachment to the external lysine | 120 | Intravital vascular mapping of tumour growth or embryogenesis | [137] |
| Cy5.5 dye and Gadolinium complexes | PhMV | Covalent attachment to the internal cysteine | ∼20 and ∼130 (respectively) | Near-infrared fluorescence and magnetic resonance imaging of prostate cancer | [90] |
| Gadolinium complexes | CPMV | Covalent attachment to the external lysine | ∼225 | Magnetic resonance imaging (MRI) | [138] |
| Green fluorescent protein (GFP) | PVX | Genetic fusion to N-terminus of the viral coat protein | 1:3 fusion protein to coat protein ratio | Molecular imaging of tumour cells/tissues | [139] |
| SulfoCy5 dye | TMV | Covalent attachment to internal glutamate or external tyrosine | 68–555 and 124–613 (respectively) | Optical mapping of particles cellular processing and uptake kinetics | [140] |
| Targeting ligand (non-invasive delivery) | |||||
| Asp-Gly-Glu-Ala (DGEA) peptide plus Cy7.5 dye and Dy complex | TMV | Covalent attachment to external tyrosine with Cy7.5 and Dy conjugated to internal glutamate | ∼20% surface coverage with ligand and 380 Cy7.5 and 980 Dy molecules | Targeting integrin α2β1 for non-invasive bimodal (near infrared and MRI) imaging of prostate cancers | [88] |
| Folic acid plus doxorubicin | JgCSMV | Covalent attachment to external lysine and doxorubicin loaded by infusion | 300–350 folic acid and 2579 doxorubicin moieties | Tissue-specific breast cancer therapy | [85] |
| EGFL7-binding peptide E7p72 plus A647 dye | CPMV | Covalent attachment to the external lysine | ∼25 A647 dye (E7p72 not quantified) | Intravitreal imaging of tumour neovasculature | [141] |
| EGFR-binding peptide GE11 plus A647 | PVX | Covalent attachment to external lysine | 40% surface coverage with ligand and 300 A647 molecules | Targeted imaging of EGFR+ cancer cells | [142] |
| TAT peptide plus GFP siRNA | TMV | Covalent attachment to external tyrosine plus external siRNA loading via electrostatic interaction with TMV-TAT | 45–60% surface coverage with TAT ligand (siRNA loading not quantified) | Gene knockdown in GFP-expressing metastatic hepatocellular carcinoma (proof-of-concept) | [143] |
AIDS: Acquired immunodeficiency syndrome. DGEA: Asp-Gly-Glu-Ala sequence. eGFP: Enhanced green fluorescent protein. eYFP; Enhanced yellow fluorescent protein. EGFR: Epidermal growth factor receptor. EGFL7: Epidermal growth factor-like domain 7. HER2: Human epidermal growth factor receptor 2. HIV: Human immunodeficiency virus. MRI: Magnetic resonance imaging. Ni-NTA: Nickel-nitrilotriacetic acid. TAT: Transacting activator of transduction. TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand.
2.3. VNPs Classical formulations and administration routes
2.3.1. Classical formulations of VNPs
Being water soluble materials, VNPs are often administered to mice in aqueous buffered solutions through intravenous (I.V.), subcutaneous (S.C.), intramuscular (I.M.), intraperitoneal administration (I.P.), intratracheal, or intratumor injection. VNPs solutions have been also administered through the oral route; attesting to their high pH stability, systemic trafficking and bioavailability of VNPs upon oral administration has been demonstrated [144]. Berardi et al. have recently observed high stability of plant-expressed VLPs of Nudaurelia capensis omega virus in various simulated gastrointestinal media [145], suggesting their potential for oral delivery. In fact, owing to their robust structures, VNPs are good candidates for formulation technology; besides their stability in various pH ranges, VNPs remain structurally sound and tolerate high temperatures and organic solvent-water mixtures [25]. This adds great value, not just for the formulation, but also storage conditions; typically, VNPs do not require cold chains.
Efforts have been invested in studying the freeze-drying processes for viruses. Hansen et al. [146] reviewed freeze-drying approaches applied to live, attenuated viruses for vaccine development, discussing the mechanisms and strategies for virus stabilization, such as the use of cryoprotectants and adjustment of freezing rate and drying temperature. Moreover, Czyz and Pniewski [147] described a freeze-drying protocol for formulating a plant made viral protein antigen that self-assembles into a VLP and acts as an oral vaccine candidate against hepatitis B virus (HBsAg). Through modification of cryoprotectants, processing and storage conditions, authors observed the preservation of VLP structure and immunogenicity of freeze-dried HBsAg, as demonstrated during in oral immunisation trials (where similar immune response was obtained as routine vaccination). Nevertheless, little is known about the impact of these formulation and process parameters on VNPs stability throughout the lyophilization process. In a recent study by Zheng et al. [148], it was found that freeze-dried CPMV which has been used for anti-tumour in situ vaccination and treatment of canine patients [149], lost its RNA molecules in the course of freeze-drying. However, no further insights have been provided to clarify whether this only occurs for CPMV or is a generic phenomenon. In fact, the loss of RNA under VNP freeze-drying conditions would make a crucial matter of research and needs to be carefully considered when dealing with RNA cargoes critical to the intended applications (e.g. therapeutic RNA).
Another promising VNP-based formulation includes the VNP:hydrogel composites. Luckanagul et al. [150] embedded ligand-decorated TMV particles in alginate-based hydrogels to enhance local bio-adhesivity on subcutaneous implantation for regenerative medicine. Lin et al. [151] recently prepared agarose-based hydrogels encapsulating recombinant PVX that displays mineralization- and osteogenesis-associated peptides for bone tissue engineering; data demonstrated much better mineralization with nanocomposites than free peptides. TMV particles were also used as a biocompatible coating to enhance cell interactions of mesoporous silica NPs (MSNPs) in a TMV-MSNP hybrid formulation for biomedical applications [152]. A few attempts have been made so far in our labs to formulate VNP-based depots for controlled delivery applications. For example, our group formulated CPMV-dendrimer hydrogels for extended immunostimulation after I.P. administration for ovarian cancer treatment. Data indicate that the prolonged presence as a result from the slow-release of the CPMV particles from the CPMV-dendrimer hydrogels led to prolonged efficacy avoiding the need for boost administrations (repeated dosing is required to achieve potent anti-tumor efficacy when soluble CPMV is used) [153]. Another slow-release concept was developed using magnesium-based micromotors for extended and active local delivery of immunogenic bacteriophage Qβ, which resulted in prolonged immune stimulation in the intraperitoneal region and therefore enhanced immunotherapy efficacy in the treatment of ovarian tumors in an orthotopic mouse model [154]. In another study, bacteriophage Qβ displaying peptide epitopes from the human papillomavirus (HPV) was encapsulated in PLGA-based implants using a melt-processing system to formulate a single dose HPV-anti vaccine, which stimulated murine anti-HPV antibodies with neutralizing activity towards HPV [155] (unpublished data).
While only a few studies have been done on VNP depots, there are numerous reports providing insights into extended-release formulations of mammalian viruses. These include slow-release systems composed of gelatin-alginate hybrid microparticles [156] as well as hydrogels made of collagen [157], poloxamers [158] or silk-elastin-like protein polymer [159,160]. For example, silk-elastin-like protein polymer hydrogels demonstrated localized release of adenoviral vectors carrying therapeutic genes for applications in cancer gene therapy [161]. The hydrogel formulated viral vector exhibited lower liver invasion and 4 to 8-fold higher gene expression levels compared to its soluble counterpart; and therefore, the hydrogel formulation led to significantly greater efficacy as evident by reduced tumor burden in mouse models. In the frame of local delivery systems for viruses, it is worth mentioning our recent development of a transdermal microneedle patch for autonomous administration of a VNP-based cancer immunotherapy [162]. This study applied intratumoral delivery of CPMV to B16F10 melanomas using an innovative microneedle patch technology; integration of magnesium particles into the patch enables active delivery of the therapeutic ingredient (here the VNP); the propulsion of the therapeutic enables homogenous delivery deep into the tumor tissue. In comparison with the conventional needle injection of CPMV solution, active microneedle injection enhanced tumour regression, extended animal survival and improved antitumor immune responses locally and systemically.
In sum, the structural robustness of VNPs offers the opportunity for new applications in various dosage forms for future clinical use. Since VNPs technology is moving stepwise towards the clinic, there is a need to consider exploring pharmaceutical formulation technology to increase the chances for further translational development. At this preclinical stage, extensive investigation of VNPs formulation in various dosage forms would allow tuning VNPs shelf-life stability and compatibility with the clinically relevant routes of administration to optimize product efficacy or safety and project future cost and patient convenience.
2.3.2. Routes of administration for VNPs
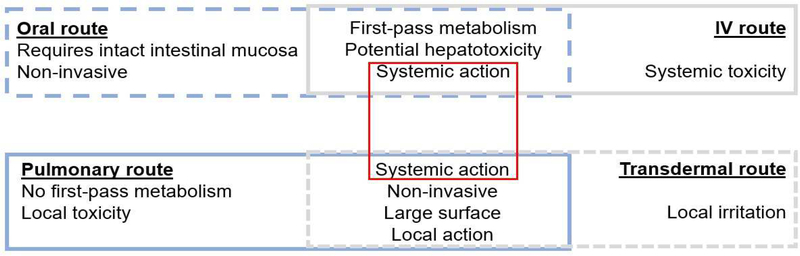
The routes of administration have different impact on the fates of NPs in vivo [163–166], since each route exhibits specific features when compared to others (Figure 2). Most of the clinically exploited routes have been used for VNPs administration to animal models for in vivo investigations. The study by Rioux et al. is a good example of respiratory administration of VNPs for lung diseases such as influenza infections [167]. It was observed that the intranasal instillation of the trivalent inactivated flu vaccine (TIV) adjuvanted by PapMV led to enhanced pulmonary antibody responses and more robust mucosal immunity against influenza infection than TIV alone. Another elegant illustration of the influence of administration route on VNPs tissue distribution was reported by Gonzalez et al. [168]. The authors observed that, following I.P. and I.V. inoculation, CPMV particles were considerably present in all the dendritic cell types (including lymphoid, myeloid, and plasmocytoid cell populations); while CPMV particles were mainly found in the lymphoid cell type following oral gavage.
Figure 2:
Key characteristics of the main routes for administration of biomedical NPs [37,172]
In a toxicological study, Vardhan et al. compared mice treated with 100–200 mg/kg oral and 40–80 mg/kg I.V. administrations of SeMV [169]. Irrespective of the administration route or the doses, no overt histopathological, haematological or biochemical changes were observed, suggesting good biocompatibility for SeMV particles. Similar results were observed with CPMV particles conjugated to Gd3+ or Tb3+ complexes. These particles showed no signs of toxicity following I.V. administration of 1–100 mg/kg [170], except a slight leukopenia trend at the highest dose, which however may be attributed to Gd3+ or Tb3+ ions used for bioimaging. Another interesting illustration of toxicological relevance of VNP administration is the work reported by Denis et al. [171]. The authors observed no local toxicity following S.C. administration of PapMV loaded with M2e influenza epitope (M2e), while S.C. injection of free M2e led to the formation of granulomas at the site of injection; this data demonstrates the potential of VNPs to attenuate or impede therapeutic ingredient’s toxicity. This has been observed in particular when toxic drugs, such as chemotherapies are being delivered by VNPs or other NPs (see discussion in section 3.1.2). In addition to injection route, formulation impacts the toxicology of the VNPs – for example, Luckanagul et al. reported that the immune response for subcutaneous TMV decreased markedly upon incorporation in alginate-hydrogel, leading to the absence of signs of toxicity [150].
Beyond the conventional routes of administration, as discussed above, we note the potential delivery of VNPs through the mucosal routes (e.g. oral, nasal, and vaginal mucosa). In fact, VNPs may be highly suitable for these applications given their small size and zwitterionic surface properties [173, 174]. Rapid diffusion of VNPs through mucus was first observed studying mammalian viruses such as human papilloma virus (HPV) and Norwalk virus [173]; and these observations have inspired nanotechnology design of NPs to incorporate features to mimic the virus characteristics yielding mucus-penetrating NP formulations [175–178]. In a recent study, Berardi et al. [179] studied diffusion properties of CPMV through mucin glycoprotein gels (mucin glycoprotein is the main component of mucus). Data suggest a non-sticky nature of CPMV and demonstrate great potential for its delivery via the mucosal routes. Although mucosal routes are not commonly exploited in the clinic, mucosal administration of biologics and vaccines has gained attention based on the potential to elicit potent IgA antibody responses critical for elimination of mucosal infections; the book by Rosales-Mendoza and González-Ortega [180] is a good reference for interested readers.
3. Pharmacological considerations of VNPs
The increasing interest in VNPs for biomedical applications underlines the need for better understanding of the consequences that may arise from their interactions with the biological systems. As much as data have demonstrated the effectiveness of VNPs as vaccine adjuvants, drug delivery systems and imaging platforms [181], it is critical to anticipate potential adverse effects to ensure optimal medical outcomes. Sufficient evidence established that the fate of NPs in biological systems depends upon both particle characteristics (e.g. size, shape, surface charge, composition and stability) and particle-unrelated factors, such as routes of administration and biodistribution profiles [39,182]. This implies that the identified parameters are key to the adjustment of the interactions between VNPs and biological structures to control formulation’s toxicity or biocompatibility. Herein, we refer VNPs’ toxicity to the ability to adversely affect the physiology or biological structure of tissues and organs, while biocompatibility refers to the absence of toxicity signs [172]. In this section, we highlight some of the important observations from the in vitro and in vivo evaluation of VNPs pharmacokinetics, toxicity and biocompatibility profiles.
3.1. Biological interactions and cytocompatibility
3.1.1. VNP-cell interactions
Many studies have demonstrated the abilities of VNPs to bind to and be taken up by mammalian cells, distribute to the subcellular structures and affect the biological functions [80,89,93,183 –187]. For example, CPMV has shown attractive interactions with antigen presenting cells (APCs) [168]. Interestingly, this targeting of APCs is mediated in part through molecular interaction with the protein vimentin, a type III intermediate intracellular filament, that is secreted and surface expressed on a subset of immune cells [188–190]. Koudelka et al. demonstrated the implications of vimentin surface protein in the entry of CPMV particles into different mammalian cells, including not only immune cells but also inflamed endothelial cells and cancer cell lines that are positive for surface vimentin staining [189,191]. In fact, the CPMV-vimentin interaction is known to be part of the cell entry mechanisms for a number of mammalian viruses, including the Theiler’s murine encephalomyelitis virus [192] and the porcine reproductive and respiratory syndrome virus [193]. The binding of the plant virus CPMV to the mammalian protein vimentin can be explained by the fact that CPMV is a plant picornavirus and as such shares structural and genomic similarities with mammalian viruses [189,191].
CPMV interactions with surface vimentin on APCs have laid the foundation for CPMV-based immunotherapies (as detailed under the immunological section). Similarly, the affinity of CPMV for vimentin on the surface of professional APCs (e.g. dendritic cells and macrophages) was reported to be beneficial for drug targeting to eliminate chronic infectious diseases [194]. In addition, the overexpression of vimentin in atherosclerotic lesions or inflammatory vasculatures offers the opportunity to exploit the CPMV-vimentin interactions for early detection, follow up and non-invasive treatment of cardiovascular disorders [168]. Lastly, because vimentin is highly expressed during the epithelial mesenchymal transition [195], surface-expressed vimentin enables targeting of CPMV to cancer cells (e.g. cervical, breast, and colon cancers) [190].
Plant VNP–mammalian cell/receptor interactions are not unique to CPMV. Another plant VNP not from the picornavirus family, SeMV, was found to enter a range of cancer cells, and this was attributed also to interactions with different surface proteins, including vimentin, voltage-dependent anion-selective channel protein, and annexin A2 isoform 2 [196]. Another example of unique (and somewhat unexpected) VNP-cell interactions worth mentioning is the tropism of PVX to B cell lymphomas. Shukla et al. [122] recently reported that PVX homes to malignant B cells, specifically Non-Hodgkin’s Lymphoma (NHL). The study showed that PVX co-localizes to metastatic lymphoma in an orthotopic murine NHL model. PVX interacts preferentially interacts with malignant B cells and when conjugated with the anti-mitotic agent monomethyl auristatin (MMAE) induces efficient cell killing of malignant but not healthy B cells or other malignant cell types. The tropism and specificity are striking – however at this point it is unknown whether this NHL tropism is shape-mediated and could be explain by lymphatic transport or whether there is a specific receptor that guides targeting and NHL cell entry.
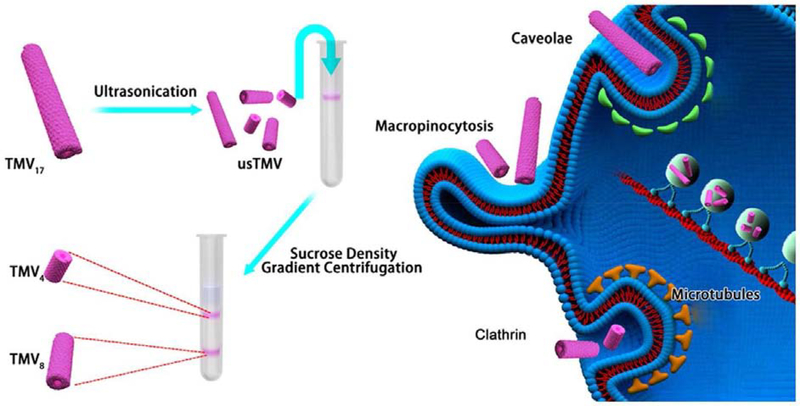
While specific interactions have been observed and reported and/or can be engineered into the VNP; the literature indicates that the native plant VNPs – just like synthetic NPs – are taken up by cells through a combination of different pathways (such as microtubules transport, micropinocytosis and endocytosis mediated by caveolar, clathrin or integrin receptors). For example, Plummer and Manchester [197] observed that CPMV entered both human epithelial cells and murine RAW264.7 macrophages through a combination of caveolar endocytosis and micropinocytosis pathways; data revealed co-localization of CPMV with an endosomal marker (Rab5). Tian et al. [198] witnessed integrin-mediated endocytosis of TMV particles by human epithelial cells as a result of their conjugation to cyclic Arg-Gly-Asp (cRGD) ligand, which suggests the potential influence of surface composition on cell entry mechanisms. In another study, Liu et al. [199] elucidated the structure-function relationship comparing TMV nucleoprotein assemblies of distinct aspect ratio to dissect the impact of aspect ratio on cell uptake and trafficking. Using an epithelial cell type (HeLa) and an endothelial cell type (HUVEC), the authors demonstrated that cell internalization mechanisms are not only cell-type dependent but also particle-shape dependent: TMV rods with aspect ratios 4 and 8 have both entered HeLa via microtubules transport, while their entry in HUVEC was mediated by clathrin endocytosis. However, TMV rods with aspect ratio 17 (TMV wild type) were internalized by the two cell types (HeLa and HUVEC) through a combination of caveolae endocytosis and microtubules transport (Figure 3). The outcome from this study decouples cell entry mechanisms from particle charge and composition leverage, but also triggers the need for assessing the uptake mechanisms of different types of VNPs using a range of cell lines.
Figure 3:
Illustrating the manufacture of TMV-based rods of different aspect ratios, and the discrepancies in their cell entry mechanisms. TMV4, TMV8 and TMV17 indicate to particles of aspect ratio 4, 8 and 17 (TMV wild type). Reproduced with permission from [199]
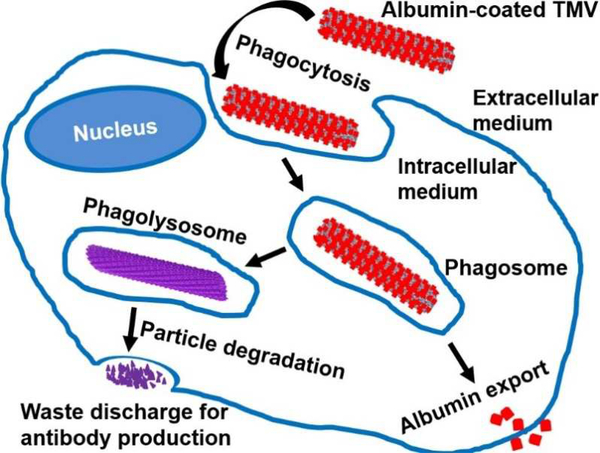
Following cell entry, VNPs undergo intracellular trafficking through various compartments and enzymes located in organelles. Our group and others constantly witnessed the intracellular metabolization of VNPs. This is evidenced, for instance, by the endosomal release of fluorophores from CPMV [140] as well as protein cargo removal from engineered TMV particles (Figure 4) [200]; illustrating the VNPs potential for intracellular cargo release [184]. This cargo release can be explained by the proteolytic activity within the acidic endolysosomal environment. Because of this hostile/harsh environment, both pH labile as well as pH stabile cargoes are quickly released, and thus happen to achieve the desired course of action within the cell. This was demonstrated in studies comparing different chemical bonds used to conjugate CPMV to doxorubicin (DOX). These include the comparative studies of CPMV-DOX containing pH labile hydrazone bond vs. stabile amide bond [92], as well as amide bond vs. disulphide bond [201]; either nanoparticle formulation achieved drug delivery and cytotoxicity.
Figure 4:
Schematic illustration of intracellular trafficking of VNPs: endocytosis and intracellular fate/metabolization of serum albumin-coated tobacco mosaic virus (TMV) by a macrophage. Adapted from [200] with permission from the Royal Society of Chemistry.
There is evidence that VNPs traffic through the endolysosomal pathway; however, the fact that VNPs have been successful in delivering fragile materials such as nucleic acids (Table 2) also indicates that some VNPs enter cells through alternate pathways or escape from the endosomal vesicles. However, reported data are contradictory: While some reports state that lipofectamine or cell penetrating peptides are needed to achieve efficient delivery of therapeutic nucleic acids (such as mRNA delivered by CCMV [131] or siRNA delivered by CCMV [130] or TMV [143]), some reports indicate that VNPs can effectively deliver genes without the aid of transfecting adjuvants. For instance, TMV loaded with mRNA encoding for enhanced green fluorescent protein (eGFP) exhibited good transfection efficiency in BHK-21 cells [128], and further induced antibodies against green fluorescent protein in BALB/c mice [129], which demonstrated the potential of TMV-mediated gene delivery for vaccine development. Similarly, VLPs from native CCMV coat protein were used to encapsulate mRNA encoding for eGFP; and, while no transfecting adjuvants were incorporated, the findings demonstrated excellent transfection efficiency in a range of mammalian cells (HEK293, HeLa and HK2 cells) [202]. Overall, these data underline the potential of VNPs for gene and drug therapy, but also highlight that a deeper understanding of the intracellular fates is needed to effectively tailor VNP-based therapies.
3.1.2. In vitro biocompatibility of VNPs
Methods for in vitro nanotoxicity assessment generally involve the determination of cell viability (live/dead ratio) or analysis of cytotoxicity mechanisms, which include oxidative stress and DNA damage. The techniques for NPs toxicity assessment have been reviewed [203–206]. The viability protocols are categorised into cell proliferation, necrosis, apoptosis or stress assays [206]. Several VNPs have been studied for targeted drug delivery and imaging applications. In most cases, the viral carrier itself showed no toxicity toward cells but was effective in delivering the cargo to achieve the drug-mediated cytotoxic effects; cell toxicity of VNPs used for imaging applications was not reported (Table 3). Nonetheless, beyond assessment of cell viability, data are scarce, and more research is needed to elucidate the biocompatibility of VNPs.
Table 3:
Summary of in vitro studies evaluating the biocompatibility and toxicity of cargo-loaded and native VNPs
| Plant VNP | Formulation composition | Dose | Cell type | Biocompatibility-/toxicity-related outcomes | IC50 | Ref. |
|---|---|---|---|---|---|---|
| Drug delivery studies | ||||||
| CPMV | Native CPMV and CPMV loaded with mitoxantrone (MTO) with and without combination of TNF-related apoptosis inducing ligand (TRAIL) | 0.05–5000 nM MTO, with 20–50 MTO molecules/CPMV | U87-MG cells | Up to 107 particles per cell, native CPMV showed no cytotoxicity, while MTO - loaded CPMV (with/out TRAIL) exhibited marked cytotoxicity over 72 hrs | MTO 212.8 nM; Free MTO + TRAIL 65.9 nM; CPMV-MTO 269.0 ± 70.2 nM; CPMV-MTO + TRAIL 153.1 nM | [121] |
| HCRSV | Doxorubicin (DOX)-loaded HCRSV particles decorated with folic acid (FA) | 0.005–30 μg/mL DOX, with 900 DOX molecules/protein cage | ovarian cancer cells OVCAR-3 and normal cells CCL-186 | Following 2 hrs of incubation, no cytotoxicity was observed on normal cells, but rather protection against the cytotoxic effects of doxorubicin | DOX 0.48 μg/mL; FA-HCRSV-DOX 0.11 μg/mL vs. OVCAR-3 cells DOX 1.95 μg/mL; HCRSV-DOX 3.4 μg/mL; FA-HCRSV-DOX 3.2 μg/mL vs. CCL-186 cells |
[86] |
| PVX | PEGylated PVX loaded with doxorubicin (DOX) | 10–50 μM DOX, with 850–1000 DOX molecules/PVX | A2780. MDA-MB-231 and HeLa cells l | Following 24 hrs of incubation, empty PVX showed no cell toxicity, while DOX-loaded PVX was cytotoxic but to lesser extent than free DOX | DOX 0.13 μM; PEG-PVX-DOX 0.78 μM | [207] |
| PhMV | PhMV loaded with photosensitizer (Zn-EpPor) | 0.19 mg/mL | PC-3 cell line | After 48 hrs of incubation, unloaded and Zn-EpPor-loaded PhMV showed no cytotoxicity in the dark, while the latter was cytotoxic following 30 min illumination at 430 nm | ZnEpPor 0.05 μM; PhMV-ZnEpPor 0.03 μM | [208] |
| TMV | Phenanthriplatin(Pt)-loaded TMV | 0.05–100 μM Pt; with 1,000,000 TMV particles per cell | A2780, A2780/CP70, OV81.2, LNCAP, MCF-7, 8988T and MDA-MB-231 cells | While native TMV showed no cytotoxicity, Pt-loaded TMV was cytotoxic | Similar for both Pt and Pt-TMV: 0.293.59 μM depending on cell lines | [80] |
| TMV | Native and serum albumin (SA)-coated TMV loaded with doxorubicin (DOX) | 3.2 nM–2 μM DOX, with 1476–1664 DOX /TMV | MDA-MB231 and 4T1 cells | Native and SA-coated TMV showed no cytotoxicity, while DOX-loaded native and SA-coated TMVs were cytotoxic against cancer cells over 20 hrs | DOX 0.30 μM; TMV-amide-DOX 0.43 μM; TMV-hydrazone-DOX 0.79 μM; SA-TMV-amide-DOX 33.76 μM; SA-TMV-hydrazone-DOX 36.94 μM | [186] |
| Imaging studies | ||||||
| PhMV | PhMV loaded with Cy5.5 and Gd3+ and decorated with PEG and targeting peptides | 0.1–0.4 mg/mL PhMV | PC-3 cell line | The modified PhMV showed no significant cytotoxicity after 12–24 hrs of incubation at the concentrations of 107–2×108 particles/cell | - | [90] |
| TMV | PEGylated and Peptide (DGEA)-decorated TMV loaded with Dy3+ complex and Cy7.5 dye | 0.1–0.4 mg/mL | PC-3 cell lines | No significant reduction in cell viability was observed after 12–24 hrs of incubation at the concentrations of 105–4×106 particles/cell | - | [88] |
| TMV | Polydopamine-decorated TMV loaded with Gd3+ complex | 0.5 mg/mL | PC-3 and 4T1 cell lines | Without irradiation (with 808 nm laser), TMV particles showed no cytotoxicity after 6 hrs of incubation | - | [94] |
NPs in general do not enhance efficacy of therapeutics – however NP-mediated delivery changes the biodistribution of the cargo allowing drug to be concentrated in target tissue while avoiding healthy tissues; therefore, increasing therapeutic outcomes. Similarly, VNPs have demonstrated the ability to impede drug toxicity on normal cells. An illustrative example is HCRSV particles loaded with doxorubicin and decorated with folic acid, this targeted VNP-drug formulation showed no cytotoxicity on normal cells, but good cytotoxic activity on multiple cancer cell lines [86]. Furthermore, often NP- and VNP-drug conjugates exhibit lower IC50 values in tissue culture experiments. For example, Le et al. [207] reported on doxorubicin-loaded PVX, which showed lower cytotoxicity on cancer cells compared to free doxorubicin. Similarly, free doxorubicin exhibited greater cytotoxicity compared to doxorubicin-loaded PhMV when tested against a panel of cancer cell lines: MDA-MB-231 (IC50 0.63 vs 0.98 μM for free DOX vs. PhMV-DOX), A2780 (IC50 0. 35 vs 1.16 μM for free DOX vs. PhMV-DOX), SKOV-3 (IC50 0.33 vs 1.84μM for free DOX vs. PhMV-DOX) and PC-3 (IC50 1.15 vs 3.85 μM for free DOX vs. PhMV-DOX) cells [118]. These differences can be explained by the distinct cell uptake routes and intracellular processing of the cargo comparing VNP/NP-delivered vs. free drug. Similar observations have been made using synthetic nanoparticles such as liposomes [209]. On the other hand, Franke et al. [210] observed that cisplatin-loaded TMV exhibited markedly high cytotoxicity on cisplatin-resistant ovarian cancer cells. This phenomenon may be explained also by distinct cell uptake and trafficking routes of free vs. VNP-delivered drug; the latter strategy may overcome drug transporters mediating drug resistance to free drug entering the cells via the cell membrane – in contrast the VNPs enter via a combination of endocytosis and micropinocytosis (see Figure 3).
Consistently, studies have shown that native VNPs are non-toxic toward mammalian cells. This also holds true for VNP delivering light-activated cytotoxic agents (for photodynamic or photothermal therapy), which showed cytotoxicity only upon illumination (but not in the dark) [94,208]. Toxicity is conferred only through the delivered active ingredient. VNP carrier formulation chemistry and mechanism of drug loading and release will affect efficacy and biocompatibility. For instance, Cao et al. observed bimodal release kinetics when loading RCNMV with doxorubicin drug using two different techniques: infusion and electrostatic attachment [81]. The release of electrostatically surface-bound molecules occurred quickly while infused molecules were released later at slower rate, being partly driven by RCNMV’s pores sensitivity to changes in pH and divalent cations concentration [61]. Pitek et al. observed that doxorubicin conjugated to TMV through amide linkages showed 2-fold higher cytotoxicity (IC50 0.32 and 0.43 μM for MDA-MB231 and 4T1 cells) than doxorubicin conjugated via hydrazone bonds (IC50 0.61 and 0.80 μM for the aforementioned cells) [92]. This is solely due to the difference in drug release kinetics; amide bonds being cleaved by cellular amidases (leading to relatively faster release), while hydrazone bridges might have required timely exposure to endosomal acidification for complete pH-trigged release – making acid-dependent release a useful strategy for non-invasive delivery. Toward this end, our group recently also designed PhMV particles encapsulating doxorubicin equipped with pH-sensitive hydrazone linker for acid-triggered drug release behaviour [118]. Because of pH-dependent/tissue-specific drug release (selective delivery to acidic tumor microenvironment and endosomes), PhMV formulations exhibited 3.4-fold higher anticancer efficacy in breast tumor mouse model compared to free doxorubicin. Such targeted drug delivery approaches hold promise to overcome cardiotoxicity associated with many chemotherapy regimens [211].
Albeit the reported cell viability data support the biocompatibility of VNPs, it is important to mention the lack of studies discussing biomarker analysis to detect signs of nonnecrotic cellular disturbances, which is recommended prior to categorizing NPs as inert [203]. Drawing from clinically established synthetic nanoplatforms, such as iron oxide NPs (IONPs), an intriguing example of diligent cytocompatibility profiling could be the study by Pongrac et al. [212]. The authors tested IONPs on murine neural stem cells and observed various adverse effects at subcellular levels, i.e. loss of mitochondrial homeostasis, DNA damage, etc., while cell viability remained unchanged after 24 hours incubation with up to 200 μg/mL. In the field of VNP bioengineering, one of the few toxicity studies addressing nonnecrotic cellular perturbations was reported by Li et al. [213]. The authors observed that, when TMV’s RNA was administered by electroporation with Lipofectamine™ 2000, expression of glucose-regulated protein GRP78 (a marker of endoplasmic reticulum stress) was induced; further, they observed autophagy in human epithelial carcinoma cells (HeLa cells). This data highlights potential interactions between viral components and host cell biomolecules, but also demonstrates further the protective effects of viral protein cage as a vehicle. Native TMV did not induce autophagy. However, to our best knowledge, no study has attempted to interrogate the structure-function relationship of VNP shape, size, and surface chemistry on cell interactions. Although toxicity is not expected, treatment with plant virus carriers may induce stress. It would therefore be insightful to evaluate whether plant virus exposure to mammalian cells alters expression of proteins involved in pro-inflammatory responses (e.g. HSP90, IL-18, IL-1), DNA damage and repair (e.g. PCNA, RAD32), and apoptosis (e.g. CASP1, CASP8, or ERG1); which are part of nanotoxicity mechanisms as extensively described for synthetic nanoparticles [214–216]. Particle characteristics, such as size, surface chemistry and composition, have been reported to be the acellular parameters that determine the NP-induced oxidative stress; the mainstay of multiple pathophysiological effects such as genotoxicity, inflammation and fibrosis [217]. Smaller NPs were reported to induce higher oxidative stress owing to surface area enhancement (i.e. increased number of accessible reactive groups/sites) [218,219]. A contradictory opinion was postulated when lung toxicity of single-walled carbon nanotubes was attributed to nanotubular aggregation instead of individual particles of high aspect ratios [220]. Nonetheless, the endogenous induction of oxidative stress due to cell function perturbations upon NPs internalization (i.e. mitochondria responses particularly) [221,222] underlines the key role of cell entry in the stress paradigm. In this context, the particle size, charge, shape and other properties driving NPs cell uptake appear to be paramount important. Thus, the tuneable structure of VNPs offers great opportunity for in-depth exploration of cell nanotoxicity mechanisms. Thorough investigation of the influence of VNPs surface functionalities and structural properties on cell functions would enable setting up the design principles to inform synthetic nanotechnologists.
Although plant viruses do not replicate in mammalian cells, their intracellular trafficking needs to be carefully examined considering cell metabolization processes. Within cells VNPs are generally assumed to be broken down into nucleic acids (generally labile RNAs) and coat proteins, which then are further broken down in peptides and amino acids. While these processes may induce some level of cellular stress, one might think that because VNPs are broken down into biological building blocks, they may have a higher degree of biocompatibility compared to synthetic materials of non-biological origins, which either take much longer to break down (e.g. carbon nanotubes [223]) or change the environment as they break down (e.g. poly(lactide-co-glycolide)/PLGA and iron oxide NPs/IONPs). For instance, IONPs biodegradation leads to increasing loads of divalent iron cations (Fe+2) [224]. Due to Fe+2 intracellular accumulation, IONPs generate reactive oxygen species (ROS) and induce oxidative stress as well as apoptosis through perturbation of mitochondrial and nuclear functions (including DNA damage) [225–228]. Contrary to IONPs, PLGA degradation by-products (e.g. lactic and glycolic acids) are known to be safe; lactic and glycolic acids are eliminated through normal cell metabolization (the respiration Kreb’s cycle) into carbon dioxide and water [229,230]. Nevertheless, local accumulation of these acidic by-products due to poor elimination can result in acidification of the cells/tissues, which can disrupt the local biological response [231]. These illustrative safety concerns pertaining formulation degradation by-products highlight the need for in-depth investigations to fully elucidate the fate of VNPs and their by-products.
In fact, studies have demonstrated that the intracellular processing of VNPs is the ground for their usage as epitope display technology for vaccines and immunotherapy: immune cells process VNPs and delivered peptide epitopes leading to presentation of the latter major histocompatibility complex (MHC) I or II [232]. For instance, Makarkov et al. recently witnessed the uptake and processing of plant-derived viral nanoparticles displaying influenza hemagglutinin-derived peptide epitopes in human monocyte-derived macrophages [233]. Data indicated co-localization of MHC I with the delivered peptide epitopes, which confirms the breakdown of VNPs by the cellular machinery up to peptide level. Nonetheless, no meticulous analyses have been done to interrogate the toxicological impact of VNPs peptide by-products on biological functions and activities of cells. Inspirational examples can be drawn from studies with clinically used systems, such as PLGA drug delivery systems, which have been extensively investigated for biosafety profiling at cellular level. For instance, He et al. [234,235] established the biocompatibility of PEG-PLGA-poly(L-lysine) NPs by assessing: (i) protein synthesis, cell membrane integrity and chromatin agglutination in Huh7, L02, and RAW 264.7 cells; (ii) the release of interleukin-1β, tumour necrosis factor-α and transforming growth factor-β1 from THP-1 cell-derived macrophages; as well as (iii) the potential impact on embryonic development using zebrafish embryos. Such in-depth studies are highly desired for the VNP field. The development pipeline is moving rapidly and therefore pharmacology and detailed cellular toxicity studies are urgently needed.
3.2. VNPs pharmacokinetics and in vivo biocompatibility
Although in vitro nanotoxicity data are relevant for preliminary insights anticipating in vivo behaviour, the prognosis based on in vitro data only serves as a herald to encourage in vivo testing for further confirmation [204]. In addition to the complexity of the in vivo setting, the extrapolation of cell-based data into in vivo scenario is problematic because of the extreme conditions used in vitro; usually ultra-high NPs doses exposed for a long period to investigate dose-related cell toxicity [37]. Although seen as a relatively time-consuming, complicated, and animal-sacrificing approach, in vivo testing remains critical since systematic evaluation of nanotoxicity is needed to establish the design rules for safe nanoengineering [206]. The methods for in vivo nanotoxicity assessment mostly evaluate histopathological changes and pharmacokinetic parameters such as biodistribution, haematology, metabolism (biochemical changes), and clearance [236,237]. From the literature discussing in vivo nanotoxicity assessment for VNPs (Table 4), we observed that most studies focused on histopathological, haematological and body weight variations. In the following paragraphs, we discuss the toxicological considerations of VNPs as a function of in vivo circulation, with an emphasis on VNPs tissue accumulation and clearance to provide insights into potential toxicity or biocompatibility profiles.
Table 4:
Summary of in vivo studies evaluating the biocompatibility and toxicity of VNPs in the context of biodistribution studies, immunotherapy/vaccines, drug delivery, or imaging
| Formulation composition | Dose (Administration route) | Animal model (organ/tissue target) | Biocompatibility-/toxicity-related outcomes | Ref. |
|---|---|---|---|---|
| Biodistribution study | ||||
| Native CCMV | 50 μg in 200 μL (IV) | Female Balb/c mice (lung, liver, spleen and kidney) | No remarkable histopathological signs were observed over 24 hrs | [238] |
| Native PVX | 10–200 μg (IV) | Cerquaglia Farm chicken (blood, chicken embryos) | No signs of toxicity or teratogenicity at the doses of 1 ng–10 μg/embryo. Slight haemolysis rate of 1.8 and 2.7% at higher doses (100 and 200 μg) | [239] |
| Native SeMV | 40–80 mg/kg (IV) and 100–200 mg/kg (oral) | Female Swiss albino mice (blood, brain, kidneys, liver, lungs, spleen) | Biochemical and haematological parameters remained unchanged after 6 and 72 h, except mild leukopenia at the highest dose. No histopathological changes were observed | [169] |
| Native TBSV | 10–200 μg (IV) | Cerquaglia Farm chicken (blood, chicken embryos) | No effects on erythrocytes integrity. No signs of toxicity or teratogenicity at the doses of 1 ng–10 μg/embryo | [239] |
| Native and PEGylated TMV rods and spheres | 10 mg/kg (IV) | Healthy Balb/c mice (liver and spleen) | No histopathological changes observed over 14 days post-injection. No clotting or haemolysis noted after 1 hr of incubation of 2.5 mg/mL TMV with 5×108 RBCs/mL | [93] |
| Immunotherapy and vaccine | ||||
| PapMV coat protein (CP) encapsulating M2e influenza epitope (M2e) | 100 μg/injection (SC) | Balb/c mice (subcutaneous tissue/injection site) | PapMV-CP-M2e showed no local toxicity, while alum-M2e generated obvious granulomas at injection site | [171] |
| Trivalent inactivated flu vaccines (TIV) adjuvanted with PapMV | 21 μg/injection (intranasal or SC) | Balb/c mice (respiratory mucosa) | No induction of tumour necrosis factors or inflammatory reactions | [167] |
| Complexes of rubella tetraepitope A (A4) and spherical particles (SP) from thermal remodelling of TMV | 100 μg per IM injection | Female Balb/c mice (blood/sera) | A4-SP showed no lethality (acute toxicity), and the body weights remained unchanged with no pathological signs nor physiological changes (no chronic toxicity) after three doses over 42 days | [240] |
| Drug delivery | ||||
| Nickel-coordinated PVX loaded with tumor necrosis factor-related apoptosis-inducing protein (TRAIL) | 12–15 μg/kg per injection (injected intratumorally) | Female NCR nu/nu mice and TNBC cell lines | Ni-PVX showed no cell toxicity, while TRAIL-loaded Ni-PVX was cytotoxic after 12 h incubation. The two formulations induced no body weight changes over 30 days | [136] |
| Phenanthriplatin-loaded TMV | Equivalent to 1 mg/kg phenanthriplatin (IV) | Balb/c mice (liver and kidney) | No body weight variation nor animal misbehaviour. No overt liver toxicity or significant changes in enzymes levels, but only marked necrosis in the kidneys due to platinum side effects | [80] |
| Imaging | ||||
| CPMV particles labeled with Gd3+ or Tb3+ complexes | 1–100 mg/kg (IV) | Balb/c mice (blood, and tissues from all mouse organs) | No signs of tissue degeneration, necrosis or apoptosis over 24 hrs. Haematology was normal, except the leukopenia trend at highest doses | [170] |
| Tissue engineering | ||||
| Alginate hydrogel containing native and RGD-mutant TMV | 6.35×2mm gel disk containing TMV 0.1% (SC) | Male Balb/c mice (blood, liver, lung, brain, heart, and spleen) | No chronic and/or major inflammatory and toxicity reactions in the RES, and blood cell numbers remained normal after 4 weeks of gel implantation | [150] |
3.2.1. VNP pharmacokinetics and biodistribution
In general, NPs are known to distribute to almost all the tissues and organs following their administration through any routes [241]. Depending on NPs properties, routes of administration and body physiology, NPs accumulate in different tissues and organs at various concentrations [242]. Since NPs systemic exposure is common to most administration routes (Figure 2), the fate of NPs in the bloodstream is frequently investigated in biodistribution studies owing to its remarkable impact on NPs tissue accumulation and clearance [243]. In fact, upon entry in the bloodstream, NPs interact with plasma proteins and a protein corona is formed surrounding the NP; this protein corona affects the NP’s in vivo behaviour due to the change in particle size and surface characteristics [4]. While VNPs are also recognized as foreign nanoparticles by the body, data indicate however that fewer corona proteins/less protein corona is formed on the proteinaceous nanoparticles vs. synthetic nanoparticles [244]. This may be explained by the zwitterionic nature of the proteinaceous nanoparticles, owing to the presence of both, basic and acidic amino acids, which minimizes the VNP interactions with other biomolecules (i.e. plasma proteins) sharing similar zwitterion properties [176,179]. Pitek et al. [244] revealed that the amount of protein corona formed on TMV was 6-fold lower compared to the protein corona formed on synthetic NPs; and the TMV protein corona was made mainly of immune system proteins, i.e. complement proteins and immunoglobulins. It was observed that protein corona affected the TMV–cell interactions, and this was dependent on the cell type under investigation: enhanced interaction was observed with HeLa cells, while no influence was observed on TMV-macrophage (SC and RAW264.7) interactions [244]. In the same study, the molecular recognition of TMV conjugated to PEG and targeting ligands (for integrins or fibrinogen) was found to be affected by protein corona. Surface modification with appropriate ligands is therefore key to controlling TMV particles interactions with plasma proteins (i.e. protein corona formation), promoting their dispersion in plasma. PEGylation was reported to be effective in minimizing the amount of protein corona on particles surface.
Many studies have focused on detailing that protein corona composition is critical to the in vivo fate of NPs. Protein corona containing opsonins (such as complement proteins, immunoglobulins and laminin) induces fast recognition and quick uptake by the immune cells [245,246]. This can initiate a complement cascade immune response, induce cytokine secretion and generate systemic immune response, which can result in immunotoxicity [247,248]. While polymer-coatings can reduce the immunogenicity of NPs including VNPs; data indicate that PEGylation may not be effective to completely eliminate immune surveillance – e.g. Hu et al. [90] witnessed the formation of immunoglobulins and complement proteins corona on PEGylated PhMV particles (Figure 5A). In a different approach, stealth coatings can be applied: for example, serum albumin coatings on TMV were hown to significantly enhance TMV’s biocompatibility by overcoming immune recognition and avoiding capture by neutralizing antibodies [249]. These properties also conferred increased blood circulation time of serum albumin-coated TMV [200]. The serum albumin-coated TMV formulation was also explored as a carrier for doxorubicin and Gd3+ complexes for cancer therapy and magnetic resonance imaging (MRI), respectively [92]. While these findings set the foundation for serum albumin surface decoration as a strategy to improve VNPs biocompatibility, no further studies have interrogated the efficiency of serum albumin surface technology when coating VNPs with different sizes, shapes or aspect ratios; which could provide insights into optimal design rules of biocompatible NPs.
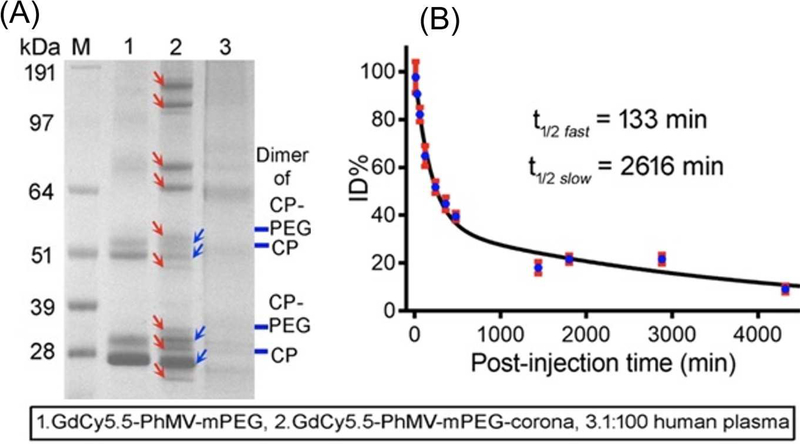
Figure 5:
(A) SDS-PAGE fingerprint of protein corona formed on PEGylated PhMV particles after 1hour incubation with human plasma. (B) Pharmacokinetic profile of PEGylated PhMV (coloaded with Cy5.5 and Gd3+) in Balb/C mice following I.V. injection of 200 μg. Reprinted from [90], Copyright 2019, with permission from American Chemical Society.
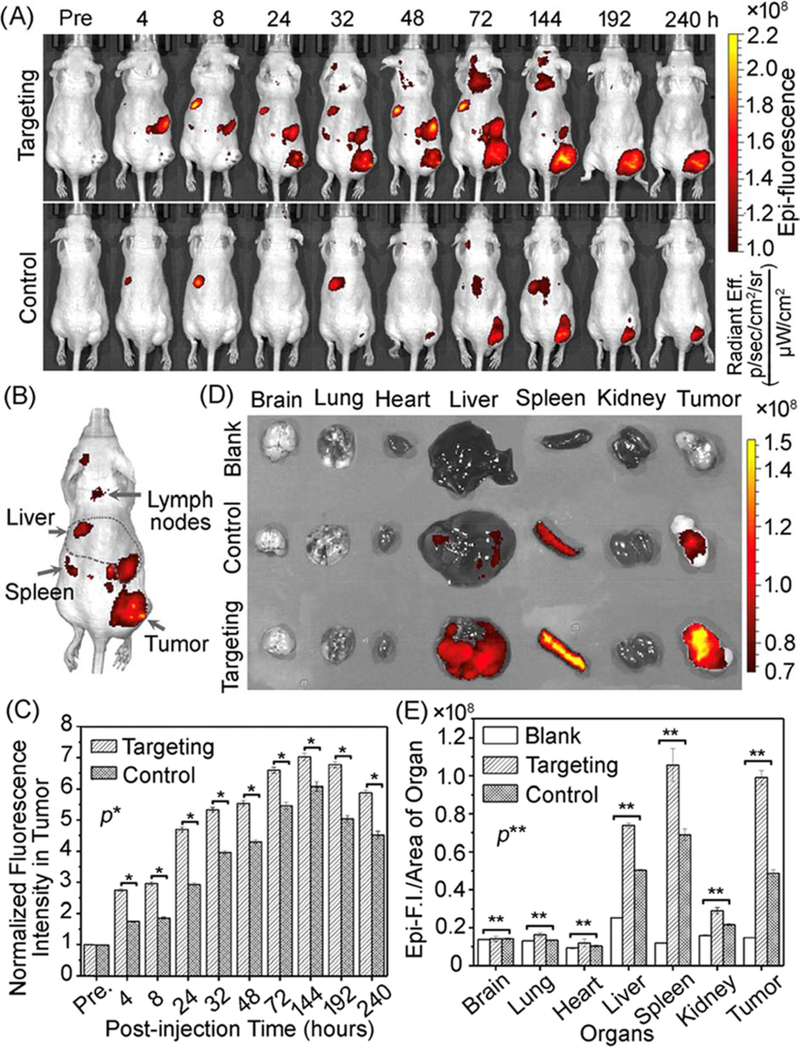
Since the interactions with plasma proteins and cells depend on particle surface composition, VNPs blood circulation and clearance profiles vary markedly from one VNP to another. For example, Lee et al. [250] observed that non-PEGylated PVX displayed one-phase plasma elimination pattern, whereas PEGylated PVX exhibited two-phase decay from the bloodstream. The study demonstrated distinct half-lives for PEGylated PVX depending on the type of PEG used: the initial fast and slow clearance half-lives (tI1/2 and tII1/2, respectively) for PVX conjugated to branched 5 kDa PEG were 14 and 1142 min respectively, while PVX attached to linear 5 kDa PEG exhibited tI1/2 =11 min and tII1/2 =409 min – quite different from PVX with linear 20 kDa PEG (tI1/2 =27 min and tII1/2 =231 min). Similarly, PEGylated TMV particles showed different plasma circulation/clearance when compared to TMV modified with serum-albumin; the reported half-lives for the two nano-formulations were 10 and 100 min, respectively [251]. The circulation time of native CPMV, which has an icosahedral shape and negative surface charge, was found to be slightly longer than that of PVX particles, which have a filamentous shape and overall positive charge, with half-lives of 20.8 and 12.5 min, respectively [108]. Among all the VNPs, PEGylated PhMV particles have so far shown the most long-lasting plasma circulation time, with tI1/2 = 133 and tII1/2 = 2616 min (Figure 5B) [90]; which is also much longer than some of the clinically established synthetic NPs (i.e. dextran-coated Fe3O4 NPs, with tI1/2 =9.7 min and tII1/2 =150 min) [252]. In general, extended plasma circulation is desired to increase the chance for enhanced tumour tissue accumulation. For the PhMV platform, Hu et al. [90] demonstrated that these VNPs can be tailored for imaging of prostate tumors in mice over time periods as long as ~10 days (dual-modal optical-MRI was demonstrated) (Figure 6), which makes PhMVs an intriguing platform for longitudinal imaging applications to follow disease progression or treatment response.
Figure 6:
Non-invasive near-infrared fluorescence (NIRF) bioimaging using Cy5.5-GdPhMV particles decorated with DGEA peptide (or with PEG, control group) for targeting prostate tumors in athymic nude mice following I.V. injection of 200 μg. (A) Longitudinal visualization of PhMV particles using NIRF imaging. (B) In vivo localization of PhMV particles in the RES organs. (C) Long-term quantitation of fluorescence signals in prostate tumors. (D) Ex vivo NIRF imaging of major organs 240 hours post-injection. (E) Organs distribution/biodistribution profiles of Cy5.5-Gd-PhMV-DGEA particles using ex vivo NIRF imaging data – control group being untreated animals. Reprinted from [90], Copyright 2019, with permission from American Chemical Society.
However long circulation may not always be the key to treatment success; Madden et al. observed that doxorubicin-loaded RCNMV exhibited faster plasma clearance (t1/2 = 42–60 min) but much greater tumor homing (ratio of tumor AUC0-Tlast to plasma AUC0-Tlast = 19–21) than the marketed PEGylated doxorubicin-liposomes (Lipodox®) (t1/2 = 528 min and ratio tumor AUC0-Tlast to plasma AUC0-Tlast = 0.14), when tested in melanoma A375 and ovarian carcinoma SKOV3ip1 models [253]. Efforts are often dedicated to extending plasma circulation, but one should rather directly focus on site-specific/non-invasive delivery; especially since longer plasma residence might be a double-edged sword for VNP formulations designed to interact with the immune system (e.g. VNPs – as discussed later). Thus, there is a need for careful structure-bioactivity optimization of VNPs to anticipate any unwanted in vivo performance.
In addition to plasma clearance mainly due to uptake by immune cells, another in vivo challenge for VNPs/NPs is the unavoidable off-target tissue accumulation, which can lead to loss of efficacy or increased tissue toxicity. It is well established that NPs tissue accumulation is driven by two factors: phagocytosis by immune cells and infiltration through fenestrated endothelia in reticuloendothelial system (RES, which includes liver, spleen, etc.) [254]. For example, PEGylated PVX particles exhibited extended plasma circulation, but their biodistribution was consistent with clearance mechanism by the RES organs [250]. In fact, being considered as foreign particles to the body, VNPs make no exception to the general clearance and accumulation mechanisms of NPs. Efforts have been made to tailor VNPs surface chemistry and shape to tune particles circulation, accumulation and clearance for enhanced efficacy, reduced toxicity or improved biocompatibility of VNP-based formulations (Table 4). For instance, Bruckman et al. compared the pharmacokinetics, biodistribution and biocompatibility profiles of fluorescently labelled sphere-like, rod-shaped native and PEGylated TMV particles [93]. While circulation time is short no matter which particle formulation was tested, differences were apparent with longer blood circulation time for TMV rods (phase I half-life 3.5 min) compared to the sphere (phase I half-life 2.3 min), and that PEGylated rod exhibited phase I half-life of 6.3 min (i.e. 2-fold higher plasma half-life than native particles); which was expected as PEGylation is known to minimize protein corona formation. All the three particles were found to distribute to the RES organs at similar extents, but tissue clearance patterns were different: sphere-like TMV particles were cleared within 24 hours while TMV rods were still detectable up to 96 hours. Despite the difference in the course of clearance, the three formulations induced no histopathological changes in the RES organs and no clotting or haemolysis was observed [93], suggesting good histocompatibility and hemocompatibility. Indeed, fast clearance of VNPs from the RES organs is a desirable behaviour for safe drug delivery or imaging applications, avoiding any potential toxicity due to extended cargo accumulation. In this regard, with 24–96 hours of tissue accumulation, most VNPs appear to be cleared – this is consistent with clearance time of some of the soft synthetic systems, e.g. PLGA [255]; and in contrast to hard synthetic NPs such as metallic NPs [256] and carbon nanotubes [257], which may persist in tissues (for more than a month) and lead to adverse effects.
Comparisons of biodistribution between different VNPs have been reported. For example, Shukla et al. compared the biodistribution of icosahedral CPMV and filamentous PVX: PVX accumulated mainly in the spleen while most CPMV particles were found in the liver 24 hours after IV injection [108]. It was also noted that PVX exhibited increased transport properties into solid tumors. The comparison of CPMV and PVX, however, is problematic because the observed differences in biodistribution could be attributed to shape, charge or surface chemistry. PVX and CPMV have different nanoparticles shapes and volumes; normalization of the administered dose therefore may lead to observed changes (one could normalize to the number of particles or amount of protein). While CPMV has an overall negative surface charge, PVX has an overall positive surface charge [108], and this may attribute to increased tumor transport properties. At the same time, differences may be due to shape and different transport behaviour; elongated nanoparticles avoid phagocytosis and have enhanced margination properties [258–260]. Lastly, more recently Shukla et al. reported tropism of PVX to B cell lymphomas (see section 3.1.1) [122], and this may point toward some unknown molecular interactions that may lead to the observed distinct biodistribution profiles.
To some degree one may argue that the differences noted could be mainly attributed to differences in shape; as this has been commonly observed using synthetic nanoparticles [112,258–261]. For example, shape-mediated effects were observed with synthetic silica NPs of 200–1800 nm size, where thinner disks showed the potential to be more effective in targeting the diseased microvasculature than spheres and rods [262]. In another study, Finbloom et al. [116] witnessed the shape-depend efficacy of different VLPs in glioma-bearing mice, comparing TMV disks versus nanophage filamentous rods and MS2 spheres (sphere-like icosahedrons) loaded with doxorubicin. Amongst, TMV disks afforded the highest animal survival rates followed by the MS2 spheres. This could be due to the shape-related discrepancies in particle diffusion within the tumour tissue [263], but again one can think about the influence of surface chemistries (as the compared nanomaterials had different chemical compositions). In this context, our previous study [113] comparing the impact of aspect ratio (AR) on the biodistribution and tumor homing of nanomaterials derived from TMV appears valuably instructive. We observed that the nanorods with the lowest AR (3.5) showed the best passive tumor homing feature; while AR 7 rods decorated with RGD ligand exhibited excellent actively targeted tumor homing. Overall, it is realistic to hypothesize shape-mediated distinct tissue distribution profiles between VNPs (i.e. filaments, rod and icosahdrons); however, it is worth considering the insights from other instructive studies that raise different opinions. For example, in a comparative study between PVX and its filamentous plant virus relative (pepino mosaic virus (PepMV)), data indicated similar biodistribution patterns, but the accumulation in breast and ovarian cancer tissues was different: PepMV exhibited about 2-fold higher tumour homing than PVX [264]. The reason for greater tumour homing of PepMV has not yet been investigated. Nonetheless, from our previous observation that CPMV particles accumulated markedly in the sites of inflammation and embryo endothelium owing to their binding to vimentin [188–190], it can be anticipated that VNPs tissue distribution is not only a matter of particle sizes and shapes. There are certainly some chemical characteristics, such as surface chemistry, composition and charge, that play an important role in VNPs biodistribution.
In sum, the discrepancies in the in vivo fates of VNPs are partly related to the variability in particles structural morphology and composition, underlying different pharmacological and toxicological consequences depending on particle design. Therefore, VNPs formulations are to be analysed thoroughly and individually to evaluate any pharmacological or toxicological aspects associated with their structure and morphology attributes.
3.2.2. In vivo biocompatibility of VNPs
Data from the nanotoxicity assessment of VNPs show good biocompatibility profiles for most of the formulations investigated (Table 4), but they also highlight the need for more toxicological investigations in the growing field of VNP bioengineering. Some of the common outcomes includes the fact that both native and chemically modified VNPs show no overt signs of tissue toxicity while being used as therapeutic adjuvants or nano-vehicles for antigenic, therapeutic and/or imaging agents. An example is the study reported by Alemzadeh et al., where Johnson grass chlorotic stripe mosaic virus (JgCSMV) conjugated to folic acid (FA) was used for targeted delivery of doxorubicin to the athymic mice bearing human breast cancer xenografts [85]. In comparison with free doxorubicin, drug-loaded JgCSMV-FA particles exhibited better pharmacological profiles, encompassing effective tumor growth inhibition and reduced drug-related cardiotoxicity. These pharmacological improvements are similar to those achieved by liposomes [265]. In another study, Czapar et al. [80] observed that phenanthriplatin loaded TMV significantly inhibited tumor growth while the free phenanthriplatin drug candidates as well as cisplatin drug were inefficacious at the dose of 1.0 mg/kg. In addition, toxicological study revealed that the TMV drug delivery systems triggered no significant variations in the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT); viability indicators for all organs in general and liver functions, respectively. While liver functions were found to be maintained, the histology staining unveiled signs of necrosis in the kidneys which could be attributed to the inherent nephrotoxicity of the platinium compound. Since no weight loss or changes in animal activity were observed, the authors hypothesized a promising safety profile of phenanthriplatin-loaded TMV owing to the possibility of easily managing the reported potential nephrotoxicity; through either use of diuretics [266] or TMV surface modification with polymers (e.g. PEG) to reduce VNP kidney clearance [250]. In addition to CPMV, TMV and PVX discussed in the previous paragraphs, CCMV was also reported to be biocompatible following IV administration to female Balb/c mice [238]; since no remarkable histopathological changes were observed in the RES organs. Beyond tissue toxicity assessment, there are also a few studies investigating VNPs’ teratogenicity in chicken embryos. The native TBSV and PVX particles tested showed no signs of erythrocyte toxicity or teratogenicity at the doses of 1 ng–10 μg/embryo [239].
Careful safety profiling requires insights beyond histopathological and haematological evaluations, looking into molecular biomarker analysis to detect signs of nonnecrotic biological perturbations. In conjunction with this, previous studies with established nanoparticles, such as liposomes, would make instructive illustrations for VNPs bioengineers. For instance, Knudsen et al. [267] investigated the biocompatibility of cationic liposomes through histological, haematological and chemico-clinical evaluations. The findings revealed no significant changes after repeated doses; however, genotoxicity assessment of the same formulation unveiled DNA strand breaks as well as elevated expression of cytokines in the lung and spleen tissues. These observations are arguably part of the scarce data demonstrating the need for a thorough organ-function assessment when characterizing the safety profile of a given NP formulation, and VNPs make no exception to this diligent consideration. Extended toxicological studies are lacking to provide further insights into the VNPs’ biosafety, considering individual product attributes as well as relevant animal model specifications.
3.3. Immunological considerations of VNPs
VNPs exhibit inherent immunostimulatory properties that have set the foundation for their applications as vaccine adjuvants and immunotherapeutic agents. Owing to their optimal structural characteristics (i.e. size, shape and rigidity), most VNPs cross the lymph vessel pores and traffic into the lymph node, where they markedly stimulate the antigen-presenting cells (APCs) (Figure 7) [268]. The importance of structural organisation of VNPs has been established from the observation that disassembled coat protein was much less immunogenic than VNPs or VLPs [114,269], although the immune system of mammals identifies viral proteins as immunological aliens [124]. VNPs stimulate APCs through interactions with their pattern recognizing receptors (PRRs) such as toll-like receptors (TLRs) [270]. In general, immune cells express PRRs in response to pathogens invasion for early recognition and subsequent activation of innate immune system as well as adaptive immunity [271].
Figure 7:
Draining lymph node (dLN) homing of CPMV and PVX loaded with Alexa Fluor 647 dye. (A) Longitudinal fluorescence imaging of dLN trafficking and retention (dashed arrows) following S.C. injection into the left (CPMV 50μg/20μl) and right (PVX 50μg/20μl) footpads of FVB/N mice: indicating sustained dLN retention of CPMV as compared to PVX. (B) and (C) Confocal microscopy visualization of immunofluorescence staining (green) and imaging of CPMV and PVX accumulations (insets A and B, respectively) in the brachial dLN 12 hours following S.C. injection behind the mice’s neck: showing retention of CPMV in the subcapsular sinus (blue arrow) and T cell zones (yellow arrow), while PVX accumulate (blue arrow) in the follicle zones (red area, anti-B220 antibody staining). Reprinted from [268], Copyright 2016, with permission from Elsevier.
Compared to other nanomaterials used for epitope display, VNPs are putative vaccine adjuvants that achieve targeted delivery of foreign epitopes while interacting with the immune cell network. For example, TMV-T cell epitope display vaccine formulations exhibited superior antitumor efficacy compared to free T-cell peptide epitopes alone in C57BL/6 mice bearing EG.7-Ova and B16 melanomas [127, 272]. Other VNPs vaccine were designed using PapMV, PVX and CPMV; which were used for display and delivery of M2e influenza epitopes [171], hepatitis C virus epitope R9 [273] and HER2 CH401 epitope [125], respectively. The multivalent epitopes display capacity and their natural interactions with and activation of innate immune cells lay the foundation for the application of VNPs/VLPs in vaccinology against cancers [27,274] and infectious diseases [233,275]. Since this section broadly covers immunogenicity of VNPs, readers interested in comprehensive details regarding VNP-based vaccines can look into the recent review by Rybicki [276] or Balke and Zeltins [277], where broader applications such as allergies and autoimmune diseases are discussed.
There is an intriguing immunogenic property of VNPs that resides in their potency to induce long-lasting stimulation of system anti-tumor immunity when said VNP is injected into the tumor tissue – the concept of the in situ vaccination [278,279]. Numerous studies have demonstrated the potential of CPMV in particular as immune modulator of the tumor microenvironment (TME) [280,281]. When injected into the TME, CPMV induces activation and recruitment of innate immune cells such as monocytes, tumor infiltrating neutrophils and natural killer cells that are cytotoxic to cancer cells [282–284]. By increasing the influx of APCs upon tumor antigen release in the TME, these events lead to adaptive anti-tumor immunity through CD4+/CD8+ cells, which results in systemic efficacy and immunological memory against metastases [280]. It is important to mention that the immunogenicity of CPMV is not solely due to its multivalent, proteinaceous capsid. In fact, our group previously observed no matching in efficacy between native (RNA-loaded) and empty CPMV: the former showed greater animal survival rate than the latter [282], which was attributed to additional interactions between RNA and TLR-7/8 (leading to enhanced APCs boosting and cytokines activation) [270]. This is also consistent with observations made using PapMV as an in situ adjuvant for cancer immunotherapy; also here the efficacy was attributed to efficient TLR-7/8 signalling [285].
Apart from CPMV, several other VNPs were tested for in situ vaccination applications. Our group recently compared the in situ vaccine efficacy of CPMV with that of other ~30 nm icosahedral plant viruses (CCMV, PhMV, SeMV) as well as other icosahedrons such as VLPs from bacteriophage Qβ and Hepatitis B virus core particles (HBVc) [286]. The findings demonstrated unique immunological properties of CPMV; with CPMV being the most potent immune stimulant amongst all the VNPs/VLPs assessed against melanoma, ovarian cancer and colon cancer models. In agreement is an earlier study by Murray et al. [287]; where we conducted a comparative study testing in situ vaccine efficacy of CPMV versus TMV particles of different aspect ratio (300 × 18 nm native TMV versus 50 × 18 nm short TMV nanorods versus sphere-like TMV particles). According to the data, CPMV showed the most potent antitumor immunity against dermal melanoma; all the TMV types showed less potent efficacy with no significant difference related to particle shape, size or aspect ratio. The difference in immunostimulatory potency between CPMV and TMV was proposed to arise from the immune activation mechanisms, particularly the pro-inflammatory cyto/chemokine profiling: CPMV establishes immune cell signalling through IFN-γ pathway, while TMV early on triggers primarily IL-6. However, other VNPs also have been reported to have remarkable immunostimulatory properties, these include the filamentous nanoparticles formed by PapMV [285,288] and PVX [289]; the former acting mainly through IFN-α secretion while the latter stimulates IFN-γ pathway (like CPMV).
Noteworthy, all these interactions with the immune system and cells can be regarded as a double-sided sword: desired for immunotherapy applications and a challenge for drug delivery and imaging applications. TMV for example is prevalent in the human population as TMV is found in tobacco products; the anti-TMV antibodies (IgG, IgG1, IgG3, IgG4, IgA, and IgM) have been detected in the serum of healthy smokers, smokeless-tobacco users, as well as non-smokers [290]. With plant viruses being part of the food chain, there is likelihood for a pre-existing immunity. Therefore, careful consideration of the VNP platform and careful in vivo characterization should be carried out – ideally using naïve mice as well as mice pre-exposed to the VNP. As an illustration, Shukla et al. [291] observed that weekly administrations of PVX led to increasing levels of circulating anti-PVX IgM and IgG antibodies that affected PVX bioavailability. The specificity of these antibodies to PVX was confirmed by marked aggregation (i) in vitro incubation of serum from immunized mice with PVX, and (ii) in vivo – significant PVX–antibody aggregates were observed in the murine vasculature using intravital two-photon laser scanning microscopy. This data highlights, that as with other biologics, immunotoxicity must be part of the pharmacology package and particular attention must be paid to pre-existence or development of potentially neutralizing antibodies. The influence of the immunogenicity on nanocarriers efficacy and safety profiles can be illustrated by studies performed on mammalian viral vectors such as adeno-associated virus (AAV) used for gene therapy [292,293]. Although these are attenuated mammalian viruses with demonstrated transfection efficiency, induction of strong innate and adaptive immune responses still pose safety risks [294]. The use of AAV in the clinic is highly challenged by the pre-existing immunity arising from the widespread exposure to the wild type AAV variants and serotypes within the human population; the clinical efficacy is often affected by high levels of neutralizing antibodies. Interested readers are referred to some of the latest reviews discussing challenges associated with viral vector immunogenicity [295–298].
There are different approaches currently investigated at clinical or engineering levels to address AAV immunogenicity. The strategies proposed to improve the clinical outcome of AAV-based gene therapy embrace [299]: (i) selecting subjects showing low or no anti-AAV antibodies response; (ii) conducting plasmapheresis to reduce the amount of neutralizing antibodies; (iii) accomplishing isolated perfusion of organs; (iv) increasing the capsid dose, and (v) immunosuppression. For instance, a 10-patients case study demonstrated significant reduction in anti-AAV titers to undetectable levels in seropositive patients receiving immunosuppressor therapy [300]. In another study, plasmapheresis achieved effective removal of AAV binding antibodies and enhanced gene transfection efficiency (from 10 to 60%) at the same extent as observed for AAV sero-negative nonhuman primates (53%), while immunosuppression failed to improve gene expression [301]. In nanoparticle engineering, one of the promising strategies involves immunoediting to tune domains that can affect immunogenicity, e.g. here we can learn from the oncolytic viral therapy engineering: it has been shown that genetic editing allows tailoring the interaction with the immune structures, such as PRRs [302]. For example, Faust et al. [303] genetically engineered an AAV variant with depleted CpG sequences, a gene set encoding for TLR9 AAV ligand. Data indicated that the engineered AAV achieved excellent transgene expression as well as immune system invasion and reduced infiltration of effector cells. An alternative approach under investigation includes the incorporation of a TLR9-inhibitory sequence (e.g. TTAGGG from human telomeres) into the viral genome, which demonstrated the potential to avoid the murine innate immunity [293,304]. Indeed, rational mutagenesis and combinatorial libraries producing AAV capsid variants that are not detected by anti-AAV antibodies are promising, but their application requires a combination with immunosuppression to prevent de novo antibodies induction. Transient immunosuppression holds a promise to overcome cellular and humoral responses but does fail to address pre-existing neutralizing antibodies. Therefore, the shielding of viral capsid through particle surface modification (covalent attachment or coating) with polymers or biomaterials has been proposed for the engineering of immunotolerant AAV particles [305]. These principles can be extended to plant VNPs for ideal product safety and efficacy.
Nonetheless, more data are needed to fully define and predict all the aspects of immunogenicity to establish the immuno-safety of each VNP formulation. These include for instance the studies interrogating the potential immunotoxicity signs such as fever-like reactions due to the induction of cytokines/interferons, hypersensitivity due to complement activation as well as interaction with coagulation factors. In addition, the existence of diseases that can potentially affect the immune system (such as lysosomal or metabolic alterations [306]) is a further motivation for much deeper understanding of VNP-immune system interactions to anticipate possible interferences. The field of VNP bioengineering continues to rapidly evolve, transitioning from preclinical studies toward translational efforts; therefore, detailed analyses of potential immunotoxicities related to plant viral nanocarriers are highly desired to ensure successful implementation of this high-value technology in the clinic.
4. Conclusion
The increasing interests in VNP nanoengineering has prompted extensive research that has led to remarkable advances in VNP design, fabrication and manufacturing. The field is rapidly evolving, and several formulations and approaches are poised to make a clinical impact. In this report, we discussed the key concepts surrounding the toxicological considerations of VNPs, including particle properties engineering, formulation concepts and pharmacological profiles. We mainly discussed VNPs as therapeutic adjuvants, excipients or vehicles that hold the potential to display, enhance, reduce or suppress the toxicity of a given formulation. Most toxicological studies on VNPs commonly reported no overt signs of toxicity in vitro or in vivo. However, majority of these studies focused on routine cell viability assays as well as histopathological and haematological explorations of animal tissues, looking into cell apoptosis, necrosis or tissue degeneration; with no insights from extensive organ-function investigations. In addition, the lack of studies using unified protocols to interrogate the correlations between VNPs structural modifications and toxicity/biocompatibility is apparent. Yet, the robust-soft structure of VNPs can be valuably exploited to tailor particles’ properties, control biological interactions and determine the structure-nanotoxicity relationships. In depth VNPs structure-toxicity studies could be useful to inform nanoengineers of particulate or molecular mechanisms of nanotoxicity for safe development of nanomedicines. Moreover, systematic studies with several VNP systems side-by-side may provide clues whether there are underlying design concepts, i.e. appraising whether VNPs can be grouped into different classes with distinct pharmacological profiles, or there is indeed a need to study each one separately. The efficacy and safety being the main factors that anticipate product clinical success, thorough explorations of VNPs’ toxicity/biocompatibility will guarantee futuristic blossoming of VNP-based formulations as superior nanomedicines.
Research Highlights.
Plant viruses and their virus-like particles have been recognized as platform technologies for applications in nanomedicine.
Plant virus-based nanoparticles (VNPs) can be repurposed and engineered as smart bio-vehicles for targeted drug delivery and imaging; plant VNPs also find applications in vaccines and immunotherapy.
Given the growing body of data that indicate that plant VNPs are promising high value nanocarriers with potential for translational development, it is critical to gain in-depth understanding of their pharmacology to further advance the field.
The pharmacology of VNPs as a function of formulation and route of administration is discussed; most studies reported no overt toxicity in vitro or in vivo; however, majority of these studies focused on routine cell viability assays as well as histopathological and haematological explorations ; with no insights from extensive organ-function investigations.
Data highlights the potential for VNPs to impact disease management and treatment, but also underlines the need for meticulous VNP structure-nanotoxicity studies to improve our understanding of their in vivo fates and pharmacological profiles to pave the way for translation of VNPs into the clinical setting.
Acknowledgments
Funding: This work is supported in part through grants by the National Institute of Health, U01-CA218292, R01CA253615, R01-CA224605, R01-HL137674; and National Science Foundation NSF RAPID CMMI-2027668; and a Research Scholar award from the American Cancer Society, 128319-RSG-15-144-01-CDD.
Footnotes
Declaration of interest: Dr. Steinmetz is a co-founder of and has a financial interest with Mosaic ImmunoEngineering Inc. Dr. Nkanga declares no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kargozar S, Mozafari M, Nanotechnology and Nanomedicine: Start small, think big, Mater. Today Proc. 5 (2018) 15492–15500. 10.1016/j.matpr.2018.04.155. [DOI] [Google Scholar]
- [2].Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW, Therapeutic efficacy of nanoparticles and routes of administration, Biomater. Res. 23 (2019) 1–29. 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim DH, Image-guided cancer nanomedicine, J. Imaging. 4 (2018) 1–7. 10.3390/jimaging4010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barui AK, Oh JY, Jana B, Kim C, Ryu J, Cancer‐Targeted Nanomedicine: Overcoming the Barrier of the Protein Corona, Adv. Ther. 3 (2020) 1900124. 10.1002/adtp.201900124. [DOI] [Google Scholar]
- [5].Anselmo AC, Mitragotri S, Nanoparticles in the clinic: An update, Bioeng. Transl. Med. 4 (2019) 1–16. 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feng J, Markwalter CE, Tian C, Armstrong M, Prud’Homme RK, Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale, J. Transl. Med. 17 (2019) 1–9. 10.1186/s12967-019-1945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu LP, Wang D, Li Z, Grand challenges in nanomedicine, Mater. Sci. Eng. C. 106 (2020) 110302. 10.1016/j.msec.2019.110302. [DOI] [PubMed] [Google Scholar]
- [8].Wen AM, Steinmetz NF, Design of virus-based nanomaterials for medicine, biotechnology, and energy, Chem. Soc. Rev. 45 (2016) 4074–4126. 10.1039/c5cs00287g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chariou PL, Ortega-Rivera OA, Steinmetz NF, Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients, ACS Nano. (2020). 10.1021/acsnano.0c00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keeler AM, Flotte TR, Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here?, Annu. Rev. Virol. 6 (2019) 601–621. 10.1146/annurev-virology-092818-015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Naso MF, Tomkowicz B, Perry WL, Strohl WR, Adeno-Associated Virus (AAV) as a Vector for Gene Therapy, BioDrugs. 31 (2017) 317–334. 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jeevanandam J, Pal K, Danquah MK, Virus-like nanoparticles as a novel delivery tool in gene therapy, Biochimie. 157 (2019) 38–47. 10.1016/j.biochi.2018.11.001. [DOI] [PubMed] [Google Scholar]
- [13].Shukla S, Hu H, Cai H, Chan S, Boone CE, Beiss V, Chariou PL, Steinmetz NF, Plant Viruses and Bacteriophage-Based Reagents for Diagnosis and Therapy, Annu. Rev. Virol. 7 (2020) 559–87. 10.1146/annurev-virology-010720-052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Furfaro LL, Payne MS, Chang BJ, Bacteriophage Therapy : Clinical Trials and Regulatory Hurdles, Front. Cell. Infect. Microbiol. 8 (2018) 1–7. 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Krystyna D, Phage therapy : What factors shape phage pharmacokinetics and bioavailability ? Systematic and critical review, Med. Res. Rev. (2019) 1–26. 10.1002/med.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abdelkader K, Gerstmans H, Saafan A, Dishisha T, Briers Y, The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes : The Parts are Easier than the Whole, Viruses. 11 (2019) 1–16. 10.3390/v11020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huh H, Wong S, Jean JS, Slavcev R, Bacteriophage interactions with mammalian tissue : Therapeutic applications, Adv. Drug Deliv. Rev. (2019). 10.1016/j.addr.2019.01.003. [DOI] [PubMed] [Google Scholar]
- [18].Sioud M, Phage Display Libraries : From Binders to Targeted Drug Delivery and Human Therapeutics, Mol. Biotechnol. 61 (2019) 286–303. doi: 10.1007/s12033-019-00156-8. [DOI] [PubMed] [Google Scholar]
- [19].Bollyky PL, Mammalian Immune System,. Van Belleghem JD, Dąbrowska K, Vaneechoutte M, Barr JJ, Bollyky PL, Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses. 11 (2019) 10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hess KL, Jewell CM, Phage display as a tool for vaccine and immunotherapy development, Bioeng Transl Med. 5 (2020) 1–15. 10.1002/btm2.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Dong Y, Zhou J, Li X, Wang F, Application of plant viruses as a biotemplate for nanomaterial fabrication, Molecules. 23 (2018). 10.3390/molecules23092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Eiben S, Koch C, Altintoprak K, Southan A, Tovar G, Laschat S, Weiss IM, Wege C, Plant virus-based materials for biomedical applications: Trends and prospects, Adv. Drug Deliv. Rev. 145 (2019) 96–118. 10.1016/j.addr.2018.08.011. [DOI] [PubMed] [Google Scholar]
- [23].Sokullu E, Abyaneh HS, Gauthier MA, Plant/bacterial virus-based drug discovery, drug delivery, and therapeutics, Pharmaceutics. 11 (2019). 10.3390/pharmaceutics11050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chung YH, Cai H, Steinmetz NF, Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications, Adv. Drug Deliv. Rev. (2020). 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Alemzadeh E, Dehshahri A, Izadpanah K, Ahmadi F, Plant virus nanoparticles: Novel and robust nanocarriers for drug delivery and imaging, Colloids Surfaces B Biointerfaces. 167 (2018) 20–27. 10.1016/j.colsurfb.2018.03.026. [DOI] [PubMed] [Google Scholar]
- [26].Hefferon KL, Repurposing plant virus nanoparticles, Vaccines. 6 (2018). 10.3390/vaccines6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shoeb E, Hefferon K, Future of cancer immunotherapy using plant virus-based nanoparticles, Futur. Sci. OA. 5 (2019) FSO401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tremouillaux-guiller J, Moustafa K, Hefferon K, Gaobotse G, Makhzoum A, Plant-made HIV vaccines and potential candidates, Curr. Opin. Biotechnol. 61 (2020) 209–216. 10.1016/j.copbio.2020.01.004. [DOI] [PubMed] [Google Scholar]
- [29].Rycroft T, Trump B, Poinsatte-Jones K, Linkov I, Nanotoxicology and nanomedicine: making development decisions in an evolving governance environment, J. Nanoparticle Res. 20 (2018). 10.1007/s11051-018-4160-3. [DOI] [Google Scholar]
- [30].Iosub CS, Olăreţ E, AM Grumezescu AM Andronescu Holban, E., Toxicity of nanostructures—a general approach, In Nanostructures for Novel Therapy, Elsevier Inc. (2017) 793–809. [Google Scholar]
- [31].Ding X, Liu D, Booth G, Gao W, Lu Y, Virus-Like Particle Engineering: From Rational Design to Versatile Applications, Biotechnol. J. 13 (2018) 1–7. 10.1002/biot.201700324. [DOI] [PubMed] [Google Scholar]
- [32].Hefferon K, Plant virus nanoparticles: New applications and new benefits, Future Virol. 11 (2016) 591–599. 10.2217/fvl-2016-0059. [DOI] [Google Scholar]
- [33].Rohovie MJ, Nagasawa M, Swartz JR, Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery, Bioeng. Transl. Med. 2 (2017) 43–57. 10.1002/btm2.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rybicki EP, Plant molecular farming of virus-like nanoparticles as vaccines and reagents, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 12 (2020) 1–22. 10.1002/wnan.1587. [DOI] [PubMed] [Google Scholar]
- [35].ève Lebel M, Chartrand K, Leclerc D, Lamarre A, Plant viruses as nanoparticle-based vaccines and adjuvants, Vaccines. 3 (2015) 620–637. 10.3390/vaccines3030620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nikitin NA, Trifonova EA, Karpova OV, Atabekov JG, Biosafety of plant viruses for human and animals, Moscow Univ. Biol. Sci. Bull. 71 (2016) 128–134. 10.3103/S0096392516030081. [DOI] [Google Scholar]
- [37].Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM, Toxicological considerations of clinically applicable nanoparticles, Nano Today. 6 (2011) 585–607. 10.1016/j.nantod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Westmeier D, Chen C, Stauber RH, Docter D, The bio-corona and its impact on nanomaterial toxicity, Eur. J. Nanomedicine. 7 (2015) 153–168. 10.1515/ejnm-2015-0018. [DOI] [Google Scholar]
- [39].Hu X, Sun A, Kang W, Zhou Q, Strategies and knowledge gaps for improving nanomaterial biocompatibility, Environ. Int. 102 (2017) 177–189. 10.1016/j.envint.2017.03.001. [DOI] [PubMed] [Google Scholar]
- [40].Lai DY, Toward toxicity testing of nanomaterials in the 21st century: A paradigm for moving forward, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 4 (2012) 1–15. 10.1002/wnan.162. [DOI] [PubMed] [Google Scholar]
- [41].Damoiseaux R, George S, Li M, Pokhrel S, Ji Z, France B, Xia T, Suarez E, Rallo R, Mädler L, Cohen Y, Hoek EMV, Nel A, No time to lose - High throughput screening to assess nanomaterial safety, Nanoscale. 3 (2011) 1345–1360. 10.1039/c0nr00618a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA, Upconverting nanoparticles: Assessing the toxicity, Chem. Soc. Rev. 44 (2015) 1561–1584. 10.1039/c4cs00177j. [DOI] [PubMed] [Google Scholar]
- [43].Elsaesser A, Howard CV, Toxicology of nanoparticles, Adv. Drug Deliv. Rev. 64 (2012) 129–137. 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [44].Zhao J, Castranova V, Toxicology of nanomaterials used in nanomedicine, J. Toxicol. Environ. Heal. - Part B Crit. Rev. 14 (2011) 593–632. 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]
- [45].Rizvi SAA, Saleh AM, Applications of nanoparticle systems in drug delivery technology, Saudi Pharm. J. 26 (2018) 64–70. 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Doktorovova S, Shegokar R, Souto EB, Role of Excipients in formulation development and biocompatibility of lipid nanoparticles (SLNs/NLCs), Elsevier Inc., 2017. 10.1016/B978-0-323-46142-9.00030-X. [DOI] [Google Scholar]
- [47].Ma Y, Nolte RJM, Cornelissen JJLM, Virus-based nanocarriers for drug delivery, Adv. Drug Deliv. Rev. 64 (2012) 811–825. 10.1016/j.addr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- [48].Czapar AE, Steinmetz NF, Plant viruses and bacteriophages for delivery in medicine and biotechnology, Curr. Opin. Chem. Biol. 38 (2017) 108–116. 10.1016/j.cbpa.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee EJ, Lee NK, Kim IS, Bioengineered protein-based nanocage for drug delivery, Adv. Drug Deliv. Rev. 106 (2016) 157–171. 10.1016/j.addr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- [50].Huynh NAT, Hesketh EAL, Saxena P, Meshcheriakova Y, Ku YC, Hoang LAT, Johnson JAE, Ranson NAA, Lomonossoff GAP, Reddy VAS, Crystal Structure and Proteomics Analysis of Empty Virus-like Particles of Cowpea Mosaic Virus, Structure. 24 (2016) 567–575. 10.1016/j.str.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lin T, Chen Z, Usha R, Stauffacher CV, Dai JB, Schmidt T, Johnson JE, The refined crystal structure of cowpea mosaic virus at 2.8 Å resolution, Virology. 265 (1999) 20–34. 10.1006/viro.1999.0038. [DOI] [PubMed] [Google Scholar]
- [52].Liepold LO, Revis J, Allen M, Oltrogge L, Young M, Douglas T, Structural transitions in Cowpea chlorotic mottle virus (CCMV), Phys. Biol. 2 (2005). 10.1088/1478-3975/2/4/S11. [DOI] [PubMed] [Google Scholar]
- [53].Isea R, Aponte C, Cipriani R, Can the RNA of the cowpea chlorotic mottle virus be released through a channel by means of free diffusion? A test in silico, Biophys. Chem. 107 (2004) 101–106. 10.1016/S0301-4622(03)00193-5. [DOI] [PubMed] [Google Scholar]
- [54].Doan DNP, Lee KC, Laurinmäki P, Butcher S, Wong SM, Dokland T, Three-dimensional reconstruction of hibiscus chlorotic ringspot virus, J. Struct. Biol. 144 (2003) 253–261. 10.1016/j.jsb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [55].Izadpanah K, Huth W, Lesemann D‐E, Vetten HJ, Properties of Johnsongrass Chlorotic Stripe Mosaic Virus, J. Phytopathol. 137 (1993) 105–117. 10.1111/j.1439-0434.1993.tb01330.x. [DOI] [Google Scholar]
- [56].Yang S, Wang T, Bohon J, Gagné MÈL, Bolduc M, Leclerc D, Li H, Crystal structure of the coat protein of the flexible filamentous papaya mosaic virus, J. Mol. Biol. 422 (2012) 263–273. 10.1016/j.jmb.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tremblay MH, Majeau N, Gagné MEL, Lecours K, Morin H, Duvignaud JB, Bolduc M, Chouinard N, Paré C, Gagné S, Leclerc D, Effect of mutations K97A and E128A on RNA binding and self assembly of papaya mosaic potexvirus coat protein, FEBS J. 273 (2006) 14–25. 10.1111/j.1742-4658.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- [58].Krishna SS, Hiremath CN, Munshi SK, Prahadeeswaran D, Sastri M, Savithri HS, Murthy MRN, Three-dimensional structure of physalis mottle virus: Implications for the viral assembly, J. Mol. Biol. 289 (1999) 919–934. 10.1006/jmbi.1999.2787. [DOI] [PubMed] [Google Scholar]
- [59].Atabekov J, Dobrov E, Karpova O, Rodionova N, Potato virus X: Structure, disassembly and reconstitution, Mol. Plant Pathol. 8 (2007) 667–675. 10.1111/j.1364-3703.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- [60].Powers JG, Sit TL, Heinsohn C, George CG, Kim K, Lommel SA, The Red clover necrotic mosaic virus RNA-2 encoded movement protein is a second suppressor of RNA silencing, Virology. 381 (2008) 277–286. 10.1016/j.virol.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sherman MB, Guenther RH, Tama F, Sit TL, Brooks CL, Mikhailov AM, V Orlova E, Baker TS, Lommel SA, Al SET, V Irol J, Removal of Divalent Cations Induces Structural Transitions in Red Clover Necrotic Mosaic Virus, Revealing a Potential Mechanism for RNA Release ٌ, J. Virol. 80 (2006) 10395–10406. 10.1128/JVI.01137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chowdhury SR, Savithri HS, Interaction of Sesbania mosaic virus movement protein with the coat protein -implications for viral spread, FEBS J. 278 (2011) 257–272. 10.1111/j.1742-4658.2010.07943.x. [DOI] [PubMed] [Google Scholar]
- [63].Lokesh GL, Gopinath K, Satheshkumar PS, Savithri HS, Complete nucleotide sequence of Sesbania mosaic virus: A new virus species of the genus Sobemovirus, Arch. Virol. 146 (2001) 209–223. 10.1007/s007050170170. [DOI] [PubMed] [Google Scholar]
- [64].Yamamura Y, Scholthof HB, Tomato bushy stunt virus: A resilient model system to study virus-plant interactions, Mol. Plant Pathol. 6 (2005) 491–502. 10.1111/j.1364-3703.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- [65].Hearne PQ, Knorr DA, Hillman BI, Morris TJ, The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus, Virology. 177 (1990) 141–151. 10.1016/0042-6822(90)90468-7. [DOI] [PubMed] [Google Scholar]
- [66].Klug A, The tobacco mosaic virus particle: Structure and assembly, Philos. Trans. R. Soc. B Biol. Sci. 354 (1999) 531–535. 10.1098/rstb.1999.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Namba K, Pattanayek R, Stubbs G, Visualization of protein-nucleic acid interactions in a virus, J. Mol. Biol. 208 (1989) 307–325. 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- [68].Yata T, Lee KY, Dharakul T, Songsivilai S, Bismarck A, Mintz PJ, Hajitou A, Hybrid nanomaterial complexes for advanced phage-guided gene delivery, Mol. Ther. - Nucleic Acids. 3 (2014) e185. 10.1038/mtna.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sharma J, Shepardson K, Johns LL, Wellham J, Avera J, Schwarz B, Rynda-Apple A, Douglas T, A Self-Adjuvanted, Modular, Antigenic VLP for Rapid Response to Influenza Virus Variability, ACS Appl. Mater. Interfaces. 12 (2020) 18211–18224. 10.1021/acsami.9b21776. [DOI] [PubMed] [Google Scholar]
- [70].Park J, Gao H, Wang Y, Hu H, Simon DI, Steinmetz NF, S100A9-targeted tobacco mosaic virus nanoparticles exhibit high specificity toward atherosclerotic lesions in ApoE−/− mice, J. Mater. Chem. B. 7 (2019) 1842–1846. 10.1039/c8tb02276c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang Q, Lin T, Tang L, Johnson JE, Finn MG, Icosahedral virus particles as addressable nanoscale building blocks, Angew. Chemie - Int. Ed. 41 (2002) 459–462. . [DOI] [PubMed] [Google Scholar]
- [72].Colombo M, Mazzucchelli S, Montenegro JM, Galbiati E, Corsi F, Parak WJ, Prosperi D, Protein oriented ligation on nanoparticles exploiting O6- alkylguanine-DNA transferase (SNAP) genetically encoded fusion, Small. 8 (2012) 1492–1497. 10.1002/smll.201102284. [DOI] [PubMed] [Google Scholar]
- [73].Brune KD, Howarth M, New routes and opportunities for modular construction of particulate vaccines: Stick, click, and glue, Front. Immunol. 9 (2018). 10.3389/fimmu.2018.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yildiz I, Lee KL, Chen K, Shukla S, Steinmetz NF, Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus : Cargo-loading and delivery, J. Control. Release. 172 (2013) 568–578. 10.1016/j.jconrel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lockney DM, Guenther RN, Loo L, Overton W, Antonelli R, Clark J, Hu M, Luft C, Lommel SA, Franzen S, The Red clo W er necrotic mosaic W irus Capsid as a Multifunctional Cell Targeting Plant Viral Nanoparticle, Bioconjugate Chem. 22 (2011) 67–73. [DOI] [PubMed] [Google Scholar]
- [76].Steele JFC, Peyret H, Saunders K, Castells-graells R, Marsian J, Meshcheriakova Y, Lomonossoff GP, Synthetic plant virology for nanobiotechnology and nanomedicine, (2017) 1–18. 10.1002/wnan.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhang J, Zhou K, Wang Q, Tailoring the Self-Assembly Behaviors of Recombinant Tobacco Mosaic Virus by Rationally Introducing Covalent Bonding at the Protein – Protein Interface, Small. (2016) 3–7. 10.1002/smll.201503487. [DOI] [PubMed] [Google Scholar]
- [78].Cai H, Shukla S, Steinmetz NF, The Antitumor Efficacy of CpG Oligonucleotides is Improved by Encapsulation in Plant Virus-Like Particles, Adv. Funct. Mater. 1908743 (2020) 1908743. 10.1002/adfm.201908743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cardinale D, Michon T, Virus scaffolds as enzyme nano-carriers, Trends Biotechnol. 30 (2012) 369–376. 10.1016/j.tibtech.2012.04.001. [DOI] [PubMed] [Google Scholar]
- [80].Czapar AE, Zheng YR, Riddell IA, Shukla S, Awuah SG, Lippard SJ, Steinmetz NF, Tobacco Mosaic Virus Delivery of Phenanthriplatin for Cancer therapy, ACS Nano. 10 (2016) 4119–4126. 10.1021/acsnano.5b07360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cao J, Guenther RH, Sit TL, Opperman CH, Lommel SA, Willoughby JA, Loading and Release Mechanism of Red Clover Necrotic Mosaic Virus Derived Plant Viral Nanoparticles for Drug Delivery of Doxorubicin, Small. 10 (2014) 5126–5136. 10.1002/smll.201400558. [DOI] [PubMed] [Google Scholar]
- [82].Lee KL, Uhde-holzem K, Fischer R, Commandeur U, Steinmetz NF, Virus Hybrids as Nanomaterials, Virus Hybrids as Nanomater. Methods Protoc. Methods Mol. Biol. 1108 (2014) 3–21. 10.1007/978-1-62703-751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bruckman MA, Kaur G, Lee AL, Xie F, Sepulveda J, Breitenkamp R, Zhang X, Joralemon M, Russell TP, Emrick T, Wang Q, Surface modification of tobacco mosaic virus with “click” chemistry, ChemBioChem. 9 (2008) 519–523. 10.1002/cbic.200700559. [DOI] [PubMed] [Google Scholar]
- [84].Strable E, Finn MG, Chemical modification of viruses and virus-like particles, Curr. Top. Microbiol. Immunol. 327 (2009) 1–21. 10.1007/978-3-540-69379-6_1. [DOI] [PubMed] [Google Scholar]
- [85].Alemzadeh E, Dehshahri A, Dehghanian AR, Afsharifar A, Behjatnia AA, Izadpanah K, Ahmadi F, Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle, Colloids Surfaces B Biointerfaces. 174 (2019) 80–86. 10.1016/j.colsurfb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- [86].Ren Y, Sek MW, Lim LY, Folic acid-conjugated protein cages of a plant virus: A novel delivery platform for doxorubicin, Bioconjug. Chem. 18 (2007) 836–843. 10.1021/bc060361p. [DOI] [PubMed] [Google Scholar]
- [87].Destito G, Yeh R, Rae CS, Finn MG, Manchester M, Folic Acid-Mediated Targeting of Cowpea Mosaic Virus Particles to Tumor Cells, Chem. Biol. 14 (2007) 1152–1162. 10.1016/j.chembiol.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hu H, Zhang Y, Shukla S, Gu Y, Yu X, Steinmetz NF, Dysprosium-Modified Tobacco Mosaic Virus Nanoparticles for Ultra-High-Field Magnetic Resonance and Near-Infrared Fluorescence Imaging of Prostate Cancer, ACS Nano. 11 (2017) 9249–9258. 10.1021/acsnano.7b04472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sitasuwan P, Lee LA, Li K, Nguyen HG, Wang Q, RGD-conjugated rod-like viral nanoparticles on 2D scaffold improve bone differentiation of mesenchymal stem cells, Front. Chem. 2 (2014) 1–8. 10.3389/fchem.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hu H, Masarapu H, Gu Y, Zhang Y, Yu X, Steinmetz NF, Physalis Mottle Virus-like Nanoparticles for Targeted Cancer Imaging, ACS Appl. Mater. Interfaces. 11 (2019) 18213–18223. 10.1021/acsami.9b03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Park J, Chariou PL, Steinmetz NF, Site-Specific Antibody Conjugation Strategy to Functionalize Virus-Based Nanoparticles, Bioconjug. Chem. (2020). 10.1021/acs.bioconjchem.0c00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pitek AS, Hu H, Shukla S, Steinmetz NF, Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles, ACS Appl. Mater. Interfaces. 10 (2018) 39468–39477. 10.1021/acsami.8b12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bruckman MA, Randolph LN, VanMeter A, Hern S, Shoffstall AJ, Taurog RE, Steinmetz NF, Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice, Virology. 449 (2014) 163–173. 10.1016/j.virol.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hu H, Yang Q, Baroni S, Yang H, Aime S, Steinmetz NF, Polydopamine-decorated tobacco mosaic virus for photoacoustic/magnetic resonance bimodal imaging and photothermal cancer therapy, Nanoscale. 11 (2019) 9760–9768. 10.1039/c9nr02065a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ratnatilaka Na Bhuket P, Luckanagul JA, Rojsitthisak P, Wang Q, Chemical modification of enveloped viruses for biomedical applications, Integr. Biol. (United Kingdom). 10 (2018) 666–679. 10.1039/c8ib00118a. [DOI] [PubMed] [Google Scholar]
- [96].Pokorski JK, Steinmetz NF, The art of engineering viral nanoparticles, Mol. Pharm. 8 (2011) 29–43. 10.1021/mp100225y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Müller TG, Sakin V, Müller B, A spotlight on viruses—application of click chemistry to visualize virus-cell interactions, Molecules. 24 (2019). 10.3390/molecules24030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lee KL, Uhde-holzem K, Fischer R, Commandeur U, Steinmetz NF, Chemical Modification of the Inner and Outer Surfaces of Tobacco Mosaic Virus (TMV), Virus Hybrids as Nanomater. Methods Protoc. Methods Mol. Biol. 1108 (2014) 3–21. 10.1007/978-1-62703-751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Schlick TL, Ding Z, Kovacs EW, Francis MB, Dual-surface modification of the tobacco mosaic virus, J. Am. Chem. Soc. 127 (2005) 3718–3723. 10.1021/ja046239n. [DOI] [PubMed] [Google Scholar]
- [100].Brunel FM, Lewis JD, Destito G, Steinmetz NF, Manchester M, Stuhlmann H, Dawson PE, Hydrazone ligation strategy to assemble multifunctional viral nanoparticles for cell imaging and tumor targeting, Nano Lett. 10 (2010) 1093–1097. 10.1021/nl1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Biabanikhankahdani R, Bayat S, Ho KL, Alitheen NBM, Tan WS, A Simple Add-and-Display Method for Immobilisation of Cancer Drug on His-tagged Virus-like Nanoparticles for Controlled Drug Delivery, Sci. Rep. 7 (2017) 1–12. 10.1038/s41598-017-05525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wnȩk M, Górzny M, Ward MB, Wälti C, Davies AG, Brydson R, Evans SD, Stockley PG, Fabrication and characterization of gold nano-wires templated on virus-like arrays of tobacco mosaic virus coat proteins, Nanotechnology. 24 (2013). 10.1088/0957-4484/24/2/025605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Koho T, Ihalainen TO, Stark M, Uusi-Kerttula H, Wieneke R, Rahikainen R, Blazevic V, Marjomäki V, Tampé R, Kulomaa MS, Hytönen VP, His-tagged norovirus-like particles: A versatile platform for cellular delivery and surface display, Eur. J. Pharm. Biopharm. 96 (2015) 22–31. 10.1016/j.ejpb.2015.07.002. [DOI] [PubMed] [Google Scholar]
- [104].Atabekov J, Nikitin N, Arkhipenko M, Chirkov S, Karpova O, Thermal transition of native tobacco mosaic virus and RNA-free viral proteins into spherical nanoparticles, J. Gen. Virol. 92 (2011) 453–456. 10.1099/vir.0.024356-0. [DOI] [PubMed] [Google Scholar]
- [105].Karpova O, Nikitin N, Chirkov S, Trifonova E, Sheveleva A, Lazareva E, Atabekov J, Immunogenic compositions assembled from tobacco mosaic virus-generated spherical particle platforms and foreign antigens, J. Gen. Virol. 93 (2012) 400–407. 10.1099/vir.0.036293-0. [DOI] [PubMed] [Google Scholar]
- [106].Bruckman MA, Vanmeter A, Steinmetz NF, Nanomanufacturing of Tobacco Mosaic Virus-Based Spherical Biomaterials Using a Continuous Flow Method, ACS Biomater. Sci. Eng. 1 (2015) 13–18. 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Bruckman MA, Hern S, Jiang K, Flask CA, Yu X, Steinmetz NF, Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents, J. Mater. Chem. B. 1 (2013) 1482–1490. 10.1039/c3tb00461a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Shukla S, Ablack AL, Wen AM, Lee KL, Lewis JD, Steinmetz NF, Increased tumor homing and tissue penetration of the filamentous plant viral nanoparticle potato virus X, Mol. Pharm. 10 (2013) 33–42. 10.1021/mp300240m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lee KL, Hubbard LC, Hern S, Yildiz I, Gratzl M, Steinmetz NF, Shape matters: The diffusion rates of TMV rods and CPMV icosahedrons in a spheroid model of extracellular matrix are distinct, Biomater. Sci. 1 (2013) 581–588. 10.1039/c3bm00191a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wen AM, Wang Y, Jiang K, Hsu GC, Gao H, Lee KL, Yang AC, Yu X, Simon DI, Steinmetz NF, Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging, J. Mater. Chem. B. 3 (2015) 6037–6045. 10.1039/c5tb00879d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Firme CP, Bandaru PR, Toxicity issues in the application of carbon nanotubes to biological systems, Nanomedicine Nanotechnology, Biol. Med. 6 (2010) 245–256. 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- [112].Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE, Shape effects of filaments versus spherical particles in flow and drug delivery, Nat. Nanotechnol. 2 (2007) 249–255. 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shukla S, Eber FJ, Nagarajan AS, Difranco NA, Schmidt N, Wen AM, Eiben S, Twyman RM, Wege C, Steinmetz NF, The impact of aspect ratio on the biodistribution and tumor homing of rigid soft-matter nanorods, Adv. Healthc. Mater. 4 (2015) 874–882. 10.1002/adhm.201400641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Murray AA, Wang C, Fiering S, Steinmetz NF, In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma, Mol. Pharm. 15 (2018) 3700–3716. 10.1021/acs.molpharmaceut.8b00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Witus LS, Francis MB, Using Synthetically Modified Proteins to Make New Materials, Acc. Chem. Res. 44 (2011) 774–783. 10.1021/ar2001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Finbloom JA, Aanei IL, Bernard JM, Klass SH, Elledge SK, Han K, Ozawa T, Nicolaides TP, Berger MS, Francis MB, Evaluation of Three Morphologically Distinct Virus-Like Particles as Nanocarriers for Convection-Enhanced Drug Delivery to Glioblastoma, Nanomaterials. 8 (2018). 10.3390/nano8121007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wege C, Koch C, From stars to stripes: RNA-directed shaping of plant viral protein templates—structural synthetic virology for smart biohybrid nanostructures, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 12 (2020) 1–44. 10.1002/wnan.1591. [DOI] [PubMed] [Google Scholar]
- [118].Hu H, Steinmetz NF, Doxorubicin-Loaded Physalis Mottle Virus Particles Function as a pH-Responsive Prodrug Enabling Cancer Therapy, Biotechnol. J. 2000077 (2020). 10.1002/biot.202000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Franke CE, Czapar AE, Patel RB, Steinmetz NF, Tobacco Mosaic Virus-Delivered Cisplatin Restores E ffi cacy in Platinum-Resistant Ovarian Cancer Cells, Mol. Pharm. 15 (2018) 2922–2931. 10.1021/acs.molpharmaceut.7b00466. [DOI] [PubMed] [Google Scholar]
- [120].Lockney DM, Guenther RN, Loo L, Overton W, Antonelli R, Clark J, Hu M, Luft C, Lommel SA, Franzen S, The Red clover necrotic mosaic virus Capsid as a Multifunctional Cell Targeting Plant Viral Nanoparticle, Bioconjugate Chem. 22 (2011) 67–73. 10.1021/bc100361z. [DOI] [PubMed] [Google Scholar]
- [121].Lam P, Lin RD, Steinmetz NF, Delivery of mitoxantrone using a plant virus-based nanoparticle for the treatment of glioblastomas, J. Mater. Chem. B. 6 (2018) 5888–5895. 10.1039/c8tb01191e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shukla S, Roe AJ, Liu R, Veliz FA, Commandeur U, Wald DN, Steinmetz NF, Affinity of plant viral nanoparticle potato virus X (PVX) towards malignant B cells enables cancer drug delivery, Biomater. Sci. (2020). 10.1039/d0bm00683a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Patel BK, Wang C, Lorens B, Levine AD, Steinmetz NF, Shukla S, Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO ‑ 1 Vaccine Elicits an Antigen-Speci fi c Cytotoxic T Cell Response, (2020). 10.1021/acsabm.0c00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Denis J, Majeau N, Acosta-ramirez E, Savard C, Bedard M, Simard S, Lecours K, Bolduc M, Pare C, Willems B, Shoukry N, Tessier P, Lacasse P, Lamarre A, Lapointe R, Lopez C, Leclerc D, Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope : Evidence for the critical function of multimerization, Viro. 363 (2007) 59–68. 10.1016/j.virol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [125].Shukla S, Jandzinski M, Wang C, Gong X, Bonk KW, Keri RA, Steinmetz NF, A Viral Nanoparticle Cancer Vaccine Delays Tumor Progression and Prolongs Survival in a HER2 + Tumor Mouse Model, Adv. Ther. 2 (2019) 1800139. 10.1002/adtp.201800139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, Benvenuto E, Capone I, Superiore I, Chimeric Plant Virus Particles as Immunogens for Inducing Murine and Human Immune Responses against Human Immunodeficiency Virus Type 1, J. Virol. 75 (2001) 8434–8439. 10.1128/JVI.75.18.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mccormick AA, Corbo TA, Wykoff-clary S, Palmer KE, Pogue GP, Chemical Conjugate TMV - Peptide Bivalent Fusion Vaccines Improve Cellular Immunity and Tumor Protection, Bioconjugate Chem. 17 (2006) 1330–1338. 10.1021/bc060124m. [DOI] [PubMed] [Google Scholar]
- [128].Maharaj PD, Mallajosyula JK, Lee G, Thi P, Zhou Y, Nanoparticle Encapsidation of Flock House Virus by Auto Assembly of Tobacco Mosaic Virus Coat Protein, Int. J. Mol. Sci. 15 (2014) 18540–18556. 10.3390/ijms151018540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mccormick AA, Kearney CM, In planta Production of Flock House Virus Transencapsidated RNA and Its Potential Use as a Vaccine, Mol Biotechnol. 57 (2015) 325–336. 10.1007/s12033-014-9826-1. [DOI] [PubMed] [Google Scholar]
- [130].Lam P, Steinmetz NF, Delivery of siRNA therapeutics using cowpea chlorotic mottle virus-like particles, Biomater. Sci. 7 (2019) 3138–3142. 10.1039/c9bm00785g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Azizgolshani O, Garmann RF, Cadena-nava R, Knobler CM, Gelbart WM, Reconstituted plant viral capsids can release genes to mammalian cells, Virology. 441 (2013) 12–17. 10.1016/j.virol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- [132].Sánchez-sánchez L, Cadena-nava RD, Palomares LA, Ruiz-garcia J, Koay MST, Cornelissen JJMT, Vazquez-duhalt R, Chemotherapy pro-drug activation by biocatalytic virus-like nanoparticles containing cytochrome P450, Enzyme Microb. Technol. 60 (2014) 24–31. 10.1016/j.enzmictec.2014.04.003. [DOI] [PubMed] [Google Scholar]
- [133].Uhde-holzem K, Mcburney M, Tiu BDB, Advincula RC, Fischer R, Commandeur U, Steinmetz NF, Production of Immunoabsorbent Nanoparticles by Displaying Single-Domain Protein A on Potato Virus X a, Macromol. Biosci. 16 (2016) 231–241. 10.1002/mabi.201500280. [DOI] [PubMed] [Google Scholar]
- [134].Pitek AS, Wang Y, Gulati S, Stewart PL, Simon DI, Steinmetz NF, Elongated plant virus-based nanoparticles for enhanced delivery of thrombolytic therapies, Mol. Pharm. 14 (2017) 3815–3823. 10.1021/acs.molpharmaceut.7b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pitek AS, Park J, Wang Y, Gao H, Hu H, Simon DI, Steinmetz NF, Delivery of thrombolytic therapy using rod-shaped plant viral nanoparticles decreases the risk of hemorrhage, Nanoscale. 10 (2018) 16547–16555. 10.1039/c8nr02861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Le DHT, Commandeur U, Steinmetz NF, Presentation and delivery of tumor necrosis factor-related apoptosis-inducing ligand via elongated plant viral nanoparticle enhances antitumor efficacy, ACS Nano. 13 (2019) 2501–2510. 10.1021/acsnano.8b09462. [DOI] [PubMed] [Google Scholar]
- [137].Leong HS, Steinmetz NF, Ablack A, Destito G, Zijlstra A, Stuhlmann H, Manchester M, Lewis JD, Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles, Nat. Protoc. 5 (2010) 21–23. 10.1038/nprot2010-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Prasuhn DE, Yeh RM, Obenaus A, Finn MG, Viral MRI contrast agents : coordination of Gd by native virions and attachment of Gd complexes by azide – alkyne cycloaddition, Chem. Commun. 12 (2007) 1269–1271. 10.1039/b615084e. [DOI] [PubMed] [Google Scholar]
- [139].Shukla S, Dickmeis C, Fischer R, Commandeur U, Steinmetz NF, In planta production of fluorescent filamentous plant virus-based nanoparticles. In: Wege C, Lomonossoff G (eds) Virus-Derived Nanoparticles for Advanced Technologies. Methods in Molecular Biology, vol 1776. Humana Press, New York, NY. 10.1007/978-1-4939-7808-3_5. [DOI] [PubMed] [Google Scholar]
- [140].Wen AM, Infusino M, De Luca A, Kernan DL, Czapar AE, Strangi G, Steinmetz NF, Interface of Physics and Biology: Engineering Virus-Based Nanoparticles for Biophotonics, Bioconjugate Chem. 26 (2015) 51–62. 10.1021/bc500524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cho C, Yu L, Nsiama TK, Kadam AN, Raturi A, Shukla S, Amadei GA, Steinmetz NF, Luyt LG, Lewis JD, Viral nanoparticles decorated with novel EGFL7 ligands enable intravital imaging of tumor, Nanoscale. 9 (2017) 12096–12109. 10.1039/c7nr02558k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nanoparticle PV, Chariou PL, Lee KL, Wen AM, Gulati NM, Stewart PL, Steinmetz NF, Detection and Imaging of Aggressive Cancer Cells Using an Epidermal Growth Factor Receptor (EGFR)-Targeted Filamentous Plant Virus-Based Nanoparticle, Bioconjugate Chem. 26 (2015) 262–269. 10.1021/bc500545z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tian Y, Zhou M, Shi H, Gao S, Xie G, Zhu M, Wu M, Chen J, Niu Z, Integration of Cell-Penetrating Peptides with Rod-like Bionanoparticles: Virus-Inspired Gene-Silencing Technology, Nano Lett. 18 (2018) 5453–5460. 10.1021/acs.nanolett.8b01805. [DOI] [PubMed] [Google Scholar]
- [144].Rae CS, Wei Khor I, Wang Q, Destito G, Gonzalez MJ, Singh P, Thomas DM, Estrada MN, Powell E, Finn MG, Manchester M, Systemic trafficking of plant virus nanoparticles in mice via the oral route, Virology. 343 (2005) 224–235. 10.1016/j.virol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- [145].Berardi A, Castells-Graells R, Lomonossoff GP, High stability of plant-expressed virus-like particles of an insect virus in artificial gastric and intestinal fluids, Eur. J. Pharm. Biopharm. 155 (2020) 103–111. 10.1016/j.ejpb.2020.08.012. [DOI] [PubMed] [Google Scholar]
- [146].Hansen LJJ, Daoussi R, Vervaet C, Remon J, De Beer TRM, Freeze-drying of live virus vaccines: A review, Vaccine. 30 (2015). 10.1016/j.vaccine.2015.08.085. [DOI] [PubMed] [Google Scholar]
- [147].Czyż M, Pniewski T, Thermostability of Freeze‐Dried Plant‐Made VLP‐Based Vaccines, IntechOpen. (2016). doi: 10.5772/63503. [DOI] [Google Scholar]
- [148].Zheng Y, Lee PW, Wang C, Thomas LD, Stewart PL, Steinmetz NF, Pokorski JK, Freeze-Drying to Produce Efficacious CPMV Virus-like Particles, Nano Lett. 19 (2019) 2099–2105. 10.1021/acs.nanolett.9b00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, Crary-Burney M, Ariaspulido H, Veliz FA, Steinmetz NF, Fiering SN, Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation, Mol. Pharm. 15 (2018) 3717–3722. 10.1021/acs.molpharmaceut.8b00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Luckanagul JA, Lee LA, You S, Yang X, Wang Q, Plant virus incorporated hydrogels as scaffolds for tissue engineering possess low immunogenicity in vivo, J. Biomed. Mater. Res. - Part A. 103 (2015) 887–895. 10.1002/jbm.a.35227. [DOI] [PubMed] [Google Scholar]
- [151].Lin YY, Schuphan J, Dickmeis C, Buhl EM, Commandeur U, Fischer H, Attachment of Ultralow Amount of Engineered Plant Viral Nanoparticles to Mesenchymal Stem Cells Enhances Osteogenesis and Mineralization, Adv. Healthc. Mater. 2001245 (2020) 1–13. 10.1002/adhm.202001245. [DOI] [PubMed] [Google Scholar]
- [152].Marín-Caba L, Chariou PL, Pesquera C, Correa-Duarte MA, Steinmetz NF, Tobacco Mosaic Virus-Functionalized Mesoporous Silica Nanoparticles, a Wool-Ball-like Nanostructure for Drug Delivery, Langmuir. 35 (2019) 203–211. 10.1021/acs.langmuir.8b03337. [DOI] [PubMed] [Google Scholar]
- [153].Czapar AE, Tiu BDB, Veliz FA, Pokorski JK, Steinmetz NF, Slow-Release Formulation of Cowpea Mosaic Virus for In Situ Vaccine Delivery to Treat Ovarian Cancer, Adv. Sci. 5 (2018) 1–8. 10.1002/advs.201700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wang C, Fernández de Ávila BE, Mundaca-Uribe R, Lopez-Ramirez MA, Ramírez-Herrera DE, Shukla S, Steinmetz NF, Wang J, Active Delivery of VLPs Promotes Anti-Tumor Activity in a Mouse Ovarian Tumor Model, Small. 1907150 (2020) 1–11. 10.1002/smll.201907150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Shao S, Ortega-rivera OA, Pokorski JK, Steinmetz NF, A scalable manufacturing approach to single dose vaccination against HPV (unpublished). [DOI] [PMC free article] [PubMed]
- [156].Kalyanasundaram S, Feinstein S, Nicholson JP, Leong KW, Garver RI, Coacervate microspheres as carriers of recombinant adenoviruses, Cancer Gene Ther. 6 (1999) 107–112. [DOI] [PubMed] [Google Scholar]
- [157].Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu D, Ma C, Nesbit M, Crombleholme TM, Sosnowski BA, Pierce GF, FGF2-Targeted Adenovirus Encoding Platelet-Derived Growth Factor-B Enhances de Novo Tissue Formation, Mol. Ther. 2 (2000) 153–160. 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- [158].Wang Y, Liu S, Li CY, Yuan F, A novel method for viral gene delivery in solid tumors, Cancer Res. 65 (2005) 7541–7545. 10.1158/0008-5472.CAN05-1112. [DOI] [PubMed] [Google Scholar]
- [159].Dandu R, H.G. Ã, Delivery of bioactive agents from recombinant polymers, Prog. Polym. Sci. 32 (2007) 1008–1030. 10.1016/j.progpolymsci.2007.05.015. [DOI] [Google Scholar]
- [160].Hatefi A, Cappello J, Ghandehari H, Adenoviral Gene Delivery to Solid Tumors by Recombinant Silk Y Elastinlike Protein Polymers, Pharm. Res. 24 (2007) 7–13. 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- [161].Greish K, Araki K, Li D, Malley BWO, Dandu R, Frandsen J, Cappello J, Ghandehari H, Silk-Elastinlike Protein Polymer Hydrogels for Localized Adenoviral Gene Therapy of Head and Neck Tumors, Biomacromolecules. 10 (2009) 2183–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Boone CE, Wang C, Lopez-ramirez MA, Beiss V, Shukla S, Chariou PL, Kupor D, Rueda R, Wang J, Steinmetz NF, Active Microneedle Administration of Plant Virus Nanoparticles for Cancer In Situ Vaccination Improves Immunotherapeutic Efficacy, ACS Appl. Nano Mater. (2020). 10.1021/acsanm.0c01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wu T, Tang M, Review of the effects of manufactured nanoparticles on mammalian target organs, J. Appl. Toxicol. 38 (2018) 25–40. 10.1002/jat.3499. [DOI] [PubMed] [Google Scholar]
- [164].Fu C, Liu T, Li L, Liu H, Chen D, Tang F, The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes, Biomaterials. 34 (2013) 2565–2575. 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- [165].Ashajyothi C, K Handral H, Kelmani C, A Comparative In Vivo Scrutiny of Biosynthesized Copper and Zinc Oxide Nanoparticles by Intraperitoneal and Intravenous Administration Routes in Rats, Nanoscale Res. Lett. 13 (2018). 10.1186/s11671-018-2497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Bin Zhai Z, Meng AM, Liu PX, Zhang LA, Fan FY, Toxicologic effects of gold nanoparticles in vivo by different administration routes, Int. J. Nanomedicine. 5 (2010) 771–781. 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Rioux G, Mathieu C, Russell A, Bolduc M, Laliberté-Gagné ME, Savard P, Leclerc D, PapMV nanoparticles improve mucosal immune responses to the trivalent inactivated flu vaccine, J. Nanobiotechnology. 12 (2014) 1–6. 10.1186/1477-3155-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Gonzalez MJ, Plummer EM, Rae CS, Manchester M, Interaction of Cowpea mosaic virus (CPMV) nanoparticles with antigen presenting cells in vitro and in vivo, PLoS One. 4 (2009) e7981. 10.1371/journal.pone.0007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Vishnu Vardhan GP, Savithri HS, Murthy MRN, Hema M, Biodistribution and toxicity evaluation of sesbania mosaic virus nanoparticles in mice, Arch. Virol. 161 (2016) 2673–2681. 10.1007/s00705-016-2958-9. [DOI] [PubMed] [Google Scholar]
- [170].Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, Osborn K, Finn MG, Manchester M, Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo, J. Control. Release. 120 (2007) 41–50. 10.1016/j.jconrel.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Denis J, Acosta-Ramirez E, Zhao Y, Hamelin ME, Koukavica I, Baz M, Abed Y, Savard C, Pare C, Lopez Macias C, Boivin G, Leclerc D, Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform, Vaccine. 26 (2008) 3395–3403. 10.1016/j.vaccine.2008.04.052. [DOI] [PubMed] [Google Scholar]
- [172].Li X, Wang L, Fan Y, Feng Q, Cui FZ, Biocompatibility and toxicity of nanoparticles and nanotubes, J. Nanomater. 2012 (2012). 10.1155/2012/548389. [DOI] [Google Scholar]
- [173].S Olmsted S, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA, Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 81 (2001) 1930–1937. 10.1016/S0006-3495(01)75844-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Lai SK, Wang YY, Hida K, Cone R, Hanes J, Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA 107 (2010) 598–603. 10.1073/pnas.0911748107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Lai SK, Wang YY, Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61 (2009) 158–171. 10.1016/j.addr.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Abdulkarim M, Agulló N, Cattoz B, Griffiths P, Bernkop-Schnürch A, Borros SG, Gumbleton M, Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur. J. Pharm. Biopharm. 97 (2015) 230–238. 10.1016/j.ejpb.2015.01.023 [DOI] [PubMed] [Google Scholar]
- [177].Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, Hanes J, Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus, Proc. Natl. Acad. Sci. USA 104 (2007) 1482–1487. 10.1073/pnas.0608611104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J, Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine, Nanomedicine 6 (2011) 365–375. 10.1016/j [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Berardi A, Bombelli FB, Thuenemann EC, Lomonossoff GP, Viral nanoparticles can elude protein barriers: exploiting rather than imitating nature. Nanoscale 11 (2019) 2306–2316. DOI: 10.1039/C8NR09067J [DOI] [PubMed] [Google Scholar]
- [180].Rosales-Mendoza S, González-Ortega O, Nanovaccines, an innovative technology to fight human and animal diseases, Springer Nature Switzerland AG (2019). 10.1007/978-3-030-31668-6 [DOI] [Google Scholar]
- [181].Beatty PH, Lewis JD, Cowpea mosaic virus nanoparticles for cancer imaging and therapy, Adv. Drug Deliv. Rev. 145 (2019) 130–144. 10.1016/j.addr.2019.04.005 [DOI] [PubMed] [Google Scholar]
- [182].Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I, Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties, Nanoscale Res. Lett. 13 (2018). 10.1186/s11671-018-2457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Bruckman MA, Randolph LN, Gulati NM, Stewart PL, Steinmetz NF, Silica-coated Gd(DOTA)-loaded protein nanoparticles enable magnetic resonance imaging of macrophages, J. Mater. Chem. B. 3 (2015) 7503–7510. 10.1039/c5tb01014d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Abraham A, Natraj U, Karande AA, Gulati A, Murthy MR, Murugesan S, Mukunda P, Savithri HS. Intracellular delivery of antibodies by chimeric Sesbania mosaic virus (SeMV) virus like particles. Sci. Rep. 6 (2016) 21803. DOI: 10.1038/srep21803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wu M, Shi J, Fan D, Zhou Q, Wang F, Niu Z, Huang Y, Biobehavior in normal and tumor-bearing mice of tobacco mosaic virus, Biomacromolecules. 14 (2013) 4032–4037. 10.1021/bm401129j [DOI] [PubMed] [Google Scholar]
- [186].Pitek AS, Hu H, Shukla S, Steinmetz NF, Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles, ACS Appl. Mater. Interfaces. 10 (2018) 39468–39477. 10.1021/acsami.8b12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Shukla S, Wen AM, Ayat NR, Commandeur U, Gopalkrishnan R, Broome AM, Lozada KW, Keri RA, Steinmetz NF, Biodistribution and clearance of a filamentous plant virus in healthy and tumor-bearing mice, Nanomedicine. 9 (2014) 221–235. 10.2217/nnm.13.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Steinmetz NF, Maurer J, Sheng H, Bensussan A, Maricic I, Kumar V, Braciak TA, Two domains of vimentin are expressed on the surface of lymph node, bone and brain metastatic prostate cancer lines along with the putative stem cell marker proteins CD44 and CD133, Cancers (Basel). 3 (2011) 2870–2885. 10.3390/cancers3032870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M, Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin, PLoS Pathog. 5 (2009). 10.1371/journal.ppat.1000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Steinmetz NF, Cho CF, Ablack A, Lewis JD, Manchester M, Cowpea mosaic virus nanoparticles target surface vimentin on cancer cells, Nanomedicine. 6 (2011) 351–364. 10.2217/nnm.10.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Koudelka KJ, Rae CS, Gonzalez MJ, Manchester M, Interaction between a 54-Kilodalton Mammalian Cell Surface Protein and Cowpea Mosaic Virus, J. Virol. 81 (2007) 1632–1640. 10.1128/JVI.00960-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Vicart P, Laurent-winter C, Interaction of Theiler’s Virus with Intermediate Filaments of Infected Cells, J. Virol. 72 (1999). 10.1128/JVI.72.12.9553-9560.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Kim J, Fahad A, Shanmukhappa K, Kapil S, Al KIMET, V Irol J, Defining the Cellular Target(s) of Porcine Reproductive and Respiratory Syndrome Virus Blocking Monoclonal Antibody 7G10, J. Virol. 80 (2006) 689–696. 10.1128/JVI.80.2.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wen AM, Le N, Zhou X, Steinmetz NF, Popkin DL, Tropism of CPMV to Professional Antigen Presenting Cells Enables a Platform to Eliminate Chronic Infections, ACS Biomater. Sci. Eng. 1 (2015) 1050–1054. 10.1021/acsbiomaterials.5b00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yang J, Weinberg RA, Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis, Dev. Cell. 14 (2008) 818–829. 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- [196].Vishnu Vardhan GP, Hema M, Sushmitha C, Savithri HS, Natraj U, Murthy MRN, Development of sesbania mosaic virus nanoparticles for imaging, Arch. Virol. 164 (2019) 497–507. 10.1007/s00705-018-4097-y [DOI] [PubMed] [Google Scholar]
- [197].Plummer EM, Manchester M, Endocytic uptake pathways utilized by CPMV nanoparticles, Mol. Pharm. 10 (2013) 26–32. 10.1021/mp300238w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Tian Y, Gao S, Wu M, Liu X, Qiao J, Zhou Q, Jiang S, Niu Z, Tobacco Mosaic Virus-Based 1D Nanorod-Drug Carrier via the Integrin-Mediated Endocytosis Pathway, ACS Appl. Mater. Interfaces. 8 (2016) 10800–10807. 10.1021/acsami.6b02801 [DOI] [PubMed] [Google Scholar]
- [199].Liu X, Wu F, Tian Y, Wu M, Zhou Q, Jiang S, Niu Z, Size Dependent Cellular Uptake of Rod-like Bionanoparticles with Different Aspect Ratios, Sci. Rep. 6 (2016) 2–11. 10.1038/srep24567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Gulati NM, Pitek AS, Czapar AE, Stewart PL, Steinmetz NF, The in vivo fates of plant viral nanoparticles camouflaged using self-proteins: Overcoming immune recognition, J. Mater. Chem. B. 6 (2018) 2204–2216. 10.1039/c7tb03106h [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Aljabali AAA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ, CPMV-DOX delivers, Mol. Pharm. 10 (2013) 3–10. 10.1021/mp3002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Villagrana-Escareño MV, Reynaga-Hernández E, Galicia-Cruz OG, Durán-Meza AL, De La Cruz-González V, Hernández-Carballo CY, Ruíz-García J, VLPs Derived from the CCMV Plant Virus Can Directly Transfect and Deliver Heterologous Genes for Translation into Mammalian Cells, Biomed Res. Int. 2019 (2019). 10.1155/2019/4630891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Savage DT, Hilt JZ, Dziubla TD, In vitro methods for assessing nanoparticle toxicity, Methods Mol. Biol. 1894 (2019) 1–29. 10.1007/978-1-4939-8916-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Joris F, Manshian BB, Peynshaert K, De Smedt SC, Braeckmans K, Soenen SJ, Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap, Chem. Soc. Rev. 42 (2013) 8339–8359. 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- [205].Marquis BJ, Love SA, Braun KL, Haynes CL, Analytical methods to assess nanoparticle toxicity, Analyst. 134 (2009) 425–439. 10.1039/b818082b. [DOI] [PubMed] [Google Scholar]
- [206].Yang Y, Qin Z, Zeng W, Yang T, Cao Y, Mei C, Kuang Y, Toxicity assessment of nanoparticles in various systems and organs, Nanotechnol. Rev. 6 (2017) 279–289. 10.1515/ntrev-2016-0047. [DOI] [Google Scholar]
- [207].Le DHT, Lee KL, Shukla S, Commandeur U, Steinmetz NF, Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy, Nanoscale. 9 (2017) 2348–2357. 10.1039/c6nr09099k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Masarapu H, Patel BK, Chariou PL, Hu H, Gulati NM, Carpenter BL, Ghiladi RA, Shukla S, Steinmetz NF, Physalis Mottle Virus-Like Particles as Nanocarriers for Imaging Reagents and Drugs, Biomacromolecules. 18 (2017) 4141–4153. 10.1021/acs.biomac.7b01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Gregory SA, Mohrbacher A, Hussein MA, Dexamethasone Provide Significant Reduction in Toxicity Compared with Doxorubicin, Vincristine, and Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma, Cancer. 106 (2006) 848–858. 10.1002/cncr.21662. [DOI] [PubMed] [Google Scholar]
- [210].Franke CE, Czapar AE, Patel RB, Steinmetz NF, Tobacco Mosaic Virus-Delivered Cisplatin Restores Efficacy in Platinum-Resistant Ovarian Cancer Cells, Mol. Pharm. 15 (2018) 2922–2931. 10.1021/acs.molpharmaceut.7b00466. [DOI] [PubMed] [Google Scholar]
- [211].Theodoulou M, Hudis C, Cardiac Profiles of Liposomal Anthracyclines: Greater Cardiac Safety versus Conventional Doxorubicin?, Cancer. 100 (2004) 2052–2063. 10.1002/cncr.20207. [DOI] [PubMed] [Google Scholar]
- [212].Pongrac IM, Pavičić I, Milić M, Ahmed LB, Babič M, Horák D, Vrček IV, Gajović S, Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles, Int. J. Nanomedicine. 11 (2016) 1701–1715. 10.2147/IJN.S102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Li L, Wang L, Xiao R, Zhu G, Li Y, Liu C, Yang R, Tang Z, Li J, Huang W, Chen L, Zheng X, He Y, Tan J, The invasion of tobacco mosaic virus RNA induces endoplasmic reticulum stress-related autophagy in HeLa cells, Biosci. Rep. 32 (2012) 171–184. 10.1042/BSR20110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Gurunathan S, Jeyaraj M, La H, Yoo H, Choi Y, Do JT, Park C, Kim J-H, Hong K, Anisotropic Platinum Nanoparticle-Induced Cytotoxicity, Apoptosis, Inflammatory Response, and Transcriptomic and Molecular Pathways in Human Acute Monocytic Leukemia Cells, Int. J. Mol. Sci. 21 (2020). 10.3390/ijms21020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Cameron SJ, Hosseinian F, Willmore WG, A Current Overview of the Biological and Cellular Effects of Nanosilver, Nt. J. Mol. Sci. 19 (2018) 1–40. 10.3390/ijms19072030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Pedata P, Petrarca C, Garzillo EM, Di Gioacchino M, Immunotoxicological impact of occupational and environmental nanoparticles exposure : The influence of physical, chemical, and combined characteristics of the particles, Int. J. Immunopathol. Pharmacol. 29 (2016) 343–353. 10.1177/0394632015608933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Manke A, Wang L, Rojanasakul Y, Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity, Biomed Res. Int. 2013 (2013). 10.1155/2013/942916 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Sohaebuddin SK, Thevenot PT, Baker D, Eaton JW, Tang L, Nanomaterial cytotoxicity is composition, size, and cell type dependent, Part. Fibre Toxicol. 7 (2010) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Krishna V, Devey M, Hawkins S, Hails L, Davis SA, Mann S, Chang IT, Ingham E, Malhas A, Vaux DJ, Lane JD, Case CP, Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity, Biomaterials. 34 (2013) 3559–3570. 10.1016/j.biomaterials.2013.01.085. [DOI] [PubMed] [Google Scholar]
- [220].Mutlu M, Budinger GRS, Green AA, Urich D, Soberanes S, Chiarella SE, Alheid GF, Mccrimmon DR, Szleifer I, Hersam MC, Biocompatible Nanoscale Dispersion of Single-Walled Carbon Nanotubes Minimizes in vivo Pulmonary Toxicity, Nano Lett. 10 (2010) 1664–1670. 10.1021/nl9042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Sioutas C, Delfino RJ, Singh M, Exposure Assessment for Atmospheric Ultrafine Particles (UFPs) and Implications in Epidemiologic Research, Environ. Health Perspect. 113 (2005) 947–956. 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE, Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm, Nano Lett. 6 (2006) 1794–1807. [DOI] [PubMed] [Google Scholar]
- [223].Yang M, Zhang M, Biodegradation of Carbon Nanotubes by Macrophages, Front. Mater. 6 (2019) 1–7. 10.3389/fmats.2019.00225. [DOI] [Google Scholar]
- [224].Patil RM, Thorat ND, Shete PB, Bedge PA, Gavde S, Joshi MG, Tofail SAM, Bohara RA, Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles, Biochem. Biophys. Reports. 13 (2018) 63–72. 10.1016/j.bbrep.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Feng Q, Liu Y, Huang J, Chen K, Huang J, Xiao K, Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings, Sci. Rep. 8 (2018) 1–13. 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Risom L, Møller P, Loft S, Oxidative stress-induced DNA damage by particulate air pollution, Mutat. Res. - Fundam. Mol. Mech. Mutagen. 592 (2005) 119–137. 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- [227].Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M, Toxicity Evaluations of Nanoparticles: Cell “Vision” versus Superparamagnetic Iron Oxide Physicochemical Properties of Nanoparticles, ACS Nano. 5 (2011) 7263–7276. [DOI] [PubMed] [Google Scholar]
- [228].Yarjanli Z, Ghaedi K, Esmaeili A, Rahgozar S, Zarrabi A, Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation, BMC Neurosci. 18 (2017) 1–12. 10.1186/s12868-017-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Silva ATCR, Cardoso BCO, e Silva MESR, Freitas RFS, Sousa RG, Synthesis, Characterization, and Study of PLGA Copolymer <i>in Vitro</i> Degradation, J. Biomater. Nanobiotechnol . 06 (2015) 8–19. 10.4236/jbnb.2015.61002. [DOI] [Google Scholar]
- [230].Elmowafy EM, Tiboni M, Soliman ME, Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles, Springer Singapore, 2019. 10.1007/s40005-019-00439-x. [DOI] [Google Scholar]
- [231].Ramot Y, Haim-Zada M, Domb AJ, Nyska A, Biocompatibility and safety of PLA and its copolymers, Adv. Drug Deliv. Rev. 107 (2016) 153–162. 10.1016/j.addr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- [232].Leclerc D, Plant Viral Epitope Display Systems for Vaccine Development, Curr. Top. Microbiol. Immunol. 375 (2011) 47–59. 10.1007/82_2011_183. [DOI] [PubMed] [Google Scholar]
- [233].Makarkov AI, Golizeh M, Ruiz-lancheros E, Gopal AA, Costas-cancelas IN, Chierzi S, Pillet S, Charland N, Landry N, Rouiller I, Wiseman PW, Ndao M, Ward BJ, Plant-derived virus-like particle vaccines drive cross-presentation of in fl uenza A hemagglutinin peptides by human monocyte-derived macrophages, Npj Vaccines. 4 (2019). 10.1038/s41541-019-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].He Z, Sun Y, Cao J, Duan Y, Degradation behavior and biosafety studies of the mPEG-PLGA-PLL copolymer, Phys. Chem. Chem. Phys. 18 (2016) 11986–11999. 10.1039/c6cp00767h. [DOI] [PubMed] [Google Scholar]
- [235].He Z, Sun Y, Wang Q, Shen M, Zhu M, Li F, Duan Y, Degradation and bio-safety evaluation of mPEG-PLGA-PLL copolymer-prepared nanoparticles, J. Phys. Chem. C. 119 (2015) 3348–3362. 10.1021/jp510183s. [DOI] [Google Scholar]
- [236].Kumar V, Sharma N, Maitra SS, In vitro and in vivo toxicity assessment of nanoparticles, Int. Nano Lett. 7 (2017) 243–256. 10.1007/s40089-017-0221-3. [DOI] [Google Scholar]
- [237].Clichici S, Filip A, In vivo Assessment of Nanomaterials Toxicity, Nanomater. - Toxic. Risk Assess. (2015). 10.5772/60707. [DOI] [Google Scholar]
- [238].Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, Douglas T, Young MJ, Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo, Int. J. Nanomedicine. 2 (2007) 715–733. [PMC free article] [PubMed] [Google Scholar]
- [239].Blandino A, Lico C, Baschieri S, Barberini L, Cirotto C, Blasi P, Santi L, In vitro and in vivo toxicity evaluation of plant virus nanocarriers, Colloids Surfaces B Biointerfaces. 129 (2015) 130–136. 10.1016/j.colsurfb.2015.03.039. [DOI] [PubMed] [Google Scholar]
- [240].Trifonova EA, Zenin VA, Nikitin NA, Yurkova MS, Ryabchevskaya EM, Putlyaev EV, Donchenko EK, Kondakova OA, Fedorov AN, Atabekov JG, Karpova OV, Study of rubella candidate vaccine based on a structurally modified plant virus, Antiviral Res. 144 (2017) 27–33. 10.1016/j.antiviral.2017.05.006. [DOI] [PubMed] [Google Scholar]
- [241].Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJAM, What do we (need to) know about the kinetic properties of nanoparticles in the body?, Regul. Toxicol. Pharmacol. 49 (2007) 217–229. 10.1016/j.yrtph.2007.07.006. [DOI] [PubMed] [Google Scholar]
- [242].Li M, Al-Jamal KT, Kostarelos K, Reineke J, Physiologically based pharmacokinetic modeling of nanoparticles, ACS Nano. 4 (2010) 6303–6317. 10.1021/nn1018818. [DOI] [PubMed] [Google Scholar]
- [243].Almeida JPM, Chen AL, Foster A, Drezek R, In vivo biodistribution of nanoparticles, Nanomedicine. 6 (2011) 815–835. 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- [244].Pitek AS, Wen AM, Shukla S, Steinmetz NF, The Protein Corona of Plant Virus Nanoparticles Influences their Dispersion Properties, Cellular Interactions, and in Vivo Fates, Small. 12 (2016) 1758–1769. 10.1002/smll.201502458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE, Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy, Adv. Drug Deliv. Rev. 61 (2009) 428–437. 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Owens DE, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int. J. Pharm. 307 (2006) 93–102. 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [247].Dobrovolskaia MA, McNeil SE, Immunological properties of engineered nanomaterials, Nanosci. Technol. A Collect. Rev. from Nat. Journals. (2009) 278–287. 10.1142/9789814287005_0029. [DOI] [Google Scholar]
- [248].Karmali PP, Simberg D, Interactions of nanoparticles with plasma proteins: Implication on clearance and toxicity of drug delivery systems, Expert Opin. Drug Deliv. 8 (2011) 343–357. 10.1517/17425247.2011.554818. [DOI] [PubMed] [Google Scholar]
- [249].Gulati NM, Pitek AS, Steinmetz NF, Stewart PL, Cryo-electron tomography investigation of serum albumin-camouflaged tobacco mosaic virus nanoparticles, Nanoscale. 9 (2017) 3408–3415. 10.1039/c6nr06948g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Lee KL, Shukla S, Wu M, Ayat NR, El Sanadi CE, Wen AM, Edelbrock JF, Pokorski JK, Commandeur U, Dubyak GR, Steinmetz NF, Stealth filaments: Polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X, Acta Biomater. 19 (2015) 166–179. 10.1016/j.actbio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Pitek AS, Jameson SA, Veliz FA, Shukla S, Steinmetz NF, Serum albumin “camouflage” of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics, Biomaterials. 89 (2016) 89–97. 10.1016/j.biomaterials.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Abdollah MRA, Carter TJ, Jones C, Kalber TL, Rajkumar V, Tolner B, Gruettner C, Zaw-thin M, Bagun J, Ellis M, Robson M, Pedley RB, Mulholland P, De Rosales RTM, Chester KA, Fucoidan Prolongs the Circulation Time of Dextran-Coated Iron Oxide Nanoparticles, ACS Nano. 12 (2018) 1156–1169. 10.1021/acsnano.7b06734. [DOI] [PubMed] [Google Scholar]
- [253].Oberhardt B, Franzen S, Pharmacokinetics and efficacy of doxorubicin-loaded plant virus nanoparticles in preclinical models of cancer, Nanomedicine. 12 (2017) 2519–2532. [DOI] [PubMed] [Google Scholar]
- [254].Gaumet M, Vargas A, Gurny R, Delie F, Nanoparticles for drug delivery: The need for precision in reporting particle size parameters, Eur. J. Pharm. Biopharm. 69 (2008) 1–9. 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [255].Panagi Z, Beletsi A, Evangelatos G, Livaniou E, Ithakissios DS, Avgoustakis K, Effect of dose on the biodistribution and pharmacokinetics of PLGA and PLGA-mPEG nanoparticles, Int. J. Pharm. 221 (2001) 143–152. 10.1016/S0378-5173(01)00676-7. [DOI] [PubMed] [Google Scholar]
- [256].Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP, Evaluation of the toxicity of intravenous delivery of auroshell particles (Gold-Silica Nanoshells), Int. J. Toxicol. 31 (2012) 584–594. 10.1177/1091581812465969. [DOI] [PubMed] [Google Scholar]
- [257].Liu Z, Davis C, Cai W, He L, Chen X, Dai H, Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 1410–1415. 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Da Silva-Candal A, Brown T, Krishnan V, Lopez-Loureiro I, Ávila-Gómez P, Pusuluri A, Pérez-Díaz A, Correa-Paz C, Hervella P, Castillo J, Mitragotri S, Campos F, Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions, J. Control. Release. 309 (2019) 94–105. 10.1016/j.jconrel.2019.07.026. [DOI] [PubMed] [Google Scholar]
- [259].Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR, Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers, Mol. Ther. 16 (2008) 1450–1458. 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Cooley M, Sarode A, Hoore M, Fedosov DA, Mitragotri S, Sen Gupta A, Influence of particle size and shape on their margination and wall-adhesion: implications in drug delivery vehicle design across nano-to-micro scale, Nanoscale. 10 (2018) 15350–15364. 10.1039/c8nr04042g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Toy R, Peiris PM, Ghaghada KB, Karathanasis E, Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles, Nanomedicine. 9 (2014) 121–134. 10.2217/nnm.13.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Adriani G, Donato M, Tullio D, Ferrari M, Hussain F, Pascazio G, Liu X, Decuzzi P, The preferential targeting of the diseased microvasculature by disk-like particles, Biomaterials. 33 (2012) 5504–5513. 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Chariou PL, Lee KL, Pokorski JK, Saidel GM, Steinmetz NF, Diffusion and Uptake of Tobacco Mosaic Virus as Therapeutic Carrier in Tumor Tissue: Effect of Nanoparticle Aspect Ratio, J. Phys. Chem. B. 120 (2016) 6120–6029. 10.1021/acs.jpcb.6b02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Le DHT, Méndez-lópez E, Wang C, Aranda MA, Steinmetz NF, Biodistribution of Filamentous Plant Virus Nanoparticles : Pepino Mosaic Virus versus Potato Virus X, Biomacromolecules. 20 (2019) 469–477. 10.1021/acs.biomac.8b01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Yin X, Luo L, Li W, Yang J, Zhu C, Jiang M, qin B, Yuan X, Yin H, Lu Y, Du Y, Chen D, You J, A cabazitaxel liposome for increased solubility, enhanced antitumor effect and reduced systemic toxicity, Asian J. Pharm. Sci. 14 (2019) 658–667. 10.1016/j.ajps.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Yao XIN, Panichpisal K, Kurtzman N, Cisplatin Nephrotoxicity : A Review, Am. J. Med. Sci. 334 (2007) 115–124. 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- [267].Knudsen KB, Northeved H, Pramod Kumar EK, Permin A, Gjetting T, Andresen TL, Larsen S, Wegener KM, Lykkesfeldt J, Jantzen K, Loft S, Møller P, Roursgaard M, In vivo toxicity of cationic micelles and liposomes, Nanomedicine Nanotechnology, Biol. Med. 11 (2015) 467–477. 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- [268].Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, Huang AY, Levine AD, Steinmetz NF, Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers, Biomaterials. 121 (2017) 15–27. 10.1016/j.biomaterials.2016.12.030. [DOI] [PubMed] [Google Scholar]
- [269].Marbrook J, Matthews REF, The Differential lmmunogenicity of Plant Viral Protein Nucleoproteins, Virology. 28 (1966) 219–228. [DOI] [PubMed] [Google Scholar]
- [270].Albakri MM, Veliz FA, Fiering SN, Steinmetz NF, Sieg SF, Endosomal toll-like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus, Immunology. 159 (2019) 183–192. 10.1111/imm.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat. Immunol. 11 (2010) 373–384. 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [272].Mccormick AA, Corbo TA, Wykoff-clary S, V Nguyen L, Smith ML, Palmer KE, Pogue GP, TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models, Vaccine. 24 (2006) 6414–6423. 10.1016/j.vaccine.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [273].Uhde-holzem K, Schlösser V, Viazov S, Fischer R, Commandeur U, Immunogenic properties of chimeric potato virus X particles displaying the hepatitis C virus hypervariable region I peptide R9, J. Virol. Methods. 166 (2010) 12–20. 10.1016/j.jviromet.2010.01.017. [DOI] [PubMed] [Google Scholar]
- [274].Cai H, Shukla S, Wang C, Masarapu H, Steinmetz NF, Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine, J. Am. Chem. Soc. 141 (2019) 6509–6518. 10.1021/jacs.9b01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Gilleland HE, Gilleland LB, Staczek J, Harty RN, Palese P, Brennan FR, Hamilton WDO, Chimeric animal and plant viruses expressing epitopes of outer membrane protein F as a combined vaccine against Pseudomonas aeruginosa lung infection, FEMS Immunol. Med. Microbiol. 27 (2000) 291–297. [DOI] [PubMed] [Google Scholar]
- [276].Rybicki EP, Plant molecular farming of virus-like nanoparticles as vaccines and reagents, WIREs Nanomed Nanobiotechnol. 12 (2020) 1–22. 10.1002/wnan.1587. [DOI] [PubMed] [Google Scholar]
- [277].Balke I, Zeltins A, Recent Advances in the Use of Plant Virus-Like Particles as Vaccines, Virus. 12 (2020) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Wang C, Steinmetz NF, CD47 Blockade and Cowpea Mosaic Virus Nanoparticle In Situ Vaccination Triggers Phagocytosis and Tumor Killing, Adv. Healthc. Mater. 8 (2019). 10.1002/adhm.201801288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Beatty PH, Lewis JD, Cowpea mosaic virus nanoparticles for cancer imaging and therapy Cowpea mosaic virus nanoparticles for cancer imaging and therapy, Adv. Drug Deliv. Rev. 145 (2019) 130–144. 10.1016/j.addr.2019.04.005. [DOI] [PubMed] [Google Scholar]
- [280].Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer, Nat. Nanotechnol. (2015). 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Peruzzi PP, Chiocca EA, A vaccine from plant virus proteins, Nat. Nanotechnol. (2015) 1–2. 10.1038/nnano.2015.306. [DOI] [PubMed] [Google Scholar]
- [282].Wang C, Beiss V, Steinmetz NF, Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties, J. Virol. 93 (2019). 10.1128/jvi.00129-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Wang C, Fiering SN, Steinmetz NF, Cowpea Mosaic Virus Promotes Anti‐Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model, Adv. Ther. 2 (2019) 1900003. 10.1002/adtp.201900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PLR, Sloan AE, Steinmetz NF, Plant virus-like particle in situ vaccine for intracranial glioma immunotherapy, Cancers (Basel). 11 (2019) 1–17. 10.3390/cancers11040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Carignan D, Herblot S, Eve M, Activation of innate immunity in primary human cells using a plant virus derived nanoparticle TLR7/8 agonist, Nanomedicine Nanotechnology, Biol. Med. 14 (2017) 2317–2327. 10.1016/j.nano.2017.10.015. [DOI] [PubMed] [Google Scholar]
- [286].Shukla S, Wang C, Beiss V, Cai H, II TW, Murray AA, Gong X, Zhao Z, Masarapu H, Zlotnick A, Ieringf SF, Steinmetz NF, The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine, Biomater. Sci. (2020). https://doi.org/DOI: 10.1039/d0bm01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Murray AA, Wang C, Fiering S, Steinmetz NF, In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma, Mol. Phar. 15 (2018) 3700–3716. 10.1021/acs.molpharmaceut.8b00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].ève Lebel M, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A, Potentiating cancer immunotherapy using Papaya mosaic virus-derived nanoparticles, Nano Lett. 16 (2016) 1826–32. 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- [289].Lee KL, Murray AA, Le DHT, Sheen MR, Shukla S, Commandeur U, Fiering S, Steinmetz NF, Combination of Plant Virus Nanoparticle-Based in Situ Vaccination with Chemotherapy Potentiates Antitumor Response, Nano Lett. 17 (2017) 4019–4028. 10.1021/acs.nanolett.7b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Liu R, Vaishnav RA, Roberts AM, Friedland RP, Humans Have Antibodies against a Plant Virus : Evidence from Tobacco Mosaic Virus, PLoS One. 8 (2013) e60621. 10.1371/journal.pone.0060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Shukla S, Dorand RD, Myers JT, Woods SE, Gulati NM, Stewart PL, Commandeur U, Huang AY, Steinmetz NF, Multiple Administrations of Viral Nanoparticles Alter in Vivo Behavior - Insights from Intravital Microscopy, ACS Biomater. Sci. Eng. 2 (2016) 829–837. 10.1021/acsbiomaterials.6b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Mingozzi F, High KA, Immune responses to AAV vectors : overcoming barriers to successful gene therapy, Blood. 122 (2013) 23–36. 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Wang D, Tai PWL, Gao G, Adeno-associated virus vector as a platform for gene therapy delivery, Nat. Rev. Drug Discov. 18 (2019) 358–378. 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Nayak S, Herzog RW, Progress and prospects : immune responses to viral vectors, Gene Ther. 17 (2009) 295–304. 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Shirley JL, De Jong YP, Terhorst C, Herzog RW, Immune Responses to Viral Gene Therapy Vectors, Mol. Ther. 28 (2020) 709–722. 10.1016/j.ymthe.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Ronzitti G, Gross D, Mingozzi F, Clement N, Human Immune Responses to Adeno-Associated Virus ( AAV ) Vectors, Front. Immunol. 11 (2020) 1–13. 10.3389/fimmu.2020.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Verdera HC, Kuranda K, Mingozzi F, AAV Vector Immunogenicity in Humans : A Long Journey to Successful Gene Transfer, Mol. Ther. 28 (2020) 723–746. 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Li C, Samulski RJ, Engineering adeno-associated virus vectors for gene therapy, Nat. Rev. Genet. 21 (2020) 255–272. 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- [299].Mingozzi F, High KA, Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors : The Race Between Clearance, Tolerance, Neutralization, and Escape, Annu. Rev. Virol. 4 (2017) 511–34. [DOI] [PubMed] [Google Scholar]
- [300].Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus M, Moullier P, Benveniste O, Masurier C, A 10 Patient Case Report on the Impact of Plasmapheresis Upon Neutralizing Factors Against Adeno-associated Virus ( AAV ) Types 1, 2, 6, and 8, Mol Ther. 19 (2011) 2084–2091. 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Chicoine LG, Montgomery CL, Bremer WG, Shontz KM, Griffin DA, Heller KN, Lewis S, Walker CM, Clark KR, Sahenk Z, Mendell JR, Rodinoklapac LR, Plasmapheresis Eliminates the Negative Impact of AAV Antibodies on Microdystrophin Gene Expression Following Vascular Delivery, Mol. Ther. 22 (2014) 338–347. 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Hirvinen M, Capasso C, Guse K, Garofalo M, Vitale A, Ahonen M, Kuryk L, Vähä-koskela M, Hemminki A, Fortino V, Greco D, Cerullo V, Expression of DAI by an oncolytic vaccinia virus boosts the immunogenicity of the virus and enhances antitumor immunity, Mol. Ther. — Oncolytics. 3 (2016) 16002. 10.1038/mto.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Faust SM, Rabinowitz JE, Wilson JM, Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, Wilson JM, CpG-depleted adeno-associated virus vectors evade immune detection, J. Clin. Invest. 123 (2013) 2994–3001. 10.1172/JCI68205.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Chan YK, Lim E, Chan R, Graveline A, Letizia A, Chiang J, Vernet A, Pezone M, Church G, Engineering AAV vectors to evade innate immune and inflammatory responses, Mol. Ther. 26. 26 (2018) 457–458. [Google Scholar]
- [305].Schaffer D, Büning H, Enhancing the clinical potential of AAV vectors by capsid engineering to evade pre-existing immunity, Front Microbiol. 2 (2011) 1–10. 10.3389/fmicb.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Melis D, Carbone F, Minopoli G, La Rocca C, Perna F, De Rosa V, Galgani M, Andria G, Parenti G, Matarese G, Cutting Edge: Increased Autoimmunity Risk in Glycogen Storage Disease Type 1b Is Associated with a Reduced Engagement of Glycolysis in T Cells and an Impaired Regulatory T Cell Function, J. Immunol. 198 (2017) 3803–3808. 10.4049/jimmunol.1601946. [DOI] [PMC free article] [PubMed] [Google Scholar]